Abstract
Purpose of Review
Hearing loss is a common congenital sensory disorder with various underlying causes. Here, we review and focus on genetic, infectious, and ototoxic causes and recent advances in inner ear therapeutics.
Recent Findings
While hearing aids and cochlear implantation are the mainstay of treatment for pediatric hearing loss, novel biological therapeutics are being explored. Recent preclinical studies report positive results in viral-mediated gene transfer techniques and surgical approaches to the inner ear for genetic hearing loss. Novel pharmacologic agents, on the other hand, show promising results in reducing aminoglycoside and cisplatin ototoxicity. Clinical trials are underway to evaluate the efficacy of antivirals for cytomegalovirus-related hearing loss, and its pathogenesis and other potential therapeutics are currently under investigation.
Summary
Individualized therapies for genetic and infectious causes of sensorineural hearing loss in animal models as well as pediatric patients show promising results, with their potential efficacy being active areas of research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hearing loss affects nearly 35 million children globally [1]. This sensory impairment negatively impacts speech and language development, in addition to school performance and social interactions [2]. Despite advances in molecular and imaging modalities in identifying the various etiologies of congenital sensorineural hearing loss (SNHL), the current means of rehabilitation are limited to hearing aids and, in more severe cases, cochlear implantation.
In recent years, novel inner ear therapeutic approaches in animal models and, in some cases, clinical trials are paving the way for biological treatment options for patients. This review discusses these recent discoveries in inner ear therapeutics, particularly in the areas of genetic-, cytomegalovirus (CMV)-, and ototoxicity-related SNHL in the pediatric population. We will also discuss challenges and considerations when testing and designing such therapies in the pediatric population, as well as the current and potential future advances in the field of inner ear therapy.
History of the Inner Ear Anatomy and Early Attempts at Its Access
The human inner ear labyrinth is an intricate organ that houses both vestibular and hearing end organs. Early descriptions of the human inner ear anatomy were reported in the mid-1500s by Vesalius and later on by Eustachi [3, 4]. Several centuries later, Contugo further defined anatomy of both the vestibular and cochlear apparatuses, and Scarpa provided a more elaborate description of the bony and membranous labyrinths [5, 6]. The human cochlea is mature at birth, and natural regeneration of the sensory epithelium is minimal to non-existent in mammals [7, 8]. Therefore, degeneration as a result of congenital or acquired disorders causes irreversible hearing loss. Therapeutic approaches to the inner ear were not attempted until 1930, when Mollison first reported injecting alcohol into the labyrinth as an ablative treatment for vertigo [9]. Since then, other therapeutic agents have been attempted, such as intratympanic streptomycin injections into the middle ear in 1956 by Schuknecht to treat Meniere’s disease, growth factor delivery close to the inner ear for treatment of steroid-refractory SNHL in 2014, and most recently gene delivery directly into the inner ear through the oval window in adult patients with SNHL [10,11,12]. Given the differences in etiology between hearing disorders in pediatric and adult patients, how these advances could apply to the pediatric population is unclear. In this review, we will discuss the potential applications and risks in children.
Pediatric Inner Ear Pathologies
The prevalence of SNHL increases throughout childhood, with approximately 2.7 per 1000 children under the age of five and rising up to 150 per 1000 adolescents having any degree of hearing impairment [13, 14]. Deprivation of auditory input can hinder language development, oral communication, speech processing, cognition, as well as psychosocial and educational development [15]. Moreover, a lack of sufficient auditory stimulus during a sensitive period of cortical maturation, particularly before the age of 3–4, can result in abnormal development of the central nervous system and speech delays [16]. Currently, the primary treatment options for mild-to-severe and severe-to-profound SNHL are hearing aids and cochlear implants (CI)s, respectively. Although a substantial number of children with profound SNHL are receiving CIs for auditory rehabilitation, outcomes can vary given a myriad of elements, such as early access to acoustical stimulus, socioeconomic factors, parent-child interactions, consistent use of implants, and etiology and duration of hearing loss [17, 18]. Additionally, human language development and speech processing require a higher level of acoustical stimulation for syntax, semantics, and pragmatics, which the electrical stimulation of CIs cannot provide [17,18,19].
Pediatric hearing loss is categorized into three main etiologies: (1) genetic mutations cause nearly 50% of hearing loss in children; (2) infectious etiologies (such as “TORCH” organisms including toxoplasmosis, rubella, CMV, herpes as well as other bacterial and viral causes) making up another 25% of all cases; and (3) environmental triggers—including but not limited to ototoxicity, prematurity, subarachnoid hemorrhage, and trauma—make up 15% of cases [2]. The remaining 25% of etiologies are unknown. We describe some of these causes and how they affect the pediatric population below.
Genetic Causes of Hearing Loss
Genetic mutations are the most common causes of congenital SNHL, as it is estimated that nearly 1% of all human genes contribute to auditory function [20]. Up to 70% of all genetic-related hearing loss is attributed to non-syndromic deafness (NSD) genes [21]. Of these, about 80% are autosomal recessive (AR), between 15 and 20% are autosomal dominant (AD), and X-linked and mitochondrial deafness account for 1% each [22]. Out of the 56 genes causing ARNSD, more than 700 different mutations have been identified thus far, lending themselves to potential gene replacement therapy [23]. The most common cause of congenital ARNSD is a mutation in the Connexin 26 gene (GJB2) [23]. While GJB2-related hearing loss is usually stable over many years, some mutations (e.g., truncated) are associated with more severe and gradually progressive hearing loss [24]. In certain ethnic groups, mutations of STRC represent the second most common cause of ARNSD associated with progressive SNHL [25••]. On the other hand, mutations causing Pendred syndrome and Usher syndrome are the two most common causes of syndromic SNHL—both inherited in an AR manner with a naturally progressive clinical course [23]. Many other mutations have been associated with SNHL, and a more comprehensive discussion can be found in other reviews [23, 25••]. Unlike other causes of hearing loss, children with genetic hearing loss may benefit from gene replacement therapy with the goal of halting the progression of and possibly restoring hearing.
While the history of gene therapy began in the 1960s, the first clinical trial in humans using gene therapy was done in 1989 [26]. It took an additional 25 years before the first clinical gene therapy trial in the inner ear for SNHL was initiated in 2014 [12]. As the majority of current and previous inner ear gene therapy studies use preclinical animal models, their potential applications in the pediatric population will be discussed below.
CMV-Related Hearing Loss
In the USA and many parts of the world, congenital CMV (cCMV) infection has become the most prevalent infectious cause of pre- and post-lingual hearing loss [2]. As a ubiquitous virus that belongs to the family of DNA viruses Herpesviridae, CMV is the most common fetal infection in humans. cCMV is known to cause numerous perinatal abnormalities, including petechiae, hydrops, hepatosplenomegaly, microcephaly, chorioretinitis, and growth retardation [27]. It affects 0.2% to 2.5% of all live-born neonates worldwide [28,29,30,31] but has been reported as high as 5% in developing countries [32]. The association between cCMV and hearing loss was first described in 1964 and is now recognized as the most common non-genetic cause of SNHL in children [2, 33,34,35,36,37,38].
Approximately 10–15% of neonates with cCMV are symptomatic at birth, while the majority are asymptomatic (with hearing loss generally excluded as a symptom) [39]. One-third of symptomatic neonates have hearing loss, and 71% among those present with severe to profound SNHL [39]. By contrast, 1 in 10 asymptomatic neonates develops hearing loss, of which 57% are unilateral and severe-to-profound. CMV-related hearing loss is often delayed in onset (18.1% in symptomatic and 9% in asymptomatic children) and progresses over time [35, 39,40,41,42,43,44,45]. As with other forms of hearing loss, early intervention improves patient outcomes. Since the diagnosis can be challenging and relies on detecting viral shedding during the first 3 weeks of life, there are ongoing efforts to implement CMV testing in neonates who fail newborn hearing screen nationwide [46]. Numerous studies have also demonstrated a long-term efficacy of antivirals in preventing progression of CMV-related hearing loss and improving speech and language performance in symptomatic or more severely affected infants [47, 48••, 49,50,51]. Several preclinical models of CMV-related hearing loss have also shed light on mechanisms of the disease and revealed potential therapeutics [52, 53•, 54, 55], both of which we will discuss in more detail below.
Other Environmental
Ototoxicity is an important preventable cause of hearing loss in children. There are several classes of ototoxic drugs: antibiotics (aminoglycosides, macrolides, vancomycin), loop diuretics, salicylates, and chemotherapeutic agents (cisplatin, carboplatin). The prevalence of antibiotic-induced ototoxicity ranges from 2 to 50% [56], with the true incidence being an active area of research. For example, newborns with a presumptive infection who receive short-term antibiotic treatment may be at low risk, but concurrent sound exposure may accentuate its potential ototoxicity [57]. This finding contrasts those needing repeated dosing (e.g., cystic fibrosis patients) since the cumulative dose may increase ototoxicity [56]. Moreover, patients carrying the A1555G mutation in the 12S ribosomal gene are more sensitive to aminoglycoside ototoxicity [58]. The A1555G mutation accounts for 10–20% of prelingual hearing loss due to ototoxicity [59].
On the other hand, ototoxicity caused by chemotherapeutic agents (most commonly being cisplatin) varies between 4 and 90% of recipients, depending on the chemotherapeutic and other agents used [60]. Unlike aminoglycosides, cisplatin has an established dose-dependent ototoxicity relationship and audiological monitoring program for oncologic patients receiving this therapy [61]. In a select subgroup of children, the incidence can be as high as 82–90% of those receiving autologous bone marrow transplantation and a concomitant chemotherapeutic agent developed changes in hearing [60, 62]. For therapeutic options due to drug-induced ototoxicity, the focus is currently on prevention of hearing loss prior, during, and even after administration of the offending agent. Various inner ear therapies will be discussed further in the manuscript.
Inner Ear Therapy in Children
Therapies for Genetic Hearing Loss
Studies on various preclinical models using rodents and non-human primates have recently demonstrated promising results with high translational potential. Both gene therapy and genomic-editing approaches have shown positive results in mouse models of hearing loss [63, 64, 65•]. For example, a missense mutation of SLC17A8, which encodes vesicular glutamate transporter type 3 (VGLUT3), is linked to autosomal dominant progressive high-frequency SNHL in humans [66, 67]. As one of the first proof-of-principle studies, Akil et al. found that injection of an adeno-associated virus (AAV) 1 vector carrying VGLUT3 gene restored phenotypic function with evidence of preservation of ABR thresholds in VGLUT3 deficient mice. Despite the recovery of behavioral and physiological measures (startle reflex, ABR amplitudes), the rescue of synaptic morphology and spiral ganglia neuron survival was incomplete, likely due to limited transduction of hair cells as a result of the AAV1 tropism (affinity for the types of cells they transduce) [66].
A major accomplishment in the last few years has been the discovery of numerous viral capsids with high transduction efficiency in the inner ear. Several laboratories have demonstrated high transduction efficacy of various capsids (e.g., AAV-ie, Anc80L65, and AAV2.7m8 vectors) across multiple cell types in the inner ear [68••, 69, 70]. For example, increased transduction efficacy was shown in a study examining mutations of transmembrane channel-like (TMC) 1, which broadly affects sensory hair cells causing hearing and balance deficits [25••, 71]. Initial results using AAV1/2 vectors demonstrated rescue of inner hair cell function with mixed improvement of auditory responses [72]. However, the use of a highly efficient viral capsid of hair cells (Anc80L65) resulted in remarkable improvement in both the number of hair cells rescued and also auditory phenotype rescue [73]. To date, many of these studies have focused solely on examining mutations affecting hair cell survival and function; however, more common mutations that cause hearing loss in children tend to affect multiple cell types in the inner ear (e.g., GJB2). Whether those forms of hearing loss can be similarly rescued using viral capsids with high transduction efficacy remains an active area of research.
A number of fundamental elements associated with gene delivery, such as the optimal gene dosing, size limitations, delivery methods, and patient candidacy and safety particularly in children, must be assessed prior to clinical interventions. To that end, recent studies in cynomolgus monkeys using a variant of AAV9 demonstrated a dose-dependent transduction efficiency on both inner and outer hair cells, suggesting that higher doses may be needed to enhance transduction that is otherwise limited by tropism [74, 75]. Furthermore, to circumvent the limited package capacity of AAVs, a virus known to elicit little immune response [76], a dual and triple AAV paradigm was successfully employed to deliver large genetic sequences to rescue Otoferlin deficient mice [77, 78•]. Lastly, other novel techniques like CRISPR/Cas-9 technology [79], nanoparticle systems [80••] and short interfering RNAs (siRNA) [81] are actively being explored.
Therapies for CMV-Related Hearing Loss
The use of antiviral treatment in cCMV has been studied, and its efficacy in benefitting symptomatic or severely affected infants is becoming apparent. In a randomized, placebo-controlled clinical trial (RCT) completed in 2003, Kimberlin et al. showed that ganciclovir (the intravenous form of valganciclovir) prevented hearing deterioration at 6 months in symptomatically infected infants with CMV infection involving the central nervous system [47]. The same team reported in a subsequent RCT that a 6-month course of valganciclovir demonstrated modest improvement in SNHL, speech development, and neurodevelopmental outcomes for up to 24 months versus a shorter 6-week course in neonates with symptomatic cCMV [48••]. However, McCrary and colleagues showed that valganciclovir may only have short-term benefits with a subsequent progressive hearing loss despite antiviral therapy when patients were followed for an average of 3.2 years [82]. Whether valganciclovir benefits children with asymptomatic cCMV with isolated SNHL remains a topic of controversy, and clinical trials are currently underway [83]. One retrospective study showed that early initiation of therapy for a 12-month course resulted in improved hearing results for up to 1 year of follow-up [49]. Most affected ears (69%) had an improvement in hearing, of which 96% returned to normal hearing with no deterioration of the unaffected ear. A majority (80%) of those with normalized hearing had started treatment before 4 weeks of age. Given that cCMV associated SNHL can improve without therapy, it is challenging to attribute the benefits reported to valganciclovir [84]. In addition, the doses and duration used for this study have not been evaluated in a RCT, which is currently underway (ValEAR clinical trial NCT03107871).
Valganciclovir administration has the potential for significant risk. As data to support the use of antivirals in CMV-related hearing loss grows, many of these studies have recommended close monitoring for neutropenia and anemia as possible side effects of prolonged treatment with valganciclovir [48••, 51, 82], with one study showing an incidence of 28.8% and 7.5%, respectively [85]. The side effects were most notable during the first 3 months of treatment with no long-term adverse effects.
Although CMV is the most common infectious cause of SNHL, the mechanism of injury remains poorly understood. Novel murine models of CMV infection with different patterns of hearing loss have been used to better elucidate the pathogenesis [52, 53•, 55, 86•, 87, 88, 89]. Intracerebral inoculation with murine CMV has been shown to cause damage to the stria vascularis, potentially leading to poor establishment and maintenance of the endocochlear potential [87]. Another study highlighted the role of natural killer cells in protecting against CMV-induced labyrinthitis and outer hair cell loss and subsequent SNHL [86•]. In contrast, a murine model using an intraperitoneal route of CMV inoculation showed focal infection and virus-induced cochlear inflammation causing primarily loss of spiral ganglia neurons and synapses [53•].
There is a growing body of evidence that reactive oxygen species (ROS) may be involved in several types of hearing loss, including CMV infection [90,91,92,93]. Mechanistically, uncontrolled ROS production can cause oxidation of lipids, proteins, and DNA, eventually leading to apoptotic cell death [94]. Recent studies have highlighted the robust inflammatory response and possible imbalance in redox homeostasis following CMV infection, which results in a prolonged increase in ROS levels and significant damage to the organ of Corti [52, 55, 86•, 88, 89, 95]. Antioxidants have been proposed as an approach to scavenge excessive ROS to prevent or halt hearing loss. In mice, treatment with antioxidants provided partial otoprotection and preservation of cochlear hair cells and the overall hearing [52]. However, auditory thresholds remained significantly higher than uninfected controls, suggesting that antioxidants are not sufficient to completely protect the inner ear after CMV infection [52]. Decreasing inflammation by administering oral corticosteroids partially reduced loss of spiral ganglia neurons and improved auditory function compared with untreated infected mice [53•]. While most studies have been preclinical, a retrospective chart review of pediatric patients with cCMV infection revealed that antioxidants vitamins A, C, and E with magnesium (ACE-Mg) did not demonstrate significant benefits for hearing protection [50]. Cumulatively, these findings indicate that further investigation into the potential therapeutic utility and the ability to target inflammation, macrophages, and/or ROS production to protect against CMV-related hearing loss in humans is warranted.
Therapies for Ototoxin-Related Hearing Loss
There is a large body of literature on various approaches of preventing aminoglycoside and cisplatin ototoxicity. Novel approaches to prevent drug entry into cochlear hair cells have shown promise in preclinical studies, including the use of a non-ototoxic variant of the aminoglycoside [96] or using novel compounds that compete with these ototoxins entering hair cells [61]. Moreover, gene therapy has also been evaluated to deliver potentially otoprotective molecules, such as neurotrophins and Hsp70, to prevent inner ear damage by aminoglycosides through increased hair cell survival in animal models [97, 98].
Preventing formation of ROS and mitochondria-related damage has also been examined as potential treatment options [56, 99,100,101]. Administered 6 h after cisplatin chemotherapy, sodium thiosulfate resulted in a lower incidence of hearing loss in children [102•, 103•]. However, Freyer and colleagues reported a lower event-free survival and overall survival for participants with disseminated disease, raising the question of whether local administration of otoprotectants may be preferred over a systemic approach. Lastly, gene therapy has also been studied using various inhibitors as a protective mechanism against cisplatin-induced ototoxicity in animal models [104, 105].
Potential of Stem Cell Therapies for Hearing Loss
The potential for regeneration in the auditory system is an active field of investigation. After the early discovery of hair cell regeneration in both the auditory and vestibular systems of non-mammalian vertebrates such as birds and fish [106,107,108,109], Cox and colleagues reported in 2015 limited spontaneous hair cell regeneration in the neonatal mouse cochlea [110], which was long thought to be a non-regenerative organ. This indicates the presence of hair cell progenitors suggested by several prior reports [111,112,113]. While regeneration is limited to the neonatal period in mice, modulation of major signaling pathways and/or transcription factors can increase the degree of regeneration at this developmental stage [114, 115•]. Moreover, these progenitors can be expanded with defined growth factors in vitro [116], thus generating a cell line that may help guide future drug discovery. Lastly, building on early description of modest hair cell regeneration in the mature mammalian vestibular organs [117, 118], several groups have since characterized hair cell progenitors contributing to this regenerative process [119,120,121] and the potential role of the hair cell transcription factor Atoh1 in enhancing regeneration [122, 123••]. There is on-going work to evaluate the potential role of Atoh1 and other key factors by many laboratories and also biotechnology companies. A more thorough discussion can be found in other reviews [8, 124, 125].
Future of Inner Ear Therapy
The current standard of care for pediatric hearing loss is hearing aids and cochlear implants, both of which can be highly beneficial despite known limitations related to socioeconomic status and access to health care. Nonetheless, for any novel inner ear therapeutics to become applicable in the pediatric population, their potential benefits will likely need to be compared against these current standards. Additionally, pediatric hearing loss comprises of numerous causes including genetic mutations with various phenotypes (some demonstrating abrupt changes while others are stable over many years). Ultimately, inner ear therapies chosen for SNHL will depend on the underlying pathology as is discussed previously. Here, we will discuss three main areas to consider.
Delivery of Therapeutics in Children
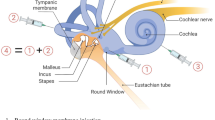
In the past decade, major strides in research on therapeutics and approaches to the inner ear have been achieved. Most studies have been done in preclinical animal models; to extrapolate these results to humans including children, it is important to understand the labyrinthine microanatomy. The rate and the redistribution of each substance, whether it is viral capsids for gene therapy or antioxidants to negate ototoxicity, as it transverses the fluid and cellular compartments of the inner ear will vary depending on the size, half-life, consistency of the vehicle, and the anatomical area of injection. Homeostatic mechanisms within the inner ear also matter, as recent animal studies demonstrated that the regulation of fluid dynamics will affect the final distribution of agents based on the approach to introduce inner ear therapeutics [126•]. When comparing dye delivery into the posterior semicircular canal or through the round window membrane of mice (Fig. 1), the former produced higher levels of dye delivery within the cochlea, while the latter led to higher levels of dye distribution in the brain [126•]. While the patency of the cochlear aqueduct in humans is a topic of discussion, the possibility exists that this connection between the central nervous system and the inner ear is patent in children, thereby allowing for therapeutics to enter the brain [127, 128]. Therefore, the best approach to deliver therapy to humans, specifically children, remains to be determined.
Potential therapies for hearing loss. Schematic of potential genetic and pharmacologic therapies for hearing loss. Recent studies have begun to examine different routes of gene and pharmacologic delivery, including via the posterior semicircular canal, oval window, and round window, (black arrows). Therapies can also be administered systemically (large blue arrow). Anti-viral and anti-oxidant therapeutics are being studied as treatment for infection- and ototoxin-related cochlear inflammation and hearing loss. (Reproduced and modified from: Talaei S, et al. Front Cell Neurosci, 2019. 13: p. 471; https://www.frontiersin.org/articles/10.3389/fncel.2019.00471/full; Creative Commons user license https://creativecommons.org/licenses/by/4.0/) [105•]
Additionally, while many therapeutics can be given transtympanically to adults in the clinic setting, this approach is not easily achievable in young children, who often require anesthesia even for minimally invasive procedures. Given the inherent risk of anesthesia, particularly in those younger than 12 months, repeat dosing may prove to be more challenging in children, necessitating approaches employing a singular therapeutic delivery or the use of a drug vehicle allowing sustained release.
Timing of Treatment
Another important consideration is the timing of intervention. For progressive hearing loss such as those caused by Pendred syndrome or other genetic mutations, one may consider intervening earlier when target cells in the labyrinth remain viable and thus amenable to gene replacement. However, this motivation is weighed against the potential risk of hastening the progression of hearing loss by accessing the labyrinth, which is likely already vulnerable to injury. Moreover, the stakes are particularly high for children suffering from hearing loss in the pre-lingual and peri-lingual period (< 4 years old). As stated before, one must always return to the fundamental question: can this new therapy complement or replace hearing aids and/or cochlear implants, which can be quite effective and safe in treating this patient population?
On the other hand, children with post-lingual and slowly progressive hearing loss may have a larger therapeutic window for testing new therapies, again provided that therapeutics or the approach do not risk propagating the hearing deficits before improvements are seen. Should the child be monitored for hearing changes and provided with inner ear therapy once the hearing loss becomes non-serviceable, or should the intervention be administered prior to such progression? Answers will likely depend on the etiology, age of the patient, and potential risks of the therapy.
Parental Concerns
While clinical trials evaluating the efficacy of biological therapeutics, including gene therapy, have begun in adults, the applicability of these interventions to children is unknown. Conscious decision-making will require involvement of the parent, child, and the surgical team. The emotional strain on parents of whether or not to treat with novel therapies can create a feeling of guilt and responsibility for the well-being of their child; these are facets of the parent-child relationship that cannot be ignored. For example, approximately 30–40% of deaf children have additional special needs [129,131,131]. Thus, many will require post-treatment care and monitoring by a multi-disciplinary team of surgeons, audiologists, speech pathologists, psychologists, and educational specialists.
Conclusion
It has been almost 40 years since the first CI was performed in pediatric patients [132]. Since that time, the field has evolved, and much advancement has been made in diagnosing and understanding the pathophysiology of pediatric hearing loss. While the results in animal models of human hearing loss are promising, one should carefully weigh the risks and benefits of novel technology before translating into clinical trials, especially in the pediatric population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Childhood hearing loss: act now, here’s how!. World Health Organization 2016.
Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med. 2006;354(20):2151–64.
Vesalius A. De humani corporis fabrica. 1543.
Eustachi B. Epistola de auditus organis. In: Opuscula Anatomica. Venice: Vincentius Luchinus excudebat. p. 1564.
Scarpa A. Disquisitiones Anatomicae de Audit et Olfactu. Ticini: Typographeo Petri Galeatii; 1789.
Cotugno D. De aquaeductibus auris humanae internae anatomica dissertatio. 1775, Neapoli et Bononiae: Typographia Sanctae Thomae Aquinatis.
Moore JK, Linthicum FH Jr. The human auditory system: a timeline of development. Int J Audiol. 2007;46(9):460–78.
Atkinson PJ, Kim GS, Cheng AG. Direct cellular reprogramming and inner ear regeneration. Expert Opin Biol Ther. 2019;19(2):129–39.
Mollison WM. Treatment of vertigo by destruction of the labyrinth with absolute alcohol. Guy’s Hosp Rep. 1930;80:470.
Schuknecht, HF. Ablation therapy for the relief of Meniere’s disease. Laryngoscope. 1956;66:859–70.
Nakagawa T, K.K, Usami S, et al. A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med. 2014;12:219.
Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier. Hear Res. 2016;338:52–63.
Su BM, Chan DK. Prevalence of hearing loss in US children and adolescents: findings from NHANES 1988-2010. JAMA Otolaryngol Head Neck Surg. 2017;143(9):920–7.
Jun HJ, Hwang SY, Lee SH, Lee JE, Song JJ, Chae S. The prevalence of hearing loss in South Korea: data from a population-based study. Laryngoscope. 2015;125(3):690–4.
Kral A, O'Donoghue GM. Profound deafness in childhood. N Engl J Med. 2010;363(15):1438–50.
Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23(6):532–9.
Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303(15):1498–506.
Quittner AL, Cruz I, Barker DH, Tobey E, Eisenberg LS, Niparko JK, et al. Effects of maternal sensitivity and cognitive and linguistic stimulation on cochlear implant users’ language development over four years. J Pediatr. 2013;162(2):343–8 e3.
Geers AE, Moog JS, Biedenstein J, Brenner C, Hayes H. Spoken language scores of children using cochlear implants compared to hearing age-mates at school entry. J Deaf Stud Deaf Educ. 2009;14(3):371–85.
Friedman TB, Griffith AJ. Human nonsyndromic sensorineural deafness. Annu Rev Genom Hum Genet. 2003;4:341–402.
Duman D, Tekin M. Autosomal recessive nonsyndromic deafness genes: a review. Front Biosci (Landmark Ed). 2012;17:2213–36.
Hone SW, Smith RJ. Medical evaluation of pediatric hearing loss: laboratory, radiographic, and genetic testing. Otolaryngol Clin North Am. 2002;35:751–64.
Stamatiou GA, Stankovic KM. A comprehensive network and pathway analysis of human deafness genes. Otol Neurotol. 2013;34(5):961–70.
Kenna MA, Feldman HA, Neault MW, Frangulov A, Wu BL, Fligor B, et al. Audiologic phenotype and progression in GJB2 (Connexin 26) hearing loss. Arch Otolaryngol Head Neck Surg. 2010;136(1):81–7.
•• Sloan-Heggen CM, Bierer AO, Shearer AE, Kolbe DL, Nishimura CJ, Frees KL, et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum Genet. 2016;135(4):441–50. https://doi.org/10.1007/s00439-016-1648-8. This study shows results of parallel sequencing on 1119 sequential patients demonstrating a variety of genetic-related hearing loss.
Friedmann T. A brief history of gene therapy. Nat Genet, 1992. 2(2): p. 93–98.
Mestas E. Congenital cytomegalovirus. Adv Neonatal Care. 2016;16(1):60–5.
Saigal S, Lunyk O, Larke RP, Chernesky MA. The outcome in children with congenital cytomegalovirus infection. A longitudinal follow-up study. Am J Dis Child. 1982;136(10):896–901.
Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis. 1991;13(2):315–29.
Hagay ZJ, Biran G, Ornoy A, Reece EA. Congenital cytomegalovirus infection: a long-standing problem still seeking a solution. Am J Obstet Gynecol. 1996;174(1 Pt 1):241–5.
Stagno S, Dworsky ME, Torres J, Mesa T, Hirsh T. Prevalence and importance of congenital cytomegalovirus infection in three different populations. J Pediatr. 1982;101(6):897–900.
Ludwig A, Hengel H. Epidemiological impact and disease burden of congenital cytomegalovirus infection in Europe. Euro Surveill. 2009;14(9):26–32.
Declau F, Boudewyns A, van den Ende J, Peeters A, van den Heyning P. Etiologic and audiologic evaluations after universal neonatal hearing screening: analysis of 170 referred neonates. Pediatrics. 2008;121(6):1119–26.
Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–63.
Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135(1):60–4.
Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol. 2008;41(2):57–62.
Medearis DN Jr. Observations concerning human cytomegalovirus infection and disease. Bull Johns Hopkins Hosp. 1964;114:181–211.
Nance WE, Lim BG, Dodson KM. Importance of congenital cytomegalovirus infections as a cause for pre-lingual hearing loss. J Clin Virol. 2006;35(2):221–5.
Goderis J, de Leenheer E, Smets K, van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134(5):972–82.
Foulon I, Naessens A, Faron G, Foulon W, Jansen AC, Gordts F. Hearing thresholds in children with a congenital CMV infection: a prospective study. Int J Pediatr Otorhinolaryngol. 2012;76(5):712–7.
Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol. 2006;35(2):226–31.
Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130(4):624–30.
Park AH, Duval M, McVicar S, Bale JF, Hohler N, Carey JC. A diagnostic paradigm including cytomegalovirus testing for idiopathic pediatric sensorineural hearing loss. Laryngoscope. 2014;124(11):2624–9.
Royackers L, Christian D, Frans D, Ermelinde R. Hearing status in children with congenital cytomegalovirus: up-to-6-years audiological follow-up. Int J Pediatr Otorhinolaryngol. 2011;75(3):376–82.
James SH, Kimberlin DW. Advances in the prevention and treatment of congenital cytomegalovirus infection. Curr Opin Pediatr. 2016;28(1):81–5.
Diener ML, et al. Outcomes from a hearing-targeted cytomegalovirus screening program. Pediatrics. 2017;139(2).
Kimberlin DW, Lin CY, Sánchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25.
•• Kimberlin DW, Jester PM, Sanchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372(10):933–43. https://doi.org/10.1056/NEJMoa1404599. This study was a randomized, placebo-controlled trial that showed long-term sustained hearing benefits after 6 months of valganciclovir treatment for congenital CMV infection.
Pasternak Y, Ziv L, Attias J, Amir J, Bilavsky E. Valganciclovir is beneficial in children with congenital cytomegalovirus and isolated hearing loss. J Pediatr. 2018;199:166–70.
McCrary H, Del Calvo V, Purser J, Casazza G, Park A. The role of antioxidants in the treatment of congenital CMV-related hearing: a case-control study. OTO Open. 2019;3(2):2473974X19841857. https://doi.org/10.1177/2473974X19841857.
Dorfman L, Amir J, Attias J, Bilavsky E. Treatment of congenital cytomegalovirus beyond the neonatal period: an observational study. Eur J Pediatr. 2020;179:807–12.
Pecha PP, Almishaal AA, Mathur PD, Hillas E, Johnson T, Price MS, et al. Role of free radical formation in murine cytomegalovirus-induced hearing loss. Otolaryngol Head Neck Surg. 2020;162(5):709–17. https://doi.org/10.1177/0194599820901485.
• Sung CYW, Seleme MC, Payne S, Jonjic S, Hirose K, Britt W. Virus induced cochlear inflammation in newborn mice alters auditory function. JCI Insight. 2019;4(17). https://doi.org/10.1172/jci.insight.128878. This study provided support for virus-induced cochlear inflammation as part of the mechanism for CMV-related hearing loss.
Zhuang W, Li T, Wang C, Shi X, Li Y, Zhang S, et al. Berberine exerts antioxidant effects via protection of spiral ganglion cells against cytomegalovirus-induced apoptosis. Free Radic Biol Med. 2018;121:127–35.
Zhuang W, Wang C, Shi X, Qiu S, Zhang S, Xu B, et al. MCMV triggers ROS/NLRP3-associated inflammasome activation in the inner ear of mice and cultured spiral ganglion neurons, contributing to sensorineural hearing loss. Int J Mol Med. 2018;41(6):3448–56.
O’Sullivan ME, Perez A, Lin R, Sajjadi A, Ricci AJ, Cheng AG. Towards the prevention of aminoglycoside-related hearing loss. Front Cell Neurosci. 2017;11.
Garinis AC, Kemph A, Tharpe AM, Weitkamp JH, McEvoy C, Steyger PS. Monitoring neonates for ototoxicity. Int J Audiol. 2018;57(Sup4):S41–s48.
Fischel-Ghodsian N, Prezant TR, Chaltraw WE, Wendt KA, Nelson RA, Arnos KS, et al. Mitochondrial gene mutation is a significant predisposing factor in aminoglycoside ototoxicity. Am J Otolaryngol. 1997;18(3):173–8.
del Castillo FJ, Rodríguez-Ballesteros M, Martin Y, Arellano B, Gallo-Teran J, Morales-Angulo C et al. Heteroplasmy for the 1555A>G mutation in the mitochondrial 12S rRNA gene in six Spanish families with non-syndromic hearing loss. J Med Genet. 2003;40:623–36.
Landier W. Ototoxicity and cancer therapy. Cancer. 2016;122(11):1647–58.
Paken J, Govender CD, Pillay M, Sewram V. Cisplatin-associated ototoxicity: a review for the health professional. J Toxicol. 2016:713.
Coradini PP, et al. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol. 2007;29(6):355–60.
Taiber S, Avraham KB. Genetic therapies for hearing loss: accomplishments and remaining challenges. Neurosci Lett. 2019;713:134527.
Ahmed H, Shubina-Oleinik O, Holt JR. Emerging gene therapies for genetic hearing loss. J Assoc Res Otolaryngol. 2017;18(5):649–70.
• Chien WW, Isgrig K, Roy S, Belyantseva IA, Drummond MC, May LA, et al. Gene therapy restores hair cell stereocilia morphology in inner ears of deaf whirler mice. Mol Ther. 2016;24(1):17–25. https://doi.org/10.1038/mt.2015.150. This paper demonstrates the feasibility of restoring inner ear hair cell bundles as seen in an animal model of Usher syndrome.
Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75(2):283–93.
Ryu N, Sagong B, Park HJ, Kim MA, Lee KY, Choi JY, et al. Screening of the SLC17A8 gene as a causative factor for autosomal dominant non-syndromic hearing loss in Koreans. BMC Med Genet. 2016;17:6.
•• Tan F, Chu C, Qi J, Li W, You D, Li K, et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat Commun. 2019;10(1):3733. https://doi.org/10.1038/s41467-019-11687-8. This study demonstrates that AAV vectors can transduce multiple cell types within the inner ear with high efficacy.
Isgrig K, McDougald DS, Zhu J, Wang HJ, Bennett J, Chien WW. AAV2.7m8 is a powerful viral vector for inner ear gene therapy. Nat Commun. 2019;10(1):427. https://doi.org/10.1038/s41467-018-08243-1.
Landegger LD, Pan B, Askew C, Wassmer SJ, Gluck SD, Galvin A, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 2017;35(3):280–4.
Holt JR, Pan B, Koussa MA, Asai Y. TMC function in hair cell transduction. Hear Res. 2014;311:17–24.
Askew C, R.C., Pan B, Asai Y, Ahmed H, Child E, Schneider BL, Aebischer P, and Holt JR, Tmc gene therapy restores auditory function in deaf mice Sci Transl Med, 2015 8(7).
Nist-Lund CA, Pan B, Patterson A, Asai Y, Chen T, Zhou W, et al. Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders. Nat Commun. 2019;10(1):236.
Gyorgy B, Meijer EJ, Ivanchenko MV, Tenneson K, Emond F, Hanlon KS, et al. Gene transfer with AAV9-PHP.B rescues hearing in a mouse model of usher syndrome 3A and transduces hair cells in a non-human primate. Mol Ther Methods Clin Dev. 2019;13:1–13. https://doi.org/10.1016/j.omtm.2018.11.003.
Ivanchenko MV, Hanlon KS, Devine MK, Tenneson K, Emond F, Lafond JF, et al. Preclinical testing of AAV9-PHP.B for transgene expression in the non-human primate cochlea. Hear Res. 2020:107930.
Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122(1):23–36.
Akil O. Dual and triple AAV delivery of large therapeutic gene sequences into the inner ear. Hear Res. 2020:107912.
• Akil O, et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc Natl Acad Sci U S A. 2019;116(10):4496–501. This study demonstrates feasibility of rescuing hearing in a murine model using a dual AAV vector.
Zhang H, Pan H, Zhou C, Wei Y, Ying W, Li S, et al. Simultaneous zygotic inactivation of multiple genes in mouse through CRISPR/Cas9-mediated base editing. Development. 2018;145(20). https://doi.org/10.1242/dev.168906.
•• Gao X, Tao Y, Lamas V, Huang M, Yeh WH, Pan B, et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553(7687):217–21. https://doi.org/10.1038/nature25164. This study demonstrates that CRISPR-mediated gene editing can ameliorate hearing loss in a mouse model of human hearing loss in vivo.
Du X, Cai Q, West MB, Youm I, Huang X, Li W, et al. regeneration of cochlear hair cells and hearing recovery through Hes1 modulation with siRNA nanoparticles in adult guinea pigs. Mol Ther. 2018;26(5):1313–26. https://doi.org/10.1016/j.ymthe.2018.03.004.
McCrary H, Sheng X, Greene T, Park A. Long-term hearing outcomes of children with symptomatic congenital CMV treated with valganciclovir. Int J Pediatr Otorhinolaryngol. 2019;118:124–7.
Park A, Doutre S, Schleiss MR, Shoup A. All cytomegalovirus-infected children need hearing and neurologic follow-up. Clin Infect Dis. 2020;70(1):173.
Ross S, Long SS, Kimberlin DW. Closer to universal newborn screening for congenital cytomegalovirus infection but far away from antiviral therapy in all infected infants. J Pediatr. 2018;199:7–9.
Ziv L, Yacobovich J, Pardo J, Yarden-Bilavsky H, Amir J, Osovsky M, et al. Hematologic adverse events associated with prolonged valganciclovir treatment in congenital cytomegalovirus infection. Pediatr Infect Dis J. 2019;38(2):127–30.
• Almishaal AA, Mathur PD, Hillas E, Chen L, Zhang A, Yang J, et al. Natural killer cells attenuate cytomegalovirus-induced hearing loss in mice. PLoS Pathog. 2017;13(8):e1006599. https://doi.org/10.1371/journal.ppat.1006599. This study provided insight into the hose immune response during CMV-induced hearing loss by showing how natural killer cells may play a critical otoprotective role.
Carraro M, Almishaal A, Hillas E, Firpo M, Park A, Harrison RV. Cytomegalovirus (CMV) infection causes degeneration of cochlear vasculature and hearing loss in a mouse model. J Assoc Res Otolaryngol. 2017;18(2):263–73.
Schachtele SJ, et al. Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J Neuro-Oncol. 2011;17(3):201–11.
Yuehua Q, Longzhen Z, Kailin X, Lingyu Z, Lingjian M, Jun W, et al. Inflammatory lesions of cochlea in murine cytomegalovirus-infected mice with hearing loss. Cell Biochem Biophys. 2012;62(2):281–7.
Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007;42(9):1454–63.
Le Prell CG, et al. Assessment of nutrient supplement to reduce gentamicin-induced ototoxicity. J Assoc Res Otolaryngol. 2014;15(3):375–93.
Thatcher A, le Prell C, Miller J, Green G. ACEMg supplementation ameliorates progressive Connexin 26 hearing loss in a child. Int J Pediatr Otorhinolaryngol. 2014;78(3):563–5.
Kamogashira T, Fujimoto C, Yamasoba T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. Biomed Res Int. 2015;2015:617207.
Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochhar A, de Silva MG, et al. Advances in molecular and cellular therapies for hearing loss. Mol Ther. 2008;16(2):224–36.
Xiao J, Deng J, Lv L, Kang Q, Ma P, Yan F, et al. Hydrogen peroxide induce human cytomegalovirus replication through the activation of p38-MAPK signaling pathway. Viruses. 2015;7(6):2816–33.
Huth ME, Han KH, Sotoudeh K, Hsieh YJ, Effertz T, Vu AA, et al. Designer aminoglycosides prevent cochlear hair cell loss and hearing loss. J Clin Invest. 2015;125(2):583–92.
Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, et al. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18(6):1111–22.
Takada Y, Beyer LA, Swiderski DL, O'Neal AL, Prieskorn DM, Shivatzki S, et al. Connexin 26 null mice exhibit spiral ganglion degeneration that can be blocked by BDNF gene therapy. Hear Res. 2014;309:124–35.
Kitcher SR, et al. ORC-13661 protects sensory hair cells from aminoglycoside and cisplatin ototoxicity. JCI Insight. 2019:4(15).
O'Sullivan ME, Cheng AG. Mind your ears: a new antidote to aminoglycoside toxicity? J Med Chem. 2018;61(1):81–3.
He Y, Li W, Zheng Z, Zhao L, Li W, Wang Y, et al. Inhibition of protein arginine methyltransferase 6 reduces reactive oxygen species production and attenuates aminoglycoside- and cisplatin-induced hair cell death. Theranostics. 2020;10(1):133–50.
• Brock PR, et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med. 2018;378(25):2376–85. Randomized control trial from Europe in children with liver cancer that received cisplatin therapy, showing that children in the sodium thiosulfate group were half as likely to experience permanent hearing loss compared with children who received cisplatin alone.
•• Freyer DR, Chen L, Krailo MD, Knight K, Villaluna D, Bliss B, et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. The Lancet Oncology. 2017;18(1):63–74. https://doi.org/10.1016/s1470-2045(16)30625-8. First randomized control study out of the United States testing the ability of sodium thiosulfate to prevent cisplatin-induced hearing loss in children with diverse types of cancer.
Jie H, Tao S, Liu L, Xia L, Charko A, Yu Z, et al. Cochlear protection against cisplatin by viral transfection of X-linked inhibitor of apoptosis protein across round window membrane. Gene Ther. 2015;22(7):546–52.
Cooper LB, Chan DK, Roediger FC, Shaffer BR, Fraser JF, Musatov S, et al. AAV-mediated delivery of the caspase inhibitor XIAP protects against cisplatin ototoxicity. Otol Neurotol. 2006;27(4):484–90.
Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci. 1990;10(8):2502–12.
Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–4.
Lombarte A, Yan HY, Popper AN, Chang JS, Platt C. Damage and regeneration of hair cell ciliary bundles in a fish ear following treatment with gentamicin. Hear Res. 1993;64(2):166–74.
Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240(4860):1774–6.
Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141(4):816–29.
Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012;109(21):8167–72.
Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Géléoc GSG, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8(1):18–31.
White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–7.
Atkinson PJ, Dong Y, Gu S, Liu W, Najarro EH, Udagawa T, et al. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J Clin Invest. 2018;128(4):1641–56.
• Kuo BR, et al. In vivo cochlear hair cell generation and survival by coactivation of beta-catenin and Atoh1. J Neurosci. 2015;35(30):10786–98. This study showed that modulating certain key factors such as Atoh1 can enhance hair cell generation and survival.
McLean WJ, Yin X, Lu L, Lenz DR, McLean D, Langer R, et al. Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep. 2017;18(8):1917–29.
Forge A, Li L, Corwin J, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259(5101):1616–9.
Forge A, Li L, Nevill G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol. 1998;397(1):69–88.
Bucks SA, Cox BC, Vlosich BA, Manning JP, Nguyen TB, Stone JS. Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice. Elife. 2017;6.
Wang T, Chai R, Kim GS, Pham N, Jansson L, Nguyen DH, et al. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat Commun. 2015;6:6613.
Wang T, Niwa M, Sayyid ZN, Hosseini DK, Pham N, Jones SM, et al. Uncoordinated maturation of developing and regenerating postnatal mammalian vestibular hair cells. PLoS Biol. 2019;17(7):e3000326.
Jen HI, Hill MC, Tao L, Sheng K, Cao W, Zhang H, et al. Transcriptomic and epigenetic regulation of hair cell regeneration in the mouse utricle and its potentiation by Atoh1. Elife. 2019;8.
•• Sayyid ZN, Wang T, Chen L, Jones SM, Cheng AG. Atoh1 directs regeneration and functional recovery of the mature mouse vestibular system. Cell Rep. 2019;28(2):312–24 e314. https://doi.org/10.1016/j.celrep.2019.06.028. This study demonstrated the ability of Atoh1 to enhance regeneration and function of the vestibular system in adult mice in vivo.
Roccio M, Senn P, Heller S. Novel insights into inner ear development and regeneration for targeted hearing loss therapies. Hear Res. 2019:107859.
Samarajeewa A, Jacques BE, Dabdoub A. Therapeutic potential of Wnt and Notch signaling and epigenetic regulation in mammalian sensory hair cell regeneration. Mol Ther. 2019;27(5):904–11.
• Talaei S, Schnee ME, Aaron KA, Ricci AJ. Dye tracking following posterior semicircular canal or round window membrane injections suggests a role for the cochlea aqueduct in modulating distribution. Front Cell Neurosci. 2019;13:471. https://doi.org/10.3389/fncel.2019.00471. This study demonstrates that changes in inner ear fluid homeostasis as well as the effects of surgical approach to the inner ear can affect drug redistribution.
Farrior JB, Endicott JN. Congenital mixed deafness: cerebrospinal fluid otorrhea. Ablation of the aqueduct of the cochlea. Laryngoscope. 1971;81(5):684–99.
Bianchin G, Polizzi V, Formigoni P, Russo C, Tribi L. Cerebrospinal fluid leak in cochlear implantation: enlarged cochlear versus enlarged vestibular aqueduct (common cavity excluded). Int J Otolaryngol. 2016;2016:6591684. https://doi.org/10.1155/2016/6591684.
Aaron KA, KE, Friedman RA, & Niparko JK. Cochlear implants and auditory brainstem implants for children: surgical considerations, in Clinical management of children with cochlear implants, E.L. S., Editor. 2016, Plural Publishing. p. 69–103.
Noij KS, Remenschneider AK, Kozin ED, Puram S, Herrmann B, Cohen M, et al. Direct parasagittal magnetic resonance imaging of the internal auditory canal to determine cochlear or auditory brainstem implant candidacy in children. Laryngoscope. 2015;125(10):2382–5.
Gentile A, M.B. Additional handicapping conditions among hearing-impaired students, United States: 1971–1972. Washington, DC: Office of Demographic Studies, Gallaudet University. , 1973.
Eisenberg LS, HWF, Initial experience with the cochlear implant in children. Ann Otol Rhinol Laryngol Suppl, 1982. 91(2): p. 67–73.
Acknowledgments
We thank C. Gralapp for figure illustration; and A. Park, P. Atkinson, and I. Ahmad for fruitful discussion and insightful comments on the manuscript.
Funding
This work is supported by American Neurotological Society and American Society of Pediatric Otolaryngology research grants (K.A.A.), AAOHNS resident research grant (G.S.K.), NIH/NIDCD T32DC015209, RO1DC013910, RO1DC016919, and California Institute in Regenerative Medicine DISC2–11199 (A.G.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alan G. Cheng serves on the scientific advisory board of Decibel Therapeutics.
Ksenia A. Aaron and Grace S. Kim declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Hearing Loss in Children.
Rights and permissions
About this article
Cite this article
Aaron, K.A., Kim, G.S. & Cheng, A.G. Advances in Inner Ear Therapeutics for Hearing Loss in Children. Curr Otorhinolaryngol Rep 8, 285–294 (2020). https://doi.org/10.1007/s40136-020-00300-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-020-00300-y