Abstract
Purpose of Review
There is still substantial controversy surrounding the utility of decompressive craniectomy (DC) in patients with traumatic brain injury (TBI). Some surgeons readily perform these operations, while others are more hesitant due to concerns about patient outcomes in severe TBI.
Recent Findings
In this paper, the authors outline recent literature regarding the use of DC in TBI patients, starting with a brief background on surgical methods then examining the results of recent retrospective studies, case series, and randomized trials.
Summary
Despite the controversy, and while a new randomized control trial is pending publication, DC remains an important tool in managing patients with TBI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
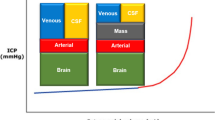
Decompressive craniotomy and decompressive craniectomy (DC) encompass a spectrum of pathological entities requiring the removal of the cranium to alleviate intracranial hypertension. With severe traumatic brain injury (TBI) the brain will often swell. When the volume of the brain exceeds that of the skull, the intracranial pressure (ICP) will increase. First line management is medical and includes keeping the head elevated, raising the tonicity of intravascular volume with mannitol and/or hypertonic saline, using analgesics and/or sedatives, and placing an external ventricular drain to remove cerebrospinal fluid (CSF). Craniotomy is the act of removing the skull for decompression of the brain with the implication that the bone will be replaced at the end of the operation unless brain swelling, or the prospect of severe brain swelling, renders craniectomy an alternative. Decompressive craniectomy is removal of the bone without replacement and has been shown to effectively decrease refractory high ICP in patients who fail medical management.
Traumatic brain injury can be divided into specific injuries, each requiring different forms of management and treatment. These subdivisions include epidural hematomas (EDH), subdural hematomas (SDH), traumatic parenchymal lesions such as contusions, posterior fossa mass lesions, depressed skull fractures, diffuse axonal injury, or diffuse brain edema.
Surgical Management of TBI
The Guidelines for the Surgical Management of Traumatic Brain Injury recommend that patients with parenchymal mass lesions from TBI and signs of progressive neurological deterioration due to refractory increased ICP should be treated operatively. Indications for operative intervention include patients with Glasgow coma scale (GCS) scores of 6–8 with frontal or temporal contusions with volume larger than 20 cc with either midline shift greater than 5 mm or cisternal compression demonstrated on CT scan, and for patients with GCS scores of 6–8 with any lesion volume greater than 50 cc. They recommend that patients without neurological compromise, who have controlled ICP, and who have no significant signs of mass effect on imaging may be managed nonoperatively with intensive monitoring and serial imaging [1••].
The guidelines recommend that patients with acute epidural hematomas be surgically evacuated if the EDH exceeds a volume of greater than 30 cc regardless of GCS score. Patients with a EDH less than 30 cc, less than 15 mm of thickness, and less than 5 mm of midline shift, with a GCS score of greater than 8 without focal deficit, may be managed nonoperatively with close observation and serial CT scanning. It is strongly recommended that patients with an acute EDH with GCS <9 with anisocoria undergo emergent surgical evacuation [2••].
The guidelines recommend that patients with acute subdural hematomas from TBI be treated with immediate operative intervention if they exhibit a SDH with a thickness of greater than 10 mm or midline shift of greater than 5 mm regardless of GCS score. It is also recommended that patients with GCS scores less than 9 with a SDH less than 10 mm in thickness or midline shift less than 8 mm should undergo emergent surgical evacuation of the lesion if the GCS score decreased by 2 or more points from the time of the injury to the time of hospital admission, or if the patient presents with asymmetric or fixed and dilated pupils. It is recommended that if surgical evacuation of an acute SDH is indicated, it should be performed using a craniotomy with or without replacement of the bone flap [3••].
Decompressive Craniectomy
If surgical indications are met, craniotomy with evacuation of mass lesion is recommended for focal lesions. DC is recommended as a treatment option if there is significant brain swelling at the time of initial craniotomy for mass lesion, or in the postinjury period if ICP elevations become refractory to medical management. DC procedures include subtemporal decompression, bifrontal/bifrontotemporal, or hemispheric DC. Temporal or frontal lobar resection may also be performed in conjunction with the craniectomy.
DC can be unilateral or bilateral depending upon the extent and location of parenchymal edema. In 2001, Whitfield et al. presented a series of 26 patients showing that bifrontal decompressive craniectomy was associated with a significant reduction in ICP from 37.5 to 18.1 mmHg. In addition, the amplitude of ICP waveforms and compensatory reserve was found to be decreased, suggesting improved pressure dynamics. Sixty-nine percent of the patients had a favorable outcome, 8 % were severely disabled, and 23 % died [4].
A more recent case series of bilateral decompressive craniectomy showed a reduction of ICP from 37.7 to 27.4 mmHg and a larger decrease to 11.2 with opening and enlargement of the dura mater. Cerebral perfusion pressure was 57.6 initially and increased to 63.3 mmHg with bone removal and 77.8 mmHg with dura opening. In total, 54 % of patients had favorable outcomes and 46 % of patients had unfavorable outcomes. The most common complication was hydrocephalus (19 %) [5].
In general, the large frontotemporoparieto (occipital) craniectomy, or “hemicraniectomy,” is used in many cases where there is diffuse injury or laterality of compressive pathology. It is important to assure that the opening is large enough to minimize strangulation of cerebral veins and venous infarction due to swelling beyond the confines of the craniectomy defect. The anterior–posterior diameter should be no smaller than 12–15 cm (Fig. 1) [6•]. For bifrontal craniectomies, removal of the falx and anterior third of the superior sagittal sinus has been described to account for expansion of brain swelling, but ICP reduction has been demonstrated without this technique [7••].
Hinge Craniotomy
An alternative surgical method to DC is the use of hinge craniotomy (HC). In a HC, the bone flap is replaced before closure. The bone flap is secured at one bone edge with a titanium plate attachment and the other edges of the bone flap have a plate attachment secured only on the bone flap, allowing the free edge to sit over the edge of the craniotomy. This allows the bone flap to expand outward, but prevents it from sinking inward toward the brain.
One early retrospective review was conducted with 20 patients undergoing HC and 30 patients undergoing DC for trauma and stroke with intracranial hypertension. There was no difference in the postoperative reduction and control of ICP between the two groups. The overall mean hospital survival, duration of mechanical ventilation and ICU stay, size of skull defect, operative time, and postoperative imaging, including Rotterdam scores, were also similar in each group [8].
Another retrospective review of 25 consecutive patients undergoing HC showed good cerebral decompression in all patients as described by the authors. The in-hospital mortality was 48 %. None of the patients required surgery for bone flap replacement or a postoperative helmet. Long-term follow-up showed that one patient required subsequent cranioplasty due to infection and one patient returned with cranial deformity. The authors concluded that HC was a viable alternative to DC [9•].
DC in Combat
DC is a valuable and potentially a life-saving procedure in wartime for military use. Surgeons can use the technique as a way to treat soldiers or civilians injured near war zones to give maximal control of ICP. Interestingly, one study using early DC for sustained, severe penetrating and closed head injury found that the patients who underwent craniectomy had worse injuries than those in whom the bone flap was replaced. Of 408 patients who had surgery, 188 underwent craniotomy. While the outcomes of patients undergoing DC were not as good as compared to those with craniotomy, the craniectomy patients showed significant clinical improvements over time. The authors recommend hemicraniectomy as a damage control procedure to protect patients from further brain swelling during transport to a facility capable of providing definitive care [10].
Ragel et al. presented craniotomy and craniectomy outcomes from the Afghanistan conflict over a two-year period from 2007 to 2009. DC was done in 28 cases and represented 31 % of all 91 craniotomies performed in Afghanistan during that period. The authors concluded that the use of DC allowed for an extended window for safe transfer to tertiary hospitals. The authors recommend use of the L.G. Kempe incision, which is a midline sagittal incision with a “T-bar” extension starting 1–2 cm anterior to the tragus at the level of the zygoma extending to sagittal incision 1 cm posterior to the coronal suture. They suggest that use of this incision can help preserve blood supply and allow for a large craniectomy to prevent brain strangulation over the bone edges, resulting in minimal brain debridement. They also advocated for the use of duraplasty with onlay substitutes for maximal brain decompression [11•].
DC Outcomes
One of the criticisms of DC is that while effective at decreasing ICP and improving survival, the quality of life of survivors remains uncertain. In 2009, Shabbar et al. looked at 29 studies reporting outcomes of hemicraniectomy for TBI that used Glasgow outcome scores (GOS). From these studies, 1422 cases were analyzed. The average 6-month postoperative mortality rate was 28.2 %. GOS were converted to a quality of life score for statistical and analytical analysis. The mean quality of life score for survivors was 0.592, which had a corresponding GOS of 4 (moderate disability). The authors posit that this finding argued against the assumption that most TBI survivors remain in a vegetative state or severely disabled following hemicraniectomy [12].
A respective review of 43 patients who underwent DC to control intracranial hypertension without a space occupying hematoma showed that ICP was significantly decreased from a mean of 37.8–12.7 mmHg. Overall mortality was 25.6 %. The overall survival rate was 74.4 %; 32.5 % of patients remained in a vegetative state or were severely disabled, and the remaining 41.9 % had a favorable outcome [13].
Some authors have also evaluated the effect DC has on brain oxygenation. A series of ten patients with severe TBI, who had continuous PbtO2 monitoring before and after delayed DC, were retrospectively analyzed to evaluate for changes in cumulative ischemic burden and therapeutic intensity levels. In this study, the ICP showed a mean decrease of 7.86 mmHg after craniectomy, and the therapeutic intensity level decreased in accordance with ICP decrease. The duration and severity of cumulative ischemic burden were significantly decreased as an effect of DC [14].
Jacob et al. focused on outcomes in the pediatric population. They described a series of 11 pediatric patients who underwent DC at a single institution over an eight-year period. One patient died (9 %) and 7 patients (70 %) had a favorable outcome (GOC of 4 or 5). The authors also pooled their outcomes with previously published results. Combined outcomes of 186 pediatric patients undergoing DC had 42 deaths (21.1 % mortality), and 112 patients had good outcomes (77.7 % of survivors with GOC of 4 or 5 at 6 months). Based on this analysis, DC showed good outcome in the majority of patients [15].
Kwok et al. conducted a retrospective review to assess for the incidence and factors associated with delayed neurologic recovery after DC. Out of 176 patients who underwent DC for nonpenetrating TBI, 104 patients (59 %) had moderate to severe disability 6 months after surgery. By 18 months after surgery, 48 % of those patients showed 1-point improvement in Glasgow outcome scale. Of the 59 patients who had an unfavorable outcome at 6 months (severe disability or vegetative state), 25 % of patients improved a favorable outcome at 18 months (moderate disability or near normal neurologic function). The two factors associated with a higher chance of delayed recovery were an absence of a nonevacuated intracerebral hematoma larger than 1 cm (OR 6.67, 95 % CI 1.12–33.3) and a higher GCS at admission (1.44, 95 % CI 1.07–1.96) [16•].
A comparison of craniotomy versus DC in patients with severe head injury (GCS 4–8) with an acute subdural hematoma was done with 102 patients. The review was done retrospectively at a single institution over nine years. 42 (41.2 %) of the patients underwent craniotomy and 60 patients underwent DC. There was no difference in baseline demographic or clinical data. There was no significant difference in outcomes or complications rates. The craniectomy group had a higher overall mortality (23.3 vs. 7.1 %; p = 0.04) [17].
A series of 201 head injured patients over a three-year period at a single institution examined outcomes of patients undergoing DC. The 30-day mortality rate was 26.4 %. Identified independent risk factors for mortality were patient age [OR 1.035 (95 % CI 1.006–1.064)] and initial GCS score [OR 0.769 (95 % CI 0.597–0.990)] [18].
A retrospective review of patients who underwent DC and comparison of injury based on presence of a mass lesion was conducted. 164 patients were reviewed; 93 had a mass lesion treated as part of the operation, and 71 patients were treated for diffuse brain swelling. Overall mortality was 22 %. 36 % of patients remained either in a vegetative state or severely disabled. The remaining 42 % patients had a good outcome. Characteristics associated with increased mortality included age greater than 50 (OR 2.36, 95 % CI 1.01–5.52), an abnormal pupillary response (OR 3.79, 95 % CI 1.29–11.14), and DC without mass evacuation (OR 0.31, 95 % CI 0.12–0.79). DC with mass evacuation had a lower mortality in this series. (14.0 % vs. 32.4 %) [19].
Recent analysis of 166 patients with fixed, dilated pupils who underwent DC compared to 41 who continued with medical management showed a significant decrease in ICP (from 36.2 to 14.3 mmHg after DC) with decreased mortality (39.7 %) compared to medical management (87.8 %), with “favorable” outcome of 34.3 % in the DC group. Of the DC patients, 77.7 % had unilateral and 22.9 % had bilateral fixed pupils compared to 61 and 39 %, respectively, of the medical management group. The mortality rates were 34.1 and 59.4 %, for unilateral and bilateral fixed, dilated pupils, respectively compared to those in the medical management group (100 and 80 %, respectively). This study indicated that ICP can be controlled in severe TBI patients with pupillary abnormalities with larger than expected numbers achieving favorable outcomes [20].
Randomized Trials for DC
The bulk of the evidence for DC presented has used retrospective reviews. Two well-known randomized trials of decompressive craniectomy for severe TBI were published. In the first, 27 children were randomized to receive standardized medical management or bitemporal craniectomy performed in the first 30 h (mean time to procedure 19.2 h) following injury. All patients had ICP recorded hourly via an intraventricular catheter. Mean ICP was 8.98 mmHg lower in the decompression group. 14 % of children in medical management group were normal or had mild disability after 6 months compared to 54 % of the children who received craniectomy [21••].
The so-called “DECRA” trial (Early Decompressive Craniectomy in Patients with Severe Traumatic Brain Injury) studied 155 patients with diffuse injury without significant mass lesions. Patients were randomized to undergo either early bifrontotemporoparietal DC after failing first-tier ICP management using a threshold of 20 mmHg or further medical management. The primary outcome was evaluated using the Extended Glasgow outcome scale at 6 months after the injury. Patients who underwent craniectomy had less time with elevated ICP, less interventions for ICP, and fewer days in the ICU; however, they had greater risk for worse outcome based on the extended Glasgow outcome scale (OR 1.84; 95 % CI 1.05–3.24) and a greater risk of an unfavorable outcome (OR 2.21; 95 % CI 1.14–4.26). Overall mortality was not significantly different in each group (19 % in craniectomy group and 18 % in standard-care group) [7••]. This study has been the criticized for several reasons. Importantly, patients in the DC group had a higher incidence of fixed pupils, when corrected for, resulted in no significant difference in outcome between the groups. This study, however, does provide evidence that early DC with bifrontal craniectomy is no more effective than continued medical management in patients with diffuse brain injury.
A systematic review of the literature published after DECRA identified three randomized, single-center trials regarding DC, only one of which compared DC with medical management. This study involved only 20 patients, equally divided between the groups, and no statistically significant difference in outcome was found between them. The other two compared DC with craniotomy for acute subdural hematomas that used alternative dural openings for decompression, such as multiple small incisions, the latter of which seemed to have an advantage over DC. Nonrandomized, prospective, and retrospective studies were also reviewed and seemed to suggest that younger patients presenting with higher Glasgow coma scores, undergoing DC within 5 h of injury, may fare better [22]. At present, even when accounting for DECRA, there is still equipoise as to the true utility of DC in affecting outcome and, even, quality of life. The review clearly underscores the need for further studies to determine the specific type of TBI patient that would most benefit from DC.
The highly anticipated randomized, controlled trial, RESCUEicp trial (The Randomised Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of Intra-Cranial Pressure) is awaiting publication. This study will examine the differences in 400 patients either undergoing decompressive craniectomy for treatment of uncontrolled intracranial hypertension versus continued medical treatment. The ICP threshold for treatment for this trial is >25 mmHg compared to the DECRA’s >20 mg Hg [23•]. An additional study is underway to study outcomes in acute subdural hematoma patients undergoing craniotomy or craniectomy at the time of initial surgical evacuation, the so-called “RescueASDH” trial (http://www.rescueasdh.org/).
Complications of DC
The patients who undergo DC often have severe injuries and may suffer numerous complications in the postoperative period. A retrospective review of 108 patients who underwent DC was analyzed to determine the complications that occured secondary to DC. Overall mortality was 23.1 %. The overall rate of having a good outcome (GOS of 4 or 5) was 50.9 %. Fifty percent of patients had a complication related to surgical decompression and 25.9 % of patients had multiple complications. The most common complication was herniation through the craniectomy defect (27.8 %) followed by subdural effusion (21.3 %), syndrome of trephined (13.0 %), post-traumatic hydrocephalus (9.3 %), contralateral intracranial hematoma (7.4 %), intracranial infection (3.7 %), CSF leak (3.7 %), and epilepsy (2.8 %) [24].
Another retrospective review of 50 patients who underwent DC showed 28 % mortality and 40 % good outcome (GOS of 4 or 5). ICP was reduced from a mean of 23.9 to 14.4 mmHg. Complications included subdural hygromas (50 %), ipsilateral hemorrhagic swelling (16 %), and hydrocephalus (10 %) [25].
Nalback et al. focused on the postoperative extra-axial collections. Their retrospective review looked at 34 patients that underwent DC for refractory ICP were being treated for hydrocephalus to elucidate the phenomenon of extra-axial fluid collections. Of these, 21 patients (62 %) developed extra-axial fluid collections and 18 (53 %) developed a collection despite ventricular drainage. The authors argue that the term external hydrocephalus does not adequately describe what is happening and, instead, suggest using the term craniectomy-associated progressive extra-axial collections with treated hydrocephalus (CAPECTH). They state that early cranioplasty can help prevent the formation and worsening of these extra-axial fluid collections [26].
A recent literature review highlighted many of the complications of DC and separated them out by time course. Early complications in the perioperative phase include expansion of contusions, formation of new subdural and epidural hematomas in the contralateral hemisphere, and external cerebral herniation. Complications that occur within the first week following surgery include subdural hygroma formation and paradoxical herniation after lumbar puncture. Late phase complications include wound-healing complications and trephination syndromes resulting in symptoms ranging from headaches to focal motor deficits to acute mental status deterioration [27•].
Aarabi et al. reviewed patients who underwent DC to evaluate risk factors for subdural hygroma formation. Two cohorts of patients who had undergone DC were identified. Thirty-nine patients developed subdural hygroma and were compared to 29 patients who did not develop a hygroma. The earliest imaging evidence of a subdural hygroma was seen during the first week following surgery. The greatest size of hygroma was seen between 3 and 4 weeks after craniectomy, and a gradual resolution was seen by the 17th week. Motor vehicle accidents were the most often linked mechanism to the development of a hygroma and falls were the least often associated. Additionally, patients with diffuse brain injury were more prone to hygroma formation than patients who had a mass evacuated. Eight percent of hygromas converted themselves into subdural hematomas and required surgical evacuation [28]. The primary theory behind the development of hydrocephalus and hygromas after DC involves alteration of CSF flow dynamics [27•] likely related to effects of atmospheric pressure on the cerebrum and disruptions of normal CSF resorption pathways.
In contrast to civilian studies, 108 patients who had undergone DC and subsequent cranioplasty during Operation Iraqi Freedom and Operation Enduring Freedom, Afghanistan, from 2002 to 2008 were reviewed for complications. The predominant mechanism of injury in this series was explosive blast injury (67 %). The average time to cranioplasty was 190 days (range 7–546 days). The overall complication rate was 24 %. Complications included perioperative infection (12 %), seizure (7.4 %), and extra-axial hematoma formation (7.4 %) [29].
A retrospective review was done on 57 patients who had hemorrhagic contusions from TBI, of which 25 patients underwent DC and 32 patients underwent medical therapy alone. A comparison of the two cohorts showed that 16 % of the patients showed an increase of contusion size in the craniectomy group compared to 12.5 % of patients in the medical group. They concluded that DC does not seem to constitute a risk factor for the evolution of hemorrhagic contusions [30].
Cranioplasty
There are three different ways to manage the bone flap after DC. The first is to discard the bone flap. If this method is used, a synthetic bone flap must be utilized at the time of reconstruction. The second option is to place the bone flap in a subcutaneous pocket over the abdomen. The third option is cryopreservation.
A recent series compared the infection rates between subcutaneous pocket storage and cryopreservation. The study reviewed 70 cases. The rate of surgical site infection was 5.1 % in the subcutaneous pocket storage group versus 16.1 % in the cryopreservation group. When comparing a subgroup of only TBI patients, the rate of surgical site infection was 0 % in the subcutaneous pocket storage group versus 28.6 % in the cryopreservation group [31].
Cranioplasty can be done with various alloplastic grafts if the original bone flap is not available. These include polymethylmethacrylate, polyetheretherketone, and titanium mesh implants as well as hydroxyapatite or calcium carbonate-based materials. A review of 26 patients who underwent cranioplasty with computer-assisted design titanium implants was conducted. The outcomes were evaluated between 6 and 12 years (mean 8.1 years). None of the implants had to be removed. 68 % of patients declared their outcomes as excellent, 24 % reported a good outcome, 0.8 % reported fair outcome, and 0 % reported a bad outcome [32].
A retrospective review identified 62 patients who underwent cranioplasty following DC to determine associated complications. The postoperative complication rate was 34 % and overall reoperation rate was 26 %. Complications included wound infection (14.5 %), bone resorption (6.5 %), wound dehiscence (3.2 %), epidural or subdural hematoma (3.2 %), and DVT (3.2 %). The only factor significantly associated with need for reoperation was the presence of a bifrontal cranial defect (67 % of 12 patients requiring reoperation versus 16 % reoperation rate for unilateral craniectomy) [33].
Logistic regression analysis of 70 patients who underwent cranioplasty after DC for trauma was conducted to determine if there was any association between the timing of cranioplasty and complications. There was no predictive time frame associated with infection or hydrocephalus development in their series. They argued that delayed cranioplasty (3–6 months) did not seem to lower post cranioplasty infection rates nor the need for cerebrospinal fluid diversion procedures. The authors advocated for earlier cranioplasty, ideally within the initial hospitalization, when feasible [34]. Alternatively, Piedra et al. studied 157 patients undergoing cranioplasty at various time intervals and found that patients undergoing cranioplasty between 16 and 20 weeks had the highest incidence of complications compared to those undergoing cranioplasty early or later than this time interval. They concluded that cranioplasty during the initial hospitalization can be performed without increasing complication risks and was associated with shorter operative times and possible cost reduction [35].
Conclusions
Decompressive craniotomy and craniectomy are effective methods to alleviate intracranial hypertension after traumatic brain injury. Methods of DC include subtemporal decompression, hemispheric, bifrontal, or hinged craniotomy with or without removal of the bone flap. Many retrospective studies have shown DC to be effective at decreasing ICP and improving survival after severe TBI, although a major prospective randomized trial has shed doubt on these findings. Factors that are associated with increased mortality include patient age and initial GCS score. Complications rates are variable and include herniation through the craniectomy defect, alterations of CSF flow resulting in subdural hygromas and hydrocephalus, syndrome of the trephined, contralateral intracranial hematomas, and intracranial infection. Reconstruction can be performed with a cryopreserved bone flap or with one placed in an abdominal subcutaneous pocket. Synthetic prostheses are also commonly used. Complications of cranioplasty include wound infection, bone resorption, wound dehiscence, and epidural or subdural hematomas. Upcoming randomized DC trials may offer further guidance on indications and patient selection, but the ultimate use of DC currently remains at the neurosurgeon’s discretion based upon individual patient factors.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of importance
•• Bullock MR, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58(3):S2–25. This article is part of an important guideline document highlighting the indications for surgery in TBI.
•• Bullock MR, et al. Surgical management of acute epidural hematomas. Neurosurgery. 2006;58(3):S2–7. This article is part of an important guideline document highlighting the indications for surgery in TBI.
•• Bullock MR, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3):S16–24. This article is part of an important guideline document highlighting the indications for surgery in TBI.
Whitfield PC, et al. Bifrontal decompressive craniectomy in the management of posttraumatic intracranial hypertension. Br J Neurosurg. 2001;15(6):500–7.
Bao Y-H, et al. Bilateral decompressive craniectomy for patients with malignant diffuse brain swelling after severe traumatic brain injury: a 37-case study. J Neurotrauma. 2010;27(2):341–7.
• Rey-Dios R, Esposito DP. Decompressive craniectomy for intracranial hypertension and stroke, including bone flap storage in abdominal fat layer. In: Ullman JS, Raskin PB, editors. Atlas of emergency neurosurgery. New York: Thieme; 2016. p. 53–72. This reference provides a technique guide for decompressive craniectomy.
•• Cooper DJ, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493–502. This article represents the first major randomized trial for DC in adult patients.
Kenning TJ, Gandhi RH, German JW. A comparison of hinge craniotomy and decompressive craniectomy for the treatment of malignant intracranial hypertension: early clinical and radiographic analysis. Neurosurg Focus. 2009;26(6):E6.
• Schmidt JH III, Reyes BJ, Fischer R, et al. Use of hinge craniotomy for cerebral decompression. J Neurosurg. 2007;107:678–82. This article provides a description of the hinge craniotomy procedure.
Bell RS, et al. Early decompressive craniectomy for severe penetrating and closed head injury during wartime. Neurosurg Focus. 2010;28(5):E1.
• Ragel BT, et al. Wartime decompressive craniectomy: technique and lessons learned. Neurosurg Focus. 2010;28(5):E2. This important article describes the procedure and complications of decompressive craniectomy in combat situations.
Danish SF, et al. Quality of life after hemicraniectomy for traumatic brain injury in adults: a review of the literature. Neurosurg Focus. 2009;26(6):E2.
Eberle BM, et al. Decompressive craniectomy: surgical control of traumatic intracranial hypertension may improve outcome. Injury. 2010;41(9):894–8.
Weiner GM, et al. Decompressive craniectomy for elevated intracranial pressure and its effect on the cumulative ischemic burden and therapeutic intensity levels after severe traumatic brain injury. Neurosurgery. 2010;66(6):1111–9.
Jacob AT, et al. Decompressive hemicraniectomy for pediatric traumatic brain injury: long-term outcome based on quality of life. Pediatr Neurosurg. 2011;47(2):81–6.
• Ho KM, Honeybul S, Litton E. Delayed neurological recovery after decompressive craniectomy for severe nonpenetrating traumatic brain injury. Crit Care Med. 2011;39(11):2495–500. This article highlights that recovery from DC and TBI is an on-going process for 18 months or more and not limited to the 6-month follow up period normally seen in TBI studies.
Chen S-H, et al. Comparison of craniotomy and decompressive craniectomy in severely head-injured patients with acute subdural hematoma. J Trauma Acute Care Surg. 2011;71(6):1632–6.
Huang Y-H, et al. Thirty-day mortality in traumatically brain-injured patients undergoing decompressive craniectomy: clinical article. J Neurosurg. 2013;118(6):1329–35.
Yuan Q, et al. Comparative study of decompressive craniectomy in traumatic brain injury with or without mass lesion. Br J Neurosurg. 2013;27(4):483–8.
Mao X, Miao G, Hao S, et al. Decompressive craniectomy for severe traumatic brain injury patients with fixed dilated pupils. Ther Clin Risk Manag. 2015;11:1627–33.
•• Taylor A, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Child’s Nerv Syst. 2001;17(3):154–62. This was the first randomized study of decompressive craniectomy.
Barthelemy EJ, Melis M, Gordon E, et al. Decompressive craniectomy for severe traumatic brain injury: a systematic review. World Neurosurg. 2016;. doi:10.1016/j.wneu.2015.12.044.
• Hutchinson PJ, Kolias AG, Timofeev I, et al. Update on the RESCUEicp decompressive craniectomy trial. Crit Care. 2011;15(Suppl 1):P312. This study has been widely anticipated in determining the utility of DC in patients refractory to first and second tier management of intra-cranial hypertension. Publication is anticipated this year.
Yang XF, et al. Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir. 2008;150(12):1241–8.
Aarabi B, et al. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104(4):469–79.
Nalbach SV, et al. Craniectomy-associated Progressive Extra-Axial Collections with Treated Hydrocephalus (CAPECTH): redefining a common complication of decompressive craniectomy. J Clin Neurosci. 2012;19(9):1222–7.
• Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009;26(6):E7. This is an important review article highlighting the complications of decompressive craniectomy.
Aarabi B, et al. Dynamics of subdural hygroma following decompressive craniectomy: a comparative study. Neurosurg Focus. 2009;26(6):E8.
Stephens FL, et al. Cranioplasty complications following wartime decompressive craniectomy. Neurosurg Focus. 2010;28(5):E3.
Sturiale CL, et al. Do traumatic brain contusions increase in size after decompressive craniectomy? J Neurotrauma. 2012;29(18):2723–6.
Inamasu J, Kuramae T, Nakatsukasa M. Does difference in the storage method of bone flaps after decompressive craniectomy affect the incidence of surgical site infection after cranioplasty? Comparison between subcutaneous pocket and cryopreservation. J Trauma Acute Care Surg. 2010;68(1):183–7.
Cabraja M, Klein M, Lehmann T-N. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009;26(6):E10.
Gooch MR, et al. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 2009;26(6):E9.
Beauchamp KM, et al. Cranioplasty after postinjury decompressive craniectomy: is timing of the essence? J Trauma Acute Care Surg. 2010;69(2):270–4.
Piedra MP, Nemecek AN, Ragel BT. Timing of cranioplasty after decompressive craniectomy for trauma. Surg Neurol Int. 2014;5:25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Mehan, Wagner, and Ullman declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Traumatic Brain Injury Surgery.
Rights and permissions
About this article
Cite this article
Mehan, N.D., Wagner, K.E. & Ullman, J.S. Decompressive Craniotomy and Craniectomy for Brain Trauma. Curr Surg Rep 4, 30 (2016). https://doi.org/10.1007/s40137-016-0151-4
Published:
DOI: https://doi.org/10.1007/s40137-016-0151-4