Abstract
Purpose of review
Multiparametric magnetic resonance imaging of the prostate (mpMRI) is a new rising diagnostic modality in prostate cancer management. The combination of anatomic and functional sequences allows for detection, localization, and characterization of prostate cancer lesions. This leads to shift from staging to other clinical applications. However, lack of standardization of scanning, detection, and reporting protocols is a major drawback.
Recent findings
Current main applications of mpMRI are detection, staging, and localization with the latter one enabling targeted biopsies of MRI-detected lesions. With this approach, more clinically significant and less insignificant cancer lesions are detected. This technique has the potential to help solving the dilemma of overdiagnosis of indolent cancers and underdiagnosis of potentially aggressive cancers. The Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) is an attempt to establish more standardization in mpMRI. This new guideline could facilitate the clinical and scientific exchange among different institutions and therefore assist in gathering more multi-center evidence.
Summary
Multiparametric magnetic resonance imaging of the prostate improves detection and staging of prostate cancer. In conjunction with targeted biopsy techniques, it allows for better characterization of the real tumor burden compared to template systematic biopsy. Potential future applications include active surveillance, detection of recurrence, metastasis and planning, and follow-up of focal therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With 180,890 estimated new cases and 26,120 estimated deaths in 2016, prostate cancer remains the most common diagnosed cancer and second common cause of cancer-related death in the United States [1]. Prostate cancer is currently detected by a transrectal ultrasound (TRUS)-guided extended sextant systematic biopsy of the prostate prompted by an elevated prostate-specific antigen (PSA) or abnormal digital rectal examination (DRE). Systematic PSA screening with this approach demonstrated controversial results with respect to improvement of survival [2, 3]. Although some data suggest an improvement in survival, this is achieved by an inacceptable quantity of overdiagnosis and overtreatment [3]. The reason for that is the high number of indolent tumors detected with this approach. On the other hand, a significant number of aggressive tumors still can be missed. These are tumors which are either outside the standard template or inside the template but are missed or under-sampled due to the non-targeted approach of the current technique. Current data comparing local radical therapy with surveillance show no difference in overall mortality after 10 years for low- and intermediate-risk prostate cancer [4]. Thus, identification of aggressive disease without detecting more indolent disease poses the main challenge in prostate cancer diagnosis.
Magnetic resonance imaging (MRI) has the potential to close this gap and has been intensively investigated during the last decade. The technique was first described in 1983 in a study evaluating pelvic organs [5]. However, its application was limited to staging purposes since only large and locally advanced tumors could be visualized. With higher magnetic field strengths and by combining anatomic and functional MRI sequences, the so-called multiparametric MRI (mpMRI), detection of prostate tumors at earlier stages became more feasible and accurate [6, 7]. Application of mpMRI in conjunction with targeted biopsies increases the detection of clinically significant prostate cancer and decreases the detection of indolent cancers and has a better concordance with final histopathology as compared to the standard extended sextant systematic biopsy approach [8••]. Furthermore, the number of patients with suspicion for prostate cancer can be significantly reduced [9••]. Despite its benefits, mpMRI is still considered experimental and its lack of standardization, high costs, and need for advanced training for readers and surgeons remain major drawbacks. Those issues must be solved before mpMRI can become a standard of care in prostate cancer diagnostics. Herein, we aimed to provide a review of prostate MRI and its common clinical uses such as lesion detection, biopsy, staging, and active surveillance.
MR Image Acquisition
When MRI was introduced in prostate cancer imaging, it was solely used for staging of already diagnosed prostate cancer. Advances in MRI technology and development of devices with higher magnetic field strengths enabled scans with higher signal-to-noise ratios (SNR). Due to higher resolution, the zonal anatomy of the prostate and smaller and locally confined prostate tumors can be delineated. Currently, 1.5 Tesla (1.5T) or 3T scanners are routinely used. If available 3T scanners are preferred due to improved SNR. The application of an endorectal coil further improves the SNR but also increases costs and patient discomfort. Although there is no consensus among experts over the use of an ERC, it is strongly recommended in scans for staging purposes especially at magnetic field strength of 1.5T [10,11,12].
The combination of anatomic and functional MRI sequences defines a multiparametric MRI. Current mpMRI protocols usually comprise T1-weighted (T1W), T2-weighted (T2W), diffusion--weighted (DWI), apparent diffusion maps (ADC) maps, high b value (≥1400 mm/s2), and dynamic contrast-enhanced (DCE) sequences. Initial use of magnetic resonance spectroscopy (MRS) was abandoned due to its limited additional benefit but high complexity.
Anatomic Pulse Sequences
T1W and T2W are the anatomic sequences of mpMRI. T1W has low contrast between the anatomic zones of the prostate as well as between tumors and healthy tissue. Thus, it is not useful in prostate cancer detection. However, it has still its value in ruling out post-biopsy hemorrhage which cannot be differentiated from tumors in T2W. Due to aggregation of methemoglobin with its paramagnetic properties, areas of hemorrhage appear hyperintense on T1W in contrast to cancerous lesions which are not visible. If hemorrhage is detected, the study should be postponed at least 6–8 weeks.
T2W is the backbone of mpMRI of the prostate. With its high SNR and spatial resolution, the different zones of the prostate, the prostatic capsule, the urethra, and the seminal vesicles can be delineated. The peripheral zone usually has a homogenously hyperintense signal while the central zone, due to its low amount of glandular tissue, appears hypointense. The transition zone has a low-intensity signal in younger patients but can evolve an inconsistent pattern with increasing age depending on the degree of benign prostatic hyperplasia (BPH). This makes it the most challenging zone for prostate cancer detection. Cancer lesions appear hypointense due to their shorter T2 relaxation time compared to healthy tissue. The sensitivity of T2W for detecting prostate cancer lesions is moderate (58–71%), while the specificity is much higher (77–98%) [13,14,15]. By adding DWI, the sensitivity can be significantly improved [16].
Functional Pulse Sequences
DWI, DCE, and MRS are the functional sequences of mpMRI. DWI determines the movement of protons inside human tissues reflected by diffusion and perfusion of water. Healthy glandular tissue with its tubular structures has a low cellularity and high diffusion. Prostate cancer destroys the ducts and replace them by tissue with high cellularity restricting the diffusion of protons. In DWI, tumors express a higher signal compared to healthy tissue. The amount of diffusion weighting is expressed by the b value. The higher the b value the higher the accuracy in prostate cancer detection especially for b values of 1000 s/mm2 or higher [17, 18]. ADC measures the flow and the distance a water molecule has moved and thus expresses perfusion and diffusion. ADC maps are calculated from different b values usually between 500 and 800 s/mm2. In contrast to standard DWI sequences, tumors appear hypointense compared to surrounding healthy tissue. Adding DWI to T2WI in a mpMRI scan significantly improves the diagnostic accuracy with a sensitivity of 80% and a specificity of 81% for clinically significant prostate cancer [19]. However, DWI has some crucial limitations. Its in-plane resolution is very low especially in high-value DWI which limits its use in prostate cancer staging. It is also susceptible to motion artifacts and magnetic field inhomogeneity caused, for example, by hip joint replacements.
Dynamic contrast enhancement is a fast T1W sequence with injection of gadolinium-based contrast agent. Prostate cancer like many other tumors express hypervascularity due to tumor angiogenesis. This leads to early enhancement and washout of the contrast agent. This can be assessed qualitatively: semi-quantitatively and qualitatively. Quantitative and semi-quantitative parameters are usually derived from gadolinium concentration–time curves and have a high sensitivity in detection of prostate cancer. Major drawbacks are the lack of standardization and consensus while there is significant variability among different devices and software applications. Qualitative parameters are easier to obtain although dependent on subjective features. Cancer lesions usually show earlier, faster, and higher enhancement and earlier washout as compared to healthy tissue [20, 21]. The main limitation of DCE is its lack of specificity since non-cancerous vascularized tissues like BPH nodules or prostatitis cannot be differentiated accurately.
Magnetic resonance spectroscopy detects resonance frequencies of protons which are specific for certain metabolites. Citrate is produced by healthy glandular tissue and secreted to the tubules where it is stored. In prostate cancer, the peak is lower due to destruction of tubular tissue. Choline, a degradation product phospholipids of cellular membranes, is increased in prostate cancers due to increased cell turnover. MRS is time consuming, needs specialized personnel, and offers limited additional value. Its application in clinical practice is thus more and more abandoned.
Clinical Uses of mpMRI
Lesion Detection and Biopsy Guidance
All MRI sequences in prostate cancer imaging have their benefits and drawbacks as discussed above. The combined mpMRI approach offers the highest accuracy and allows for detection, localization, and characterization of prostate cancer lesions. Since patients undergoing mpMRI are usually screened-positive patients often with prior negative systematic biopsy there is a higher risk of tumors in the anterior part of the prostate including the transition zone. Thus, DWI sequences are an essential part of the exam additionally to T2W and DCE. Different scanning protocols and scoring systems for detected lesions led to increasing efforts of standardization. Current consensus defines T1W, T2W, DWI, and DCE as minimal requirements for detection purposes [22]. An endorectal coil is optional but highly recommended for detecting recurrent disease or in staging exams.
Sensitivity rates, specificity rates, positive, and negative predictive values differ enormously among different studies due to variability in scoring, patient populations, gold standards, and single-center design. Generally, mpMRI tends to have a high sensitivity but low specificity. In a recent meta-analysis, sensitivity and specificity ranged between 58 and 96% and specificity 23 and 87%, respectively [23].
The first multi-center study comparing mpMRI-detected lesions with 12-core systematic biopsy (Sx) was the PROMIS trial published in January 2017 [9••]. Ahmed et al. compared mpMRI and TRUS-guided Sx findings of 740 men against template prostate mapping biopsy as the reference test. For detecting clinically significant prostate cancer (defined as Gleason ≥4 + 3 or maximum core length ≥6 mm), mpMRI displayed higher sensitivity than TRUS-guided Sx (93% vs. 48%) but less specificity (41% vs. 96%). By using mpMRI, 27% could have been avoided and 5% fewer cases of clinically insignificant cancers were detected. However, the mpMRI lesions were not biopsied pointedly in this study.
Besides improving the detection of clinically significant prostate cancer, mpMRI is to a certain extent capable of assessing tumor biology. In a small cohort of 36 patients, ADC values were significantly correlated with cellular density in prostate cancer lesions [24]. Furthermore, there is a correlation between the ADC value and the Gleason score of detected lesions with lower ADC values being associated with higher Gleason scores [19, 25, 26]. Although promising, there is a significant overlap among different Gleason scores which limits the predictive ability and clinical applicability. Moreover, ADC values in contrast to Hounsfield units in CT imaging are not standardized and are depending on scanner properties, use of ERC and magnetic field strength. Thus, obtaining biopsy histopathology remains the gold standard.
To make mpMRI an established tool in clinical practice, detected lesions on mpMRI must be confirmed histologically before a treatment decision can be made. Currently, there are three main approaches for obtaining biopsies from lesions on mpMRI: Cognitive fusion, MRI/TRUS fusion-guidance, and in-bore MRI biopsy. All three approaches have different advantages and disadvantages but there is limited and controversial on direct comparisons of all three techniques. MRI-TRUS fusion-guided biopsies are the most widely used approach due to its easy use even in an office setting (Fig. 1). Siddiqui et al. recently published a large prospective study with 1003 patients comparing (Fx) with Sx [8••]. They showed a 25% higher detection of clinically significant (defined as ≥Gleason 3 + 4) and 17% less detection of indolent prostate cancer by Fx. In a sub-analysis, they also proved a higher concordance of Fx biopsy histology with final radical prostatectomy histopathology. However, MRI-TRUS Fx has limitations. Its availability is still limited to certain centers of expertise and it has a long examination time. Besides, since it is rather a series of procedures than one single exam, it is prone to several sources of error. Poor scanning technique and lack of experience of readers can limit the ability to detect lesions. On the other hand, 5% of cancer lesions are still not detectable with current mpMRI techniques [27]. Furthermore, MRI-TRUS Fx technique has an error rate in terms of accuracy of hitting detected lesions. This is mainly due to segmentation and registration errors during the biopsy procedure. Improving every single step is of utmost importance to improve accuracy and make this technique more accepted.
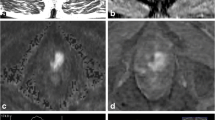
A 56-year-old man with PSA 42.73 ng/ml with one prior negative TRUS-guided biopsy. Axial (a), sagittal (b), and coronal (c) T2W MRIs show a hypointense lesion in the left to midline anterior transition (arrows) (a). The lesion shows restricted diffusion on ADC map (b) and b = 2000 DW MRI (c) (arrows) and early contrast enhancement on DCE MRI (d) (arrow). The lesion has extraprostatic extension (arrows) (a–c). MRI/TRUS fusion-guided biopsy revealed Gleason 4 + 4 prostate adenocarcinoma. Patient underwent radical prostatectomy, histopathology revealed pT3b, pN0, Gleason 4 + 4 prostatic adenocarcinoma
Local Staging
Prognostication and proper management of prostate cancer requires accurate staging [28]. Staging of local disease is dependent on identifying extracapsular extension (EPE), seminal vesicle invasion (SVI), or involvement of local pelvic structures, while staging of distant disease relies on the delineation of lymph node involvement (LNI), bone metastasis, or visceral organ spread [29, 30]. Multiparametric MRI is frequently used in the staging of localized prostate cancer, but there is little evidence for its use in the staging of distant disease. The sequences obtained are the same sequences used in prostate cancer diagnosis, and include T2W, DWI, and DCE MRI, with T1W MRI for visualizing hemorrhage from biopsy [15].
Extraprostatic Extension
The predictive ability of mpMRI for distinguishing EPE is affected by the strength of the magnetic field as well as the use of an ERC [31]. The mpMRI is typically obtained with a 3T magnet, with an ERC and surface coils for satisfactory spatial resolution necessary to detect EPE; however, a 3T magnet without ERC may be sufficient, albeit with reduced sensitivity [32]. The T2-weighted and DCE sequences are of particular importance for identifying EPE [33]. Diffusion-weighted imaging is not used for predicting EPE due to lower spatial resolution and artifacts [34]. Extraprostatic extension may be defined by enhancement, retraction, bulging, or irregularity of the capsule, loss of recto-prostatic angle, direct extraprostatic extension of the tumor, or neuro-vascular bundle involvement [35] (Figs. 1, 2). In a study, 112 patients with mpMRI of the prostate ultimately underwent radical prostatectomy for prostate cancer. Suspicion for EPE on imaging studies were retrospectively compared to the final pathology, revealing a sensitivity and specificity of 70.7 and 90.6%, respectively [32]. Subjective interpretation of EPE suspicion may play some role in these auspicious results. In our experience, suspicion for EPE on mpMRI has a sensitivity of 56% and specificity of 72%. A more pragmatic approach to predicting EPE may be based on an objective measurement. For example, we have determined that the length of a lesion on abutting the prostatic capsule, tumor contact length, may provide a more objective measure of predicting EPE. Our investigation of 379 patients undergoing radical prostatectomy revealed that the tumor contact length was an independent predictor of EPE on final pathology (odds ratio: 1.04; p = 0.001) [36].
A 51-year-old man with PSA 7.56 ng/ml with Gleason 4 + 3 prostate cancer at 12 core systematic prostate biopsy. Axial T2W MRI shows a hypointense lesion in the right apical peripheral zone (arrow) (a). The lesion shows restricted diffusion on ADC map (b) and b = 2000 DW MRI (c) (arrows) and early contrast enhancement on DCE MRI (d) (arrow). The lesion has large capsular contact which suggests extraprostatic extension (a). Patient underwent radical prostatectomy, histopathology revealed pT3b, pN0, Gleason 4 + 4 prostatic adenocarcinoma
Seminal Vesicle Invasion
Malignant involvement of the seminal vesicles is thought to occur by spreading through the ejaculatory ducts, disseminating outside the prostate to the seminal vesicles, or the non-contiguous depositing of prostate cancer in the seminal vesicles [37]. Seminal vesicle invasion presents as foci of low signal on T2-weighted imaging, low ADC values in DWI, and enhancement in DCE MRI [38]. The addition of DW imaging to T2-weighted imaging significantly improves the detection of SVI when compared to T2-weighted imaging alone [39]. A study by Soylu et al. examined 131 prostate cancer patients managed with radical prostatectomy, and retrospectively compared the pathology with the mpMRIs obtained prior to surgery. Twenty-three (18%) patients displayed pathologic SVI. The specificity T2-weighted MRI and DW imaging resulted in high specificity (97–98%), but moderate sensitivity (52–66%) [40]. This echoes the results of a similar study assessing the accuracy of mpMRI for detecting SVI. The AUC of T2W imaging in combination with DW imaging was significantly higher than the AUC of T2W imaging alone (0.897 vs. 0.779; p < 0.05) [41]. Though urologists may employ clinical data in the form of nomograms [42] to predict SVI, these predictive tools does not provide locational information of the cancer within the seminal vesicles. As such, mpMRI has been coupled with the Kattan nomogram to provide larger AUCs than either mpMRI or the Kattan nomogram alone (0.87 vs 0.76 or 0.80, respectively; p < 0.05) [43]. These encouraging results will need further investigation and external validation.
Distant Metastasis
Presently, mpMRI is not recommended for identifying lymph node involvement because of its low sensitivity [44, 45]. The specificity of MRI may be as high as 97%, and thus cross-sectional imaging has been used to some extent for identifying metastatic pelvic lymph nodes, but present literature provides a wide range of diagnostic accuracy [44, 46]. Newer lymph node-specific contrast agents, such as the ultra-small particle of iron oxide, have been developed for MR lymphangiography as a method for diagnosing nodal disease. The sensitivity and specificity of this technique is promising at 82 and 93%, respectively [47]. Multiparametric MRI is also not routinely used to detect bone metastasis; however, whole body MRI for metastatic screening has been reported to have 100% sensitivity and 100% specificity [48]. Though this presents a single test for TNM staging, cost analysis and evaluation of resource allocation has yet to be conducted.
Appropriate staging of prostate cancer is a vital step in management, and has seen marked improvements with the addition of mpMRI [49, 50]. In recent years, advances in mpMRI technology have allowed the use of higher magnetic field strengths, and improved imaging procedures, thus providing more accurate staging capabilities [51]. Though there has been a multitude of research around mpMRI in the staging of prostate cancer, the different field strengths, type of coils, and study endpoints should be considered when evaluating the study. Still, as the technology continues to improve, mpMRI may play a larger role in prostate cancer staging.
Active Surveillance
Rationale for mpMRI in Active Surveillance
Active surveillance (AS) is a cornerstone in the management of low-risk prostate cancer, and involves deferring intervention until disease progression, thus allowing men to delay the morbidities of radical treatment while maintaining similar oncologic outcomes [52,53,54]. Men in AS programs undergo frequent evaluations, such as PSA testing, DREs, and serial biopsies for monitoring cancer progression. Critical components of AS include identifying AS candidates, and effectively monitoring disease progression. The current tools of TRUS systematic biopsy, and biochemical markers, have limitations in their ability to select appropriate AS patients and detect cancer progression. Multiparametric magnetic resonance imaging, particularly in combination with MRI/TRUS fusion-guided prostate biopsy, may be used to improve these facets of AS protocols.
The potential utility of mpMRI in AS draws from its ability to aid in the diagnosis and risk stratification of prostate cancer since the functional and anatomic sequences of mpMRI allow for improved tumor detection [55]. Multiparametric MRI shows high sensitivity for detecting clinically significant cancer [15], while fusion-guided biopsies offer the ability to target mpMRI visible lesions [8••]. Herein, we will discuss the emerging role of mpMRI for determining AS candidacy and for monitoring patients on surveillance.
Selection of Patients for Active Surveillance
More than 20% of men eligible for AS by TRUS biopsy results may see Gleason score upgrades after radical prostatectomy [56, 57]. As such, mpMRI has been compared against whole-mount prostatectomy pathology to investigate its ability to select AS patients. In a study by Turkbey et al., 133 patients managed with radical prostatectomy underwent an mpMRI prior to surgery, and were then retrospectively classified as AS candidates or active treatment candidates based on whole-mount pathologic features [58]. Imaging characteristics were compared with conventional clinical assessment scoring systems (D’Amico, Epstein, and Cancer of the Prostate Risk Assessment) for AS candidacy. MpMRI demonstrated a sensitivity of 93%, a positive predictive value of 57%, and an overall accuracy of 92% (p < 0.05) for classifying patients as AS candidates, thus suggesting the potential for accurately identifying low-risk patients.
Because of the risk of upstaging at prostatectomy, AS protocols often use confirmatory biopsies prior to including the patient in a surveillance program. On confirmatory biopsy, 16–27% of patients with low-risk cancer are frequently reclassified as ineligible for AS [59, 60]. Imaging characteristics of mpMRI, such as the number of lesions, highest suspicion score, and lesion volume, have been used to create a nomogram that predicts probability of disqualification upon confirmatory biopsy [61]. External validation of this nomogram may help preclude the necessity of a confirmatory biopsy. In a similar manner, mpMRI has also been explored in centers that use fusion biopsy for confirmation prior to AS. In a study by Hu et al., 113 men meeting Epstein criteria for low-risk cancer underwent confirmatory fusion biopsy, in which 41 (36%) were reclassified based on increases in Gleason score or tumor volume. Imaging characteristics (using the UCLA 1–5 scale scoring system) were evaluated for the capacity to reclassify patients based on the results of the confirmatory biopsy. Men with image grades of 4–5 on mpMRI were more likely to be reclassified (OR 3.2, 95% confidence interval 1.4–7.1; p = 0.006) [62]. A prospective study of 60 patients with low-risk prostate cancer examined the potential of mpMRI to reclassify men on AS. Patients whose mpMRI revealed a lesion >1 cm were pathologically reclassified at a significantly higher rate than men with a normal mpMRI or men with an mpMRI consistent with low-risk disease (17.85% vs. 3.5% and 10.71% respectively; p = 0.02) [63].
The potential of mpMRI to detect prostate cancer equips fusion-guided biopsies with the ability to target suspicious lesions. Hence, fusion-guided biopsies offer advantages over traditional TRUS biopsies for establishing AS eligibility. In one study, 72 patients underwent mpMRI with subsequent fusion-guided biopsy and standard TRUS biopsy prior to enrollment into an AS program. Fusion-guided biopsy alone resulted in a higher rate of clinically significant cancer, as defined by a Gleason score ≥7, compared to the standard 12-core TRUS biopsy, highlighting the improved risk stratification provided by fusion-guided biopsy [64]. Selecting eligible patients is a paramount component of AS, and relies heavily on the exclusion of patients with high-grade disease. Multiparametric MRI and fusion-guided biopsy display the potential for playing a pivotal role in confirming AS candidates, and excluding ineligible patients.
Monitoring Active Surveillance Patients
Those who have been selected for AS may continue to leverage mpMRI for monitoring cancer progression, affording them the option for intervention as necessary. Repeat imaging permits the comparison of visible lesions, since functional or anatomic changes in the lesion may signal a progression of disease. Currently, however, there is no consensus regarding the imaging characteristics that necessitate intervention. A retrospective study of 58 patients examined serial mpMRI in patients on AS, and determined that stable mpMRIs were associated with stable Gleason scores [65]. Furthermore, the number of fusion-guided biopsies needed to detect one cancer progression was 2.89, compared to the 8.75 for the standard TRUS biopsy. In a similar manner, Felker et al. explored serial mpMRI in 49 men on AS for Gleason 6 prostate cancer. Clinical information, including baseline biopsy core length >3 mm, or follow-up PSA density >0.15 ng/ml2 demonstrated an AUC of 0.87 for predicting pathologic progression. This was compared to the progression in imaging characteristics, such an increase in suspicion score or increase in the apparent diffusion coefficient. Though mpMRI alone demonstrated an AUC of 0.63, when serial mpMRI was combined with clinical information, the AUC improved significantly to 0.91 (p = 0.044) [66].
The integration of mpMRI into fusion-guided biopsy also lends itself to monitoring disease progression. For example, in our own experience, 166 low- to intermediate-risk AS patients with mpMRI visible lesions were monitored with fusion-guided biopsies [67]. Of the 49 patients who pathologically progressed, 44.9% were identified by fusion-guided biopsy alone, whereas 30.6% were identified by standard TRUS biopsy alone (p = 0.03); the remaining 26.5% were identified by both fusion-guided biopsy and standard TRUS biopsy. This advocates for the role of fusion-guided biopsies as a more accurate method of detecting disease progression in AS patients.
The landscape of AS is evolving, particularly with the inclusion of mpMRI and fusion-guided biopsies. Emerging evidence points to the role of mpMRI in patient selection and monitoring for progression in AS protocols; however, this comes primarily from relatively small retrospective studies. Larger prospective studies will further elucidate outcomes of AS protocols that integrate mpMRI.
Conclusion
Improved scanner properties and the implementation of the multiparametric approach shifted the importance of MR imaging of the prostate from staging to different indications such as lesion detection and active surveillance. However, one major drawback of mpMRI is its lack of standardization. Many centers of excellence instituted their own image acquisition and interpretation systems complicating scientific and clinical exchange between different institutions. In order to tackle this issue, a joint effort of the American College of Radiology (ACR), the European Society of Urogenital Radiology (ESUR), and the AdMeTech Foundation introduced the Prostate Imaging Reporting and Diagnosis System Version 2 (PI-RADSv2) in 2015 [22]. Compared to its first version, the scoring system was significantly simplified which could possibly effect in better reproducibility and acceptance among clinicians outside of academic centers. This could enable larger multi-center studies and thus allow for acquiring more high-level evidence. Other future challenges include improving the precision of the fusion-guided technique by refining the segmentation and registration process as well as defining validated training protocols for urologic surgeons. Finally, although evidence is rising that mpMRI improves the detection and characterization of clinically significant prostate cancer, there are no data whether this leads to improved survival of patients diagnosed by this technique compared to the standard of care. Therefore, longitudinal studies are needed to prove that this technique impacts patient management and prognosis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9.
Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–24.
Hricak H, Williams RD, Spring DB, Moon KL Jr, Hedgcock MW, Watson RA, et al. Anatomy and pathology of the male pelvis by magnetic resonance imaging. AJR Am J Roentgenol. 1983;141(6):1101–10.
Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology. 2010;255(1):89–99.
Turkbey B, Merino MJ, Gallardo EC, Shah V, Aras O, Bernardo M, et al. Comparison of endorectal coil and nonendorectal coil T2 W and diffusion-weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mount histopathology. J Magn Reson Imaging JMRI. 2014;39(6):1443–8.
••Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390–7. This study is the first larger prospective trial comparing targeted biopsy against systematic biopsy.
••Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017. This is the first larger multi-center study comparing multiparametric magnetic resonance imaging of the prostate to a reference test (template prostate mapping biopsy).
Gupta RT, Brown AF, Silverman RK, Tay KJ, Madden JF, George DJ, et al. Can radiologic staging with multiparametric MRI enhance the accuracy of the part in tables in predicting organ-confined prostate cancer? Am J Roentgenol. 2016;207(1):87–95.
Kim BS, Kim TH, Kwon TG, Yoo ES. Comparison of pelvic phased-array versus endorectal coil magnetic resonance imaging at 3 Tesla for local staging of prostate cancer. Yonsei Med J. 2012;53(3):550–6.
Lee SH, Park KK, Choi KH, Lim BJ, Kim JH, Lee SW, et al. Is endorectal coil necessary for the staging of clinically localized prostate cancer? Comparison of non-endorectal versus endorectal MR imaging. World J Urol. 2010;28(6):667–72.
de Rooij M, Hamoen EH, Futterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol. 2014;202(2):343–51.
Delongchamps NB, Rouanne M, Flam T, Beuvon F, Liberatore M, Zerbib M, et al. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int. 2011;107(9):1411–8.
Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186(5):1818–24.
Morgan VA, Kyriazi S, Ashley SE, DeSouza NM. Evaluation of the potential of diffusion-weighted imaging in prostate cancer detection. Acta Radiol. 2007;48(6):695–703.
Katahira K, Takahara T, Kwee TC, Oda S, Suzuki Y, Morishita S, et al. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol. 2011;21(1):188–96.
Kim CK, Park BK, Kim B. High-b-value diffusion-weighted imaging at 3 T to detect prostate cancer: comparisons between b values of 1000 and 2000 s/mm2. AJR Am J Roentgenol. 2010;194(1):W33–7.
Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2010;258(2):488–95.
Barentsz JO, Engelbrecht M, Jager GJ, Witjes JA, de LaRosette J, van Der Sanden BP, et al. Fast dynamic gadolinium-enhanced MR imaging of urinary bladder and prostate cancer. J Magn Reson Imaging. 1999;10(3):295–304.
Franiel T, Ludemann L, Rudolph B, Rehbein H, Staack A, Taupitz M, et al. Evaluation of normal prostate tissue, chronic prostatitis, and prostate cancer by quantitative perfusion analysis using a dynamic contrast-enhanced inversion-prepared dual-contrast gradient echo sequence. Invest Radiol. 2008;43(7):481–7.
PI-RADStm ACoR. Prostate Imaging and Reporting and Data System 2015, version 2. 2015.
Futterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015;68(6):1045–53.
Zelhof B, Pickles M, Liney G, Gibbs P, Rodrigues G, Kraus S, et al. Correlation of diffusion-weighted magnetic resonance data with cellularity in prostate cancer. BJU Int. 2009;103(7):883–8.
Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259(2):453–61.
Salami SS, Ben-Levi E, Yaskiv O, Turkbey B, Villani R, Rastinehad AR. Risk stratification of prostate cancer utilizing apparent diffusion coefficient value and lesion volume on multiparametric MRI. J Magn Reson Imaging. 2016;. doi:10.1002/jmri.25363.
Arumainayagam N, Ahmed HU, Moore CM, Freeman A, Allen C, Sohaib SA, et al. Multiparametric MR imaging for detection of clinically significant prostate cancer: a validation cohort study with transperineal template prostate mapping as the reference standard. Radiology. 2013;268(3):761–9.
Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–31.
Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277(18):1445–51.
Partin AW, Yoo J, Carter HB, Pearson JD, Chan DW, Epstein JI, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150(1):110–4.
Somford DM, Hamoen EH, Fütterer JJ, van Basten JP, Hulsbergen-van de Kaa CA, Vreuls W, et al. The predictive value of endorectal 3 Tesla multiparametric magnetic resonance imaging for extraprostatic extension in patients with low, intermediate and high risk prostate cancer. J Urol. 2013;190(5):1728–34.
Feng TS, Sharif-Afshar AR, Smith SC, Miller J, Nguyen C, Li Q, et al. Multiparametric magnetic resonance imaging localizes established extracapsular extension of prostate cancer. Urol Oncol Semin Orig Invest. 2015;33(3):109.e15–e22.
Cornud F, Rouanne M, Beuvon F, Eiss D, Flam T, Liberatore M, et al. Endorectal 3D T2-weighted 1 mm-slice thickness MRI for prostate cancer staging at 1.5 Tesla: should we reconsider the indirects signs of extracapsular extension according to the D’Amico tumor risk criteria? Eur J Radiol. 2012;81(4):e591–7.
Petralia G, Thoeny HC. DW-MRI of the urogenital tract: applications in oncology. Cancer Imaging. 2010;10(1A):S112–23.
Renard-Penna R, Rouprêt M, Comperat E, Ayed A, Coudert M, Mozer P, et al. Accuracy of high resolution (1.5 tesla) pelvic phased array magnetic resonance imaging (MRI) in staging prostate cancer in candidates for radical prostatectomy: results from a prospective study. Urol Oncol Semin Orig Invest. 2013;31(4):448–54.
Kongnyuy M, Sidana A, George AK, Muthigi A, Iyer A, Ho R, et al. Tumor contact with prostate capsule on magnetic resonance imaging: a potential biomarker for staging and prognosis. Urol Oncol. 2017;35(1): 30.e1–e8.
Ohori M, Scardino PT, Lapin SL, Seale-Hawkins C, Link J, Wheeler TM. The mechanisms and prognostic significance of seminal vesicle involvement by prostate cancer. Am J Surg Pathol. 1993;17(12):1252–61.
Renard-Penna R, Roupret M, Comperat E, Ayed A, Coudert M, Mozer P, et al. Accuracy of high resolution (1.5 tesla) pelvic phased array magnetic resonance imaging (MRI) in staging prostate cancer in candidates for radical prostatectomy: results from a prospective study. Urol Oncol. 2013;31(4):448–54.
Kim CK, Choi D, Park BK, Kwon GY, Lim HK. Diffusion-weighted MR imaging for the evaluation of seminal vesicle invasion in prostate cancer: initial results. J Magn Reson Imaging JMRI. 2008;28(4):963–9.
Soylu FN, Peng Y, Jiang Y, Wang S, Schmid-Tannwald C, Sethi I, et al. Seminal vesicle invasion in prostate cancer: evaluation by using multiparametric endorectal MR imaging. Radiology. 2013;267(3):797–806.
Ren J, Huan Y, Wang H, Ge Y, Chang Y, Yin H, et al. Seminal vesicle invasion in prostate cancer: prediction with combined T2-weighted and diffusion-weighted MR imaging. Eur Radiol. 2009;19(10):2481–6.
Koh H, Kattan MW, Scardino PT, Suyama K, Maru N, Slawin K, et al. A nomogram to predict seminal vesicle invasion by the extent and location of cancer in systematic biopsy results. J Urol 2003;170(4, Part 1):1203–8.
Wang L, Hricak H, Kattan MW, Chen HN, Kuroiwa K, Eisenberg HF, et al. Prediction of seminal vesicle invasion in prostate cancer: incremental value of adding endorectal MR imaging to the Kattan nomogram. Radiology. 2007;242(1):182–8.
Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63(4):387–95.
Van den Bergh L, Lerut E, Haustermans K, Deroose CM, Oyen R, Isebaert S, et al. Final analysis of a prospective trial on functional imaging for nodal staging in patients with prostate cancer at high risk for lymph node involvement. Urol Oncol. 2015;33(3):109.e23–31.
Wolf JS Jr, Cher M, Dall’era M, Presti JC Jr, Hricak H, Carroll PR. The use and accuracy of cross-sectional imaging and fine needle aspiration cytology for detection of pelvic lymph node metastases before radical prostatectomy. J Urol. 1995;153(3 Pt 2):993–9.
Heesakkers RA, Hovels AM, Jager GJ, van den Bosch HC, Witjes JA, Raat HP, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008;9(9):850–6.
Pasoglou V, Larbi A, Collette L, Annet L, Jamar F, Machiels JP, et al. One-step TNM staging of high-risk prostate cancer using magnetic resonance imaging (MRI): toward an upfront simplified “all-in-one” imaging approach? The Prostate. 2014;74(5):469–77.
Fütterer JJ, Engelbrecht MR, Huisman HJ, Jager GJ, Hulsbergen-van De Kaa CA, Witjes JA, et al. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology. 2005;237(2):541–9.
Yu KK, Scheidler J, Hricak H, Vigneron DB, Zaloudek CJ, Males RG, et al. Prostate cancer: prediction of extracapsular extension with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiology. 1999;213(2):481–8.
Hoeks CMA, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SWTPJ, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261(1):46–66.
Choo R, Klotz L, Danjoux C, Morton GC, DeBoer G, Szumacher E, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167(4):1664–9.
Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–61.
Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–13.
Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–57.
Warlick C, Trock BJ, Landis P, Epstein JI, Carter HB. Delayed versus immediate surgical intervention and prostate cancer outcome. J Natl Cancer Inst. 2006;98(5):355–7.
Tosoian JJ, JohnBull E, Trock BJ, Landis P, Epstein JI, Partin AW, et al. Pathological outcomes in men with low risk and very low risk prostate cancer: implications on the practice of active surveillance. J Urol. 2013;190(4):1218–23.
Turkbey B, Mani H, Aras O, Ho J, Hoang A, Rastinehad AR, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013;268(1):144–52.
Adamy A, Yee DS, Matsushita K, Maschino A, Cronin A, Vickers A, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 2011;185(2):477–82.
Berglund RK, Masterson TA, Vora KC, Eggener SE, Eastham JA, Guillonneau BD. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008;180(5):1964–7; discussion 7–8.
Stamatakis L, Siddiqui MM, Nix JW, Logan J, Rais-Bahrami S, Walton-Diaz A, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119(18):3359–66.
Hu JC, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol. 2014;192(2):385–90.
Margel D, Yap SA, Lawrentschuk N, Klotz L, Haider M, Hersey K, et al. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: a prospective cohort study. J Urol. 2012;187(4):1247–52.
Da Rosa MR, Milot L, Sugar L, Vesprini D, Chung H, Loblaw A, et al. A prospective comparison of MRI-US fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging JMRI. 2015;41(1):220–5.
Walton Diaz A, Shakir NA, George AK, Rais-Bahrami S, Turkbey B, Rothwax JT, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urologic Oncol Semin Orig Invest. 2015;33(5):202.e1–e7.
Felker ER, Wu J, Natarajan S, Margolis DJ, Raman SS, Huang J, et al. Serial magnetic resonance imaging in active surveillance of prostate cancer: incremental value. J Urol. 2016;195(5):1421–7.
Frye TP, George AK, Kilchevsky A, Maruf M, Siddiqui MM, Kongnyuy M, et al. Magnetic resonance imaging-transrectal ultrasound guided fusion biopsy to detect progression in patients with existing lesions on active surveillance for low and intermediate risk prostate cancer. J Urol. 2017;197(3, Part 1):640–6.
Acknowledgements
Sherif Mehralivand’s postdoctoral fellowship is funded by a research grant from the “Dr. Mildred Scheel” foundation (Bonn, Germany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sherif Mehralivand, Abhinav Sidana, Mahir Maruf, Peter L. Choyke, Peter A. Pinto, and Baris Turkbey each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Urogenital Imaging.
Rights and permissions
About this article
Cite this article
Mehralivand, S., Sidana, A., Maruf, M. et al. Current Role of Magnetic Resonance Imaging in Prostate Cancer. Curr Radiol Rep 5, 57 (2017). https://doi.org/10.1007/s40134-017-0255-3
Published:
DOI: https://doi.org/10.1007/s40134-017-0255-3