Abstract
The advent of robotic surgery has revolutionized the modern treatment of a multitude of surgical diseases. With its enhanced precision, greater degrees of freedom, superior three-dimensional vision, improved resolution, and tremor elimination, robotic surgery is now playing a pivotal role in minimally invasive gynecologic, cardiothoracic, urologic, otolaryngologic, and gastrointestinal procedures. During the past decade, the field of plastic and reconstructive surgery has also started to embrace this innovative technology, especially for challenging reconstructive cases. Robotic surgery has not only enabled plastic surgeons to perform flap harvest procedures with minimal donor-site morbidity and enhanced cosmesis, but it has also allowed them to perform procedures never possible before. In this review, we illustrate the current clinical applications of robotics in plastic surgery and analyze their limitations based on the literature and our own experience in the field. We finish by presenting the technological challenges restricting the widespread use of robotics in plastic surgery, and outline some of our recent research efforts aimed at overcoming those limitations and promoting broader application of this innovative technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reconstructive plastic surgery has always been a surgical challenge. This field not only requires minimization of donor-site morbidity after flap harvest to optimize patients’ outcomes, but also necessitates superior levels of technical precision and meticulousness to achieve a reliable reconstruction. Robotic surgery has offered reconstructive plastic surgeons unique advantages to overcome all the limitations of the traditional (and endoscopic) techniques. These include, in addition to a minimally invasive approach, enhanced resolution with superior picture clarity, greater surgical precision with seven degrees of freedom of robotic instruments, and ergonomic positioning for surgeon’s comfort. In this review, we discuss the application of robotics in plastic surgery. We demonstrate how the unique feature of the robot might contribute to improved surgical outcomes with evident patient safety. We also highlight the controversies that exist on its applicability, cost, and learning curve. We finish by illustrating the research directions aiming at promoting its widespread use.
Clinical Application of Robotics in Plastic Surgery
The success of robots in various surgical specialties has inspired the senior author (JCS) to investigate their use in reconstructive plastic surgery, beginning in 2003. Over the next 10 years, three solid applications of robotics in plastic surgery were established: (1) Trans-oral robotic reconstructive surgery (TORRS) for head and neck reconstruction, enabling complex oropharyngeal reconstruction without splitting the lip or mandible; (2) robotic microvascular, micro-neural, and microlymphatic anastomoses, extending the skills of the human hand to “supra-human” levels of precision; and (3) minimal access muscle harvest achieving an “incisionless” harvest of both the latissimus dorsi and rectus abdominis muscles.
Trans-Oral Robotic Reconstructive Surgery (TORRS)
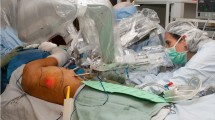
The management of oropharyngeal and base of tongue cancers remains challenging. The high incidence of morbidity following the traditional surgical approach (lip and mandible splitting) to resect such tumors has led to the use of primary chemo-radiation therapy as an alternative treatment [1, 2]. However, toxicity rates following this approach were considerably high (acute mucositis xerostomia and long-term swallowing dysfunction), and there was no improvement in functional status [3]. Thus, driven by the desire to offer a less morbid alternative to chemo-radiation, trans-oral robotic surgery (TORS) using the da Vinci Surgical System was developed and obviated the need for lip and mandible splitting. It showed great success in resecting pharyngeal and laryngeal malignant lesions achieving similar survival as compared to primary chemo-radiation therapy, but with improved functional and esthetic outcomes [4–6]. These minimally invasive resections, however, left a challenging defect to reconstruct. The reconstructive challenge is that the cylinder of the oropharynx remains almost entirely closed, severely restricting its access when attempting to inset and contour vascularized tissue. The anatomic region between the uvula and the epiglottis is particularly difficult to approach without a mandibulotomy or wide a pharyngotomy. As such, the senior author developed a minimally invasive reconstructive approach, known as TORRS [7 •]. This novel procedure allowed access to the difficult anatomy of the oropharynx whether using free flaps, local flaps, or primary closure. It facilitated suture approximation and achieved the goals of reconstruction, which are preservation of a competent velopharyngeal sphincter, a watertight seal between the pharynx and neck, and adequate sensation and volume in the tongue base. Altogether these factors preserve the physiological function of the oropharynx and larynx [8, 9]. Setup involves positioning the patient-side cart at about 60° from the head of the bed. A mouth retractor is used to establish the inter-dental opening and the endoscope, and two instrument arms are passed into the mouth, converging to the target anatomy (Fig. 1). This approach appeared to be a superior option in select cases and holds great promise in expanding the indications for minimally invasive reconstructive procedures. The senior author has proven the value of TORRS for challenging defects of the head and neck and has demonstrated both its feasibility [10] and effectiveness [11]. In addition, by adopting this technique, plastic surgeons are able to provide a reliable reconstructive support for the head and neck surgeon to robotically resect larger, deeper, and more complex tumors that would be very challenging to reconstruct through conventional methods.
TORRS. Trans-oral robotic reconstruction requires a mouth retractor to set the inter-dental opening. The robotic endoscope and two robotic instrument arms are introduced through the mouth and converge on the target oropharyngeal anatomy. External view (a) and depiction of internal view (b) are shown. c–f Case presentation: a 75-year-old man presented with a history of right neck metastatic squamous cell carcinoma. He was found to have a large recurrence. He underwent a pull-through resection, leaving a defect that extended from the tip of the tongue to the epiglottis (c view through the mouth, d the lateral pharyngotomy). The anterior inset was performed through the mouth by hand, as was the most distal portion of the pharyngeal inset through the pharyngotomy. The remaining inset, unreachable through the mouth or neck, was completed robotically (e). A second skin paddle was used to resurface the neck (f)
Robotic Microsurgery
Robotic microsurgery may constitute one of the most powerful tool in the armamentarium of the reconstructive surgeon. With complete tremor elimination and up to 5:1 motion scaling, the surgical robot achieves “supra-human” levels of precision. In no area is such precision more important than in microsurgery. Moreover, the robotic platform is equipped with high-definition three-dimensional optics providing up to 10× magnification, which constitutes a nearly ideal setup for delicate microvascular manipulations. In the senior author’s initial series of trans-oral robotic reconstruction of oropharyngeal defects [11], the robot was used to perform the microvascular anastomoses. Such anastomoses are challenging as the vessels are located in confined spaces. The facial artery (the most common recipient artery) is found beneath the hypoglossal nerve and digastric sling, often high under the body of the mandible. The space available to perform the anastomosis may be even further limited if a tracheostomy and ventilator tubing is also connected. The robot’s precision and visualization in confined spaces makes it well suited for anastomoses in such cases. Song et al. [9], who used the robot for microvascular anastomosis of a radial forearm flap (recipient vessel: facial artery) to reconstruct the defect created after resecting a tonsillar tumor (T3 N0 M0, stage III), also highlighted the advantage of the robot in allowing the micro-anastomosis to be performed in a narrow space. Setup for robotic microsurgery is relatively straightforward. The robotic arms are placed at about 45° above the target anatomy and in direct proximity to the external incision (Fig. 2).
Robotic microsurgery in TORRS. The venous anastomosis between the descending branch of the lateral circumflex femoral vein and the stump of the common facial vein was coupled with loupe magnification, and the anastomosis between the descending branch of the lateral circumflex femoral artery and superior thyroid artery was performed robotically (above). The inset is shown below
The advantages of robotics over endoscopy (disappearance of physiological tremor, three-dimensional high-definition vision with superior magnification, improved ergonomic) have led to its use for micro-neural and brachial plexus surgery. Nectoux et al. [12 •] demonstrated the feasibility of robotic micro-neural repair in their experiments on fresh nerves and determined that the robot allowed very safe and precise peripheral nerve repairs by counteracting physiological tremor and improving the overview of the surgical field. Moreover, it permitted the identification of the fascicles with greater accuracy and safety [13].
As for brachial plexus surgery, the robot might obviate the need for the long incision and the significant dissection that are generally required for access, allowing early exploration to both diagnose injuries and perform higher quality micro-neural repairs. Facca et al. [14] have presented their experimental and clinical experience in robotic-assisted surgery of the shoulder girdle and brachial plexus. In their cadaveric studies, they were able to dissect the supraclavicular brachial plexus and adjacent anatomical structures (jugular vein, omohyoid muscle, phrenic nerve, scalene muscles, and nerve roots from C4 to C7). A complete dissection and full exposure of the supraclavicular portion of the brachial plexus was successfully achieved.
Owing to its ultra-precision and 100 % tremor filtration, robotic microsurgery is currently being expanded to the field of super-microsurgery, specifically lymphedema surgery. Lymphatico-venular bypasses are typically performed end-to-end using 11-0 or 12-0 nylon sutures on a 50-µm needle [15]. These are exceptionally challenging anastomoses, and in some cases exceed the limits of human precision. As such, the supra-human precision of the robot may be of great benefit in this setting. The senior author has performed numerous lympho-venous bypass surgeries using the Da Vinci platform and found it to be favorable for this application. Moreover, the robotic platform allows fast transitioning of the visual field between near-infrared laser vision and normal bright-field vision, which is a significant advantage for lymphatics surgery when indocyanine green is used to map lymphatics.
Robotic Muscle Harvest: Latissimus Dorsi and Rectus Abdominis
The latissimus dorsi (LD) and rectus abdominis muscle flaps have been essential workhorses for reconstructive surgery since their introduction in the late 1970s [16]. Their harvest, however, requires lengthy incisions ranging from 20 to 40 cm in length. These incisions are associated with morbidities in the form of discomfort, seroma, and hernia. In addition, they are conspicuously located on the abdomen and back which results in poor esthetic results especially for breast reconstruction patients for whom cosmesis is a major determinant of final outcomes. Endoscopic and laparoscopic techniques have both been attempted to perform minimally invasive harvest procedure, but have not been adopted by plastic surgeons due to technical challenges in exposure, retraction, and lack of appropriately precise instrumentation [17–19]. A desire for a simple, reliable, and minimally invasive harvest procedure has always existed. The numerous key advantages of robotic surgery (including picture clarity, three-dimensional optics, adequate exposure, and precise instrumentation) stimulated its investigation in the harvest of these flaps. The senior author has designed and refined the technique to harvest both the latissimus dorsi and rectus abdominis muscles.
Robotic harvest of the LD muscle flap was introduced in 2010 after its investigation in a cadaver model [20]. It was then successfully performed in a series of 8 patients in 2011 [21 ••] and has since been used in over 40 LD harvests at the senior author’s institution. This novel technique was associated with a marked reduction in donor-site morbidity with demonstrable safety, efficacy, and no major complications. In addition to improved cosmesis, the robotic approach demonstrated reduced patient discomfort, decreased seroma formation, and shortened length of stay compared to the open procedure. The technique involves a short axillary incision, or simply use of the existing mastectomy incision or sentinel lymph node incision, with two additional ports and insufflation. The muscle can be harvested in its entirety and transposed through the small incision to be used as a pedicled flap (for partial breast reconstruction and implant coverage), as well as a free flap for various applications [22].
Over the past 3 years, this harvest technique has gained acceptance among reconstructive surgeons and is currently being performed worldwide, owing to its feasibility in achieving adequate coverage of the breast and providing a reliable reconstruction with concealed incisions. Its major indications include reconstruction of lateral defects following partial mastectomy, implant-based reconstruction following nipple–areola complex sparing mastectomies, and in secondary reconstruction in patients with expanders who received adjuvant radiotherapy (delayed–immediate protocol). Additionally, it can be used for chest wall deformity correction in patients with Poland syndrome [23]. The operative procedure is discussed in details elsewhere [23, 24] (Fig. 3).
Robotic harvest of the latissimus dorsi muscle flap. a Left markings and port placement. The borders of the latissimus dorsi muscle are marked according to anatomic landmarks. An axillary incision is then marked. For breast reconstruction, the sentinel lymph node incision is used. If a free flap is planned, then an incision is made that will facilitate pedicle dissection, dissection of the subcutaneous space anterior to the muscle, and placement of a port at the inferior end. Two additional ports are marked 8 cm from the end of the axillary incision and anterior to the muscle and 8 cm distal to the second port and anterior to the muscle. Right after port placement, the robotic side cart is positioned posterior to the patient with the two robotic arms and the endoscope extending over the patient in proximity to the ports. b Intraoperative views Left transposition of latissimus dorsi muscle underneath a subcutaneous skin bridge. Right latissimus dorsi muscle achieves total muscle coverage over a permanent silicone-shaped implant. c Left 90-cm2 scalp defect from resection of a squamous cell carcinoma. Right 3 weeks postoperatively, his flap is well healed, and he is marked for radiation simulation
Robotic harvest of the rectus abdominis muscle is a novel procedure that uses an intraperitoneal approach, which avoids disruption of the anterior rectus sheath and hence reduces the incidence of postoperative surgical site morbidities [25]. The first published procedure of robotic rectus muscle flap harvest was performed for a 30-year-old woman to reconstruct a lower extremity defect [26]. The authors noted multiple advantages of the robotic approach, including uninhibited 3D view of the rectus muscle and the deep inferior epigastric artery (DIEA), dexterity levels superior to human hands, and greater image clarity (as compared to traditional laparoscopy). Additionally, it was noted that the flap auto-retracts during the dissection due to gravity, which makes the muscle perforators and inscriptions easier to visualize. The operative procedure is described in details in a previous paper [27 ••]. Based on our experience, robotic harvest of the rectus muscle is a viable procedure for plastic surgeons, and can be applied for a multitude of reconstructions. Indications for harvest are classified based on the pedicle supplying the flap. Superiorly based pedicled flaps are employed for the reconstruction of anterior midline chest wall and sternal defects after oncologic surgery or wound debridement. Inferiorly based flaps on the other hand are performed to cover abdomino-pelvic defects where either space obliteration or coverage of major vessels and visceral protection are needed. Examples include abdomino-perineal resection, radical cysto-prostatectomy, pelvic exenteration, and or visceral repairs. Free flap muscle transfer can also be done robotically, and indications include primarily scalp and extremity [27 ••].
We noted several advantages of the robotic approach for rectus abdominis muscle flap harvest. As mentioned above, the traditional harvest technique requires long incisions to free the origin and insertion of the muscle. The robotic approach, however, necessitates only small incisions (for ports insertion), reducing the risk of wound complications, and improving cosmesis. More importantly, the robotic technique maintains the integrity of the anterior rectus sheath and hence decreases substantially the incidence of hernias and bulges. Overall, less tissue violation is required, and this resulted in evidently decreased postoperative pain and discomfort, shorter length of hospital stay, and more rapid functional recovery. Finally, this technique can be combined with other robotic pelvic procedures, which is of great value to multi-disciplinary robotic surgery programs where large resections are performed without a laparotomy (Fig. 4).
Robotic harvest of the rectus abdominis muscle flap. a Left markings and port placement. The contralateral costal margin and iliac crest are marked along a line connecting the anterior axillary line and the anterior superior iliac spine. The midpoint between these two landmarks and 2 cm lateral to it is the desired location of the 12-mm camera port. On either side of the camera port are the planned location of the two 8-mm instrument ports. Right All three ports are in place and the robot is ready to be docked. b Left after having an exposed and infected arthroplasty endoprosthesis, this patient received a robotically harvested rectus muscle to cover the prosthesis. Right The donor site. The scars are three small incisions on the contralateral side to the muscle being harvested. c Left after having an exposed medial ankle, this patient received also received a robotically harvested recuts muscle flap. Right The donor site is limited to the three ports because the muscle can be removed through on of the ports using a gallbladder bag
Limitations of Robotic Surgery
Cost
Cost is currently the most debated issue of robotic surgery and might be an obstacle to its widespread use in plastic surgery. The cost of the da Vinci system is $2.2 million and annual maintenance is $138,000. A great number of both teaching and community hospitals has accepted this cost in anticipation of increases in patient volume, reputational advantages, and academic/educational benefits. The ultimate determinate of the financial profit from robotic procedure is the contribution margin per case. This is computed by subtracting the cost per case from the revenue per case. For most surgeries, the revenue from a robotic case equals that of the open procedure, which is determined by the fees charged by the surgeon and the hospital, based on the DRG (there are no special robotic surgical codes). It is true that the OR cost of robotic procedures be higher due to instrumentation, staffing, and OR time; however, the ultimate hospital costs may be reduced since minimally invasive robotic surgery is associated with shorter lengths of stay and lower complication rates. The balance of revenue and cost per procedure is the ultimate determinant of the cost-effectiveness of robotic procedures. In our institution (MD Anderson Cancer Cancer), the robotic latissimus dorsi muscle flap harvest procedure costs an additional $800–$900 as compared to an open procedure [28]. Although this figure seems to prevent the widespread use of the robot in plastic surgery, when taking into account the advantages offered in terms of decreased morbidity and hospital length of stay, the final cost of robotic procedures might compare favorably to traditional approaches [28]. Ultimately, prospective long-term studies will be able to better assess the cost-effectiveness of this new technology in terms of operative time, recovery period, hospital length of stay, and morbidity rates.
Robotic Training in Plastic Surgery
Another important issue that might hinder broader application of the robotic technology in plastic surgery is its learning curve. It is important to note that a thorough understanding of robotic mechanics, kinetics, and dynamics is a key pre-requisite before performing any robotic procedure. Knowledge of its basic functionality does not suffice [28]. Therefore, surgeons need to spend a substantial amount of time and energy to understand the delicate nuances of the robotic mechanics to be able to troubleshoot the machine when it is not performing optimally. These teaching modules and credentialing systems have not been devised yet despite the vast technological development. Surgical training has remained more or less the same for more than a century. Residents and fellows learn surgery through “supervised trial and error.” This method makes training entirely dependent on the number of cases and may even lengthen the period of surgical training. It evidently cannot be applied to robotic teaching since caseloads are still limited. In this regard, simulation centers might be a better alternative for the acquisition of robotic surgical skills. Simulation allows trainees to be familiar with manipulation of instruments in a three-dimensional operating system. Moreover, advanced visual simulations and soft-tissue models recreate the textures of human tissues through force feedback (haptics) [29, 30] and allow trainees to acquire the meticulousness that is needed for robotic surgery. More importantly, trainees can be supervised through tele-mentoring and their performance/progress can be registered and monitored. This learning method allows trainees to quickly acquire the necessary robotic skills in a serene and safe environment without any compromise to patient safety. We must work as a specialty to define the competency in robotic plastic surgery and ensure it is being performed safely and according to best practices.
Technical Aspects
The current limitations of the robotic platform for microsurgery include inferior optics of the endoscope as compared to the operating microscope, in addition to instruments that are sometimes difficult to work with as compared to the fine microsurgical instrumentation, and lack of haptic feedback. These can be overcome with better quality and fixed distance lenses customized to the stereoscopic optical system of the robot. Also, developing finer robotic micro-instruments similar to the traditional microsurgical instruments is required to optimize the use of robotics in microsurgery. As for the haptic feedback, the senior author’s experience is that microsurgery is 90 % visual, and most of what we imagine we are feeling, we are actually seeing, and our brain is supplying the illusion of sensation. The 10 % of haptic feedback that is real, however, is a very important component and, at this time, constitutes a barrier to robotics playing a more active role in microsurgery. Hence, advanced teaching modules and robust learning assessment tools are needed for robotic microsurgery training to ensure its safe use. Unlike many other robotic versions of open procedures, robotic microsurgery combines the principles of conventional microsurgery (for which a number of training modules already exist [31, 32]) with an additional skill set unique to the surgical robot. To better evaluate trainees and measure this mixture of skills, the senior author created the Structured Assessment of Robotic Microsurgery Skills. This evaluation system combines the Structured Assessment of Microsurgical Skills scoring system with other established skill sets pertaining to robotic surgery [33]. The Structured Assessment of Robotic Microsurgical Skills includes three parameters to assess conventional microsurgical skills, including (1) dexterity, (2) visuospatial ability, and (3) operative flow. The robotic skills incorporate five additional parameters, including (1) camera movement, (2) depth perception, (3) wrist articulation, (4) atraumatic tissue handling, and (5) atraumatic needle handling. Each parameter is scored from 1 to 5, with 1 being the worst and 5 the best. The overall performance and overall skill level are also measured independently [34 ••]. We have recently applied this new robotic microsurgical evaluation system in a heterogeneous group of surgeons including clinical fellows, research fellows, and experienced microsurgeons to plot the maturation process of these skills [35 ••]. In this study, we successfully validated this new assessment instrument with excellent consistency and high levels of interrater reliability. We were further able to demonstrate improvement in robotic microsurgical skill across our heterogeneous group of learners. All skill areas and overall performance improved significantly for each participant. We also found that although prior experience with conventional microsurgery did improve in certain areas the acquisition of robotic technical skills, it was not necessary to gain proficiency in robotic microsurgical anastomosis. The technical aspects of robotic microsurgery can be gained by learners with no prior microsurgery or robotic experience [36]. Furthermore, subjects with no prior experience in robotic microsurgery moved through all but the highest level of Structured Assessment of Robotic Microsurgical Skills scores [37 ••, 38–41] to achieve proficiency. In our prior study of conventional microsurgical assessment data using the Structured Assessment of Microsurgical Skills [37 ••], moderately experienced subjects improved in skill through the middle and upper range of scores [37 ••, 40, 41]. Our model is the first of its kind, and we hope it will pave the road to customized education, curricular design with individual assessment, targeted feedback, and competency-based learning.
Limited Role in Esthetic Surgery
Finally, it is worth mentioning that esthetic surgery still did not (and might not) lend itself to robotic technology. A literature search did not reveal any reports describing the use of robotic surgery for cosmetic surgery [42]. The key to successful cosmetic surgery is preoperative assessment and marking, in addition to continuous tactile assessment of the tissues and sutures tension. Most of this is achieved by the surgeon’s hands that is best for sensing contour deformities and irregularities. The sense of touch is also critical to minimize tissue injury during any kind of surgical procedures. While working with a surgical robot, the surgeon relies mostly on visual cues and not on tactile feedback. Despite the fact that robotic instruments with force-feedback systems are being optimized for integrating force-sensing capabilities, they have not been met with much enthusiasm because of the constraints in size, design, cost, compatibility, and ability to withstand conventional sterilization procedures [43]. Suture tension in robotic surgery, for example, must be estimated by the degree of deformation of the respective tissues. Even though imaging technologies based on virtual and augmented reality have evolved to provide real-time navigational guidance in hope to make up for this lack of haptic feedback [44], in the end nothing can fully replace the actual sensing and feeling which is critical for cosmetic surgery. Lack of use of a robotic setup in cosmetic surgery, however, is understandable as esthetic surgery relies mostly on the artistic skills of the surgeon rather than on his technical precision and mechanical execution of pre-set surgical steps.
Conclusion
Robotic technology is the next step in the evolution of minimally invasive surgery, providing supra-human levels of precision and an unparalleled 3D visualization which can expand the capabilities of plastic surgeons and aid them in achieving safer and more complex procedures. Additionally, robotic surgery has enabled surgeons to perform procedure never possible before with demonstrable safety and effectiveness. Robotic instrumentation is also anticipated to continue improving, and this will expand further the spectrum of applications within plastic surgery, especially microsurgery, leading to the fine-tuning of procedures and ultimately to a wider adoption. The future of robotics in plastic surgery is favorable. With developing technology platforms, improved training pathways, and the anticipated decreases in cost (which eventually affect all technology revolutions), robotic plastic surgery is expected to flourish and expand.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, Forastiere A, Ang KK. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–9.
Walvekar RR, Li RJ, Gooding WE, Gibson MK, Heron D, Johnson JT, Ferris RL. Role of surgery in limited (T1-2, N0-1) cancers of the oropharynx. Laryngoscope. 2008;118:2129–34.
Nguyen NP, Vos P, Smith HJ, Nguyen PD, Alfieri A, Karlsson U, Dutta S, Lemanski C, Nguyen LM, Sallah S. Concurrent chemoradiation for locally advanced oropharyngeal cancer. Am J Otolaryngol. 2007;28:3–8.
Weinstein GS, O’malley BW, Hockstein NG. Transoral robotic surgery: supraglottic laryngectomy in a canine model. Laryngoscope. 2005;115:1315–9.
Genden EM, Desai S, Sung C-K. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck. 2009;31:283–9.
Dowthwaite SA, Franklin JH, Palma DA, Fung K, Yoo J, Nichols AC. The role of transoral robotic surgery in the management of oropharyngeal cancer: a review of the literature. ISRN Oncol. 2012;2012:945162.
• Selber JC, Sarhane KA, Ibrahim AE, Holsinger FC. Transoral robotic reconstructive surgery. Semin Plast Surg. 2014;28:35–8. This articles describes the technique of transoral robotic reconstruction, shows its advantages over the open procedure, and outlines its main indications.
De Almeida JR, Park RCW, Genden EM. Reconstruction of transoral robotic surgery defects: principles and techniques. J Reconstr Microsurg. 2012;28:465–72.
Song HG, Yun IS, Lee WJ, Lew DH, Rah DK. Robot-assisted free flap in head and neck reconstruction. Arch Plast Surg. 2013;40:353–8.
Selber JC, Robb G, Serletti JM, Weinstein G, Weber R, Holsinger FC. Transoral robotic free flap reconstruction of oropharyngeal defects: a preclinical investigation. Plast Reconstr Surg. 2010;125:896–900.
Selber JC. Transoral robotic reconstruction of oropharyngeal defects: a case series. Plast Reconstr Surg. 2010;126:1978–87.
• Nectoux E, Taleb C, Liverneaux P. Nerve repair in telemicrosurgery: an experimental study. J Reconstr Microsurg. 2009;25:261–5. This article introduces robotic micro-neural surgery and shows that telesurgery allows very safe and precise peripheral nerve repairs.
Tigan L, Miyamoto H, Hendriks S, Facca S, Liverneaux P. Interest of telemicrosurgery in peripheral nerve tumors: about a series of seven cases. Chir Main. 2014;33:13–6.
Facca S, Hendriks S, Mantovani G, Selber JC, Liverneaux P. Robot-assisted surgery of the shoulder girdle and brachial plexus. Semin Plast Surg. 2014;28:39–44.
Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg. 2010;126:752–8.
Maxwell GP, Stueber K, Hoopes JE. A free latissimus dorsi myocutaneous flap: case report. Plast Reconstr Surg. 1978;62:462–6.
Lin CH, Wei FC, Levin LS, Chen MC. Donor-site morbidity comparison between endoscopically assisted and traditional harvest of free latissimus dorsi muscle flap. Plast Reconstr Surg. 1999;104:1070–7 (Quiz 1078).
Pomel C, Missana MC, Lasser P. Endoscopic harvesting of the latissimus dorsi flap in breast reconstructive surgery: feasibility study and review of the literature. Ann Chir. 2002;127:337–42.
Fine NA, Orgill DP, Pribaz JJ. Early clinical experience in endoscopic-assisted muscle flap harvest. Ann Plast Surg. 1994;33:465–9 (Discussion 469–72).
Selber JC, Baumann DP, Holsinger CF. Robotic harvest of the latissimus dorsi muscle: laboratory and clinical experience. J Reconstr Microsurg. 2012;28:457–64.
•• Selber JC, Baumann DP, Holsinger FC. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg. 2012;129:1305–12. This article introduces the technique of robotic latissimus dorsi muscle harvest, and shows that it is effective and offers technical advantages over endoscopic harvest and aesthetic advantages over the open technique.
Ibrahim AE, Clemens MW, Sarhane KA, Selber JC. Robotic surgery in breast reconstruction: harvest of the latissimus dorsi muscle flap. Breast Reconstr Art Sci New Clin Technol. 2016. doi:10.1007/978-3-319-18726-6.
Chung J-H, You H-J, Kim H-S, Lee B-I, Park S-H, Yoon E-S. A novel technique for robot assisted latissimus dorsi flap harvest. J Plast Reconstr Aesthet Surg. 2015;68:966–72.
Clemens MW, Kronowitz S, Selber JC. Robotic-assisted latissimus dorsi harvest in delayed-immediate breast reconstruction. Semin Plast Surg. 2014;28:20–5.
Selber J, Pederson J. Muscle flaps. Telemicrosurgery robot assist microsurgy. Paris: Springer; 2012. p. 147–58.
Patel NV, Pedersen JC. Robotic harvest of the rectus abdominis muscle: a preclinical investigation and case report. J Reconstr Microsurg. 2012;28:477–80.
•• Ibrahim A, Sarhane K, Pederson J, Selber J. Robotic harvest of the rectus abdominis muscle: principles and clinical applications. Semin Plast Surg. 2014;28:26–31. This article introduces the technique of robotic harvest of the rectus abdominis muscle flap, describes its principles and clinical applications, and shows its efficacy and decreased morbidity in comparision to the traditional technique.
Selber JC. Robotic latissimus dorsi muscle harvest. Plast Reconstr Surg. 2011;128(2):88e–90e. doi:10.1097/PRS.0b013e31821ef25d.
Satava RM. Virtual reality, telesurgery, and the new world order of medicine. J Image Guide Surg. 1995;1:12–6.
Suzuki S, Suzuki N, Hayashibe M, Hattori A, Konishi K, Kakeji Y, Hashizume M. Tele-surgical simulation system for training in the use of da Vinci surgery. Stud Health Technol Inf. 2005;111:543–8.
Hino A. Training in microvascular surgery using a chicken wing artery. Neurosurgery. 2003;52:1495–7 (Discussion 1497–8).
Seyhan T, Seyan T, Ozerdem OR. Microsurgery training on discarded abdominoplasty material. Plast Reconstr Surg. 2006;117:2536–7.
Dulan G, Rege RV, Hogg DC, Gilberg-Fisher KM, Arain NA, Tesfay ST, Scott DJ. Developing a comprehensive, proficiency-based training program for robotic surgery. Surgery. 2012;152:477–88.
•• Selber JC, Alrasheed T. Robotic microsurgical training and evaluation. Semin Plast Surg. 2014;28:5–10. This article introduces and validates the Structured Assessment of Robotic Microsurgical Skills (SARMS). This surgcial evaluation tool combines the previously validated Structured Assessment of Microsurgical Skills (SAMS) with other skill domains in robotic surgery.
•• Alrasheed T, Liu J, Hanasono MM, Butler CE, Selber JC. Robotic microsurgery: validating an assessment tool and plotting the learning curve. Plast Reconstr Surg. 2014;134:794–803. This article shows that the Structured Assessment of Robotic Microsurgery Skills is a valid instrument, with excellent interrater reliability, for assessing robotic microsurgical skills, and paves the road to customized education, curricular designs with individual assessment, targeted feedback, and competency-based learning.
Karamanoukian RL, Bui T, McConnell MP, Evans GRD, Karamanoukian HL. Transfer of training in robotic-assisted microvascular surgery. Ann Plast Surg. 2006;57:662–5.
•• Selber JC, Chang EI, Liu J, Suami H, Adelman DM, Garvey P, Hanasono MM, Butler CE. Tracking the learning curve in microsurgical skill acquisition. Plast Reconstr Surg. 2012;130:550e–7e. This article shows that the Structured Assessment of Microsurgery Skills questionnaire is a valid instrument for assessing microsurgical skills, providing individualized feedback with acceptable interevaluator reliability.
Balasundaram I, Aggarwal R, Darzi LA. Development of a training curriculum for microsurgery. Br J Oral Maxillofac Surg. 2010;48:598–606.
Temple CLF, Ross DC. A new, validated instrument to evaluate competency in microsurgery: the University of Western Ontario Microsurgical Skills Acquisition/Assessment instrument (outcomes article). Plast Reconstr Surg. 2011;127:215–22.
Chan W-Y, Matteucci P, Southern SJ. Validation of microsurgical models in microsurgery training and competence: a review. Microsurgery. 2007;27:494–9.
Chan W, Niranjan N, Ramakrishnan V. Structured assessment of microsurgery skills in the clinical setting. J Plast Reconstr Aesthet Surg. 2010;63:1329–34.
Ibrahim AE, Sarhane KA, Baroud JS, Atiyeh BS. Robotics in plastic surgery: a review. Eur J Plast Surg. 2012;35:571–8.
Tan GY, Goel RK, Kaouk JH, Tewari AK. Technological advances in robotic-assisted laparoscopic surgery. Urol Clin N Am. 2009;36:237–249, ix.
Teber D, Baumhauer M, Guven EO, Rassweiler J. Robotic and imaging in urological surgery. Curr Opin Urol. 2009;19:108–13.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Plastic Surgery.
Rights and permissions
About this article
Cite this article
Ibrahim, A.E., Sarhane, K.A. & Selber, J.C. Robotics in Plastic Surgery. Curr Surg Rep 4, 9 (2016). https://doi.org/10.1007/s40137-016-0130-9
Published:
DOI: https://doi.org/10.1007/s40137-016-0130-9