Abstract
Purpose of Review
Deucravacitinib is an oral, selective TYK2 inhibitor that is currently in phase III of development for the treatment of psoriasis. This article reviews the mechanism and significance of TYK2 inhibition, as well as the current data on the safety and efficacy of deucravacitinib.
Recent Findings
Phase II results for deucravacitinib have shown that four out of six treatment groups resulted in statistically significant increases in PASI75 improvement over the placebo group. The most common adverse effects were headache, nasopharyngitis, upper respiratory tract infection, nausea, acne, and diarrhea. Three serious adverse events were noted in the treatment group, and they were gastroenteritis due to rotavirus, accidental eye injury, and dizziness.
Summary
Deucravacitinib holds great promise to meet the unfulfilled need for an efficacious oral small molecule treatment for psoriasis. However, phase III results will be needed to confirm the safety profile seen in phase I and phase II trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriasis is a common, chronic inflammatory skin disease that has a profound negative impact on a patients’ quality of life [1•]. It is known to affect 2–3% of the US population, with prevalence rates being affected by age, genetics, and geographic location [2]. The clinical manifestations of psoriasis are characterized by pruritus, scaling, and pain [3] and having psoriasis also increases the likelihood of developing multiple other comorbidities such as psoriatic arthritis, cardiovascular disease, anxiety, and depression [1•].
Although topical therapies are the first-line treatment for localized psoriasis, systemic treatment may be required for moderate-to-severe disease [4]. Current systemic treatment options for psoriasis include oral immunosuppressive drugs, biologic agents, and oral small molecule drugs [3]. Despite these treatment options, the side effect profile and/or the need for parenteral administration (intravenous or subcutaneous) makes the idea of a safe, oral small molecule treatment highly desirable.
Current Treatments
Current oral treatments have significant drawbacks. Oral immunosuppressive drugs such as methotrexate are contraindicated in patients with or at risk of hepatic disease and can lead to liver fibrosis or even cirrhosis [5]. Similarly, cyclosporine, a systemic calcineurin inhibitor, is also limited by the fact that it can lead to hypertension and renal toxicity, and thus is not a good long-term treatment option [5]. Acitretin is another option, but it is limited by teratogenicity and low effectiveness, with PASI75 response rates being around 25% [3]. Apremilast has been associated with a number of side effects including diarrhea, headaches, and upper respiratory tract infections, while also carrying a warning for depression on its label. The efficacy is similarly rather modest, with a PASI75 response rate of 33.1% at week 16 in its pivotal trial [6]. The JAK inhibitor tofacitinib has been approved for psoriatic arthritis only and has limited utility due to safety concerns [3].
Biologic therapies are currently the most effective treatment options for psoriasis. Despite the effectiveness of biologics, they are associated with some disadvantages. For example, some biologics carry label warnings of increased risk of infection from serious diseases such as tuberculosis or aspergillosis, along with increased risk for malignancies, hematologic disorders, and demyelinating disorders [3]. Another potential shortcoming with biologics is the loss of efficacy over time, which may in part be due to the development of neutralizing anti-drug antibodies, and decreased efficacy when restarting a biologic after stopping for a prolonged period of time [7]. Lastly, biologics carry high costs due to complicated manufacturing processes and have storage requirements that are more stringent than oral medications.
For these reasons, the development of newer small molecule therapies for psoriasis has continued, with the goal of producing minimally invasive treatments for this chronic condition. This would bring many advantages over biologics, such as oral or topical administration rather than injections, ease of synthesis, and lower manufacturing/handling costs [8]. Current oral treatments, as outlined above, leave much to be desired, driving the need for other small molecule therapies [9•].
JAK-STAT Signaling Pathway and the Role of TYK2
The Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) signaling pathway allows extracellular factors to influence gene expression [10]. This intracellular signaling pathway has been implicated in several autoimmune-mediated diseases, including psoriasis and psoriatic arthritis [11•].
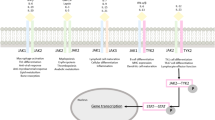
When a cytokine such as an interferon (IFN) or an interleukin (IL) binds to the receptor, a conformational change occurs, allowing the activation and combination of two JAKs [12]. This allows the JAKs to phosphorylate the receptor, which prepares the STAT proteins to attach [13]. Once attached, STAT proteins become phosphorylated and dimerized, allowing them to translocate to the nucleus to change gene expression [13]. There are four different types of JAK proteins: JAK1, JAK2, JAK3, and TYK2, and seven different STAT proteins: STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6.
One of the JAK proteins, TYK2, is an intracellular signaling enzyme that is activated by cytokines such as type I IFN, IL-6, IL-12, and IL-23 [14]. Its physiological role was first evidenced by a Japanese patient with a homozygous mutation in TYK2 who presented with signs and symptoms of hyper-IgE syndrome, such as atopic dermatitis, high circulating IgE levels, and recurrent cutaneous staphylococcal infections [15]. Moreover, this patient displayed significantly decreased immunity towards other bacterial and viral infections, presenting with Salmonella gastroenteritis, parainfluenza virus pneumonia, chronic skin Molluscum contagiosum infections, and recurrent oral HSV infections.
It was first hypothesized that this patient’s susceptibility to bacterial and viral infections was due to defect in IL-12 and IFN signaling, respectively [15]. It was also hypothesized that the elevated IgE level and the atopic dermatitis seen in this patient were due to accelerated Th2 differentiation. However, in another case report, this second patient with TYK2 deficiency did not display the elevated serum IgE levels, atopic dermatitis, or staphylococcal infections seen in the first patient [16]. But he did suffer from disseminated BCG infection, neurobrucellosis, and herpes zoster infection, which further solidified the link between TYK2 and immunity against mycobacterial and viral infections [16].
Analysis of seven other patients with TYK2 deficiency displayed impaired cellular responses to IL-12 and IFN, leading to the aforementioned mycobacterial and viral infections [17•]. These patients also showed impaired responses to IL-23 and IL-10 but responded normally to IL-6, suggesting that hyper-IgE syndrome is not an intrinsic feature of TYK2 deficiency [17•].
Conversely, TYK2 deletion in mouse models displayed increased resistance against allergic, autoimmune, and inflammatory diseases [18]. A coding variant that substitutes proline with alanine at position 1104 of the catalytic domain of TYK2 has been shown to inhibit activation of TYK2. This inactivating P9104A variant provided protection from multiple autoimmune diseases including inflammatory bowel disease, multiple sclerosis, ankylosing spondylitis, and psoriasis [18]. However, homozygosity for this P1104A variant was shown to predispose patients into developing severe mycobacterial diseases, including Mendelian susceptibility to mycobacterial disease and primary tuberculosis [19]. These mixed findings are thought to be due to selective impairment of the IL-23 pathway.
Together, these data suggest that a precise and measured inhibition of TYK2 will be necessary to provide an optimal balance between efficacy and safety. Combined with the fact that TYK2 has been identified in a genome-wide association study (GWAS) as a psoriasis susceptibility locus [20], it became a natural topic of interest for the treatment of psoriasis.
Psoriasis is thought to be a T cell–mediated disease, where IL-23/Th17 cell axis and Th17 cell–produced cytokines (IL-17 and IL-22) play an important role in abnormal skin inflammation as seen in psoriasis [21]. With the imiquimod (IMQ) mouse model for studying psoriasis-like skin inflammation, a well-studied animal model of psoriasis [22], it has been shown that TYK2 deficiency inhibits IMQ-induced psoriatic inflammation, as evidenced by decreased skin thickening [23]. Similarly, TYK2-deficient mice showed significantly reduced ear skin swelling even when injected with IL-23 [22]. These observations led to the first study of a small molecule TYK2 inhibitor, which successfully showed significant inhibition of IL-23-induced skin inflammation and cytokine production [22].
Deucravacitinib
Deucravacitinib, formally known as BMS-986165, is an oral, selective TYK2 inhibitor that is currently in phase III of development for the treatment of psoriasis and psoriatic arthritis [3]. It is being developed by Bristol-Myers Squibb and is expected in late 2022 assuming further trials proceed smoothly [3].
What makes deucravacitinib unique from other kinase inhibitors is that it stabilizes the pseudokinase JH2 domain of TYK2 in a conformational state, inhibiting the activation and activity of the catalytic domain and thus preventing downstream signaling [24]. In contrast, the other small molecule JAK inhibitors currently in development target the adenosine triphosphate (ATP) site of the catalytic JH1 domain. This selective stabilization of JH2 domain was achieved through optimization of a series of highly selective N-methyl nicotinamides and N-methyl pyridazine-3-carboxamides as a novel class of potent allosteric inhibitors [25•].
Accordingly, deucravacitinib displayed high selectivity for TYK2 pseudokinase domain in vitro when compared against 249 kinases and pseudokinases [24]. Deucravacitinib also successfully inhibited TYK2-mediated phosphorylation of STAT1 and STAT3 when stimulated by IFN-alpha and IL-23 in primary human peripheral blood mononuclear cells [24]. This high selectivity of deucravacitinib for the pseudokinase JH2 domain makes it a promising agent to deliver maximal efficacy while limiting the side effects that have plagued less selective inhibitors of the JAK-STAT pathway [26].
In vivo, mice were prophylactically treated with deucravacitinib before inducing colitis, an inflammatory bowel disease that is also driven by abnormal T cell proliferation [24]. Results showed complete protection of mice from symptoms commonly seen in colitis such as weight loss, epithelial hyperplasia, and damage in the colon [24]. Similarly, giving oral administration of deucravacitinib once daily for 2 days to lupus-prone mice resulted in dose-dependent decreased levels of type I IFN–regulated genes, and protection from nephritis [24]. These findings indicate the potential for deucravacitinib to be an effective treatment for many inflammatory and autoimmune diseases, including psoriasis, psoriatic arthritis, inflammatory bowel disease, and systemic lupus erythematosus.
Clinical Trials of Deucravacitinib in Psoriasis
Deucravacitinib completed a phase I trial involving 108 healthy subjects, displaying no serious adverse effects [26]. The frequency of non-serious adverse events was similar in the active and placebo groups, and the most common adverse events were headache, upper respiratory tract infection, nausea, and rash [26].
In the 12-week phase II randomized, double-blinded, placebo-controlled study, 267 adults with moderate-to-severe psoriasis were randomly assigned to one of six groups: 3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, 12 mg daily, or matching oral placebo in the ratio of 1:1:1:1 [27•]. The results showed that the percentage of patients with a 75% or greater reduction in the PASI score was 7% with placebo, 9% with 3 mg every other day, 39% with 3 mg daily, 69% with 3 mg twice daily, 67% with 6 mg twice daily, and 75% with 12 mg daily. Of these, dosing regimens of 3 mg daily, 3 mg twice daily, 6 mg twice daily, and 12 mg daily all showed statistically significant increases in PASI75 improvement over the placebo group (p < 0.05) [27•].
Of note, adverse effects were reported in 51% of patients in the placebo group and 55–80% of patients in the active drug groups, with the 6 mg twice daily group experiencing the most adverse effects [27•]. Higher dose ranges tended to have increased rates of adverse events, although serious adverse events remained low for all study groups. The most common adverse effects found were headache, nasopharyngitis, upper respiratory tract infection, nausea, and diarrhea. Interestingly, in this smaller trial, the trend in frequency of these adverse effects did not necessarily correlate with dose: 7 cases (16%) of nasopharyngitis were found in the 6 mg twice daily group, then 5 cases (11%) were found in the 3 mg twice daily group; 4 cases (9%) of headache were found in 3 mg daily group and also in the 3 mg every other day group; 4 cases (9%) of diarrhea were seen in the highest dose 12 mg daily group; 4 cases (9%) of upper respiratory infection were seen in the second highest dose 6 mg twice daily group; 4 cases (9%) of nausea were seen in 3 mg every other day group.
Notably, there was a higher incidence of mild-to-moderate acne seen in the active treatment groups than in placebo; 4 cases (9%) were found in the highest dose group of 12 mg daily and 2 cases (4%) were found in the second highest dose group of 6 mg twice daily [27•]. This is perhaps thought to be due to an increase in skin bacteria as a result of inhibiting the cytokines that normally regulate these organisms [27•].
For patients receiving the active drug, three different patients experienced three serious adverse effects of gastroenteritis due to rotavirus, accidental eye injury, and dizziness in a patient with a history of vestibular dysfunction [27•]. This was seen in the 3 mg every other day, 3 mg daily, and 3 mg twice daily group, respectively. For patients receiving placebo, one patient experienced two serious adverse effects of hemorrhagic anemia and hemorrhoidal hemorrhage [27•]. During the 12-week trial, no significant changes from baseline in mean values of blood counts; serum levels of liver enzymes, lipids, creatinine, or immunoglobulins; vital signal; or ECG findings were noted [27•]. Similarly, there were no reported cases of cardiovascular events or serious infections such as tuberculosis, opportunistic infections, or herpes zoster infection [27•].
Discussion
Psoriasis is a common, immune-mediated inflammatory disease that is linked to multiple physiological and psychological comorbidities. Although there is a wide range of treatments currently available, these treatments could lead to serious adverse effects and contraindications that make the development of a novel small molecule drug favorable. If successful, these small molecule treatments will have significant advantages over current therapies like biologics, such as allowing oral administration and resulting in long-term reduced costs.
The reported phase I and phase II results on the clinical efficacy and safety of deucravacitinib are promising; however, more research needs to be done in order to fully understand the mechanism and physiological consequences of TYK2 inhibition and the phase III data of this medication will help characterize potential risks. Numerous case reports of patients with TYK2 deficiency displayed susceptibility to mycobacterial and viral infections due to defect in IL-12 and IFN signaling [15, 16], although this was for complete lack of TYK2 and not partial inhibition. Furthermore, an inactivating P1104A coding variant in mice models showed mixed results: protection from multiple autoimmune diseases [18], but also an increased likelihood of developing severe mycobacterial diseases like MSMD and primary tuberculosis [19]. While a small molecule inhibitor of TYK2 such as deucravacitinib may not achieve full inhibition of the cytokine such as in the case of the P1104A variant, it is important to note the potential adverse effects that can result from inhibition of TYK2.
It is relevant to point out that another selective TYK2 inhibitor, PF-06826647, is currently in development for moderate-to-severe psoriasis. In a phase IIa trial, 212 patients with moderate-to-severe psoriasis were randomized to receive either 30 mg or 60 mg once daily of PF-06700841 or placebo for 4 weeks induction [28]. Then, they were given the same medicine at 10 mg, 30 mg, or 100 mg once weekly or placebo for 8 weeks of maintenance period. After 12 weeks, it was shown that decreases in PASI were statistically significant compared with placebo in five treatment groups out of seven [28].
The most common PF-06826647 treatment–related adverse effects were nasopharyngitis (28/189, 14.8%), upper respiratory infection (14/189, 7.4%), and headache (14/189, 7.4%) [28], which is similar to the adverse effects found in deucravacitinib. Overall, six serious adverse events were observed in five patients; of these, pneumonia and sepsis in one patient, and anemia in one patient were deemed to be PF-06826647 treatment–related [28]. However, chest pain in one patient, angina pectoris in one patient, and post-treatment death by gunshot wounds in one patient were decided to be unrelated to PF-06700841 treatment by the investigator. Importantly, no cases of herpes zoster, opportunistic infections, or any major adverse cardiac events including thromboembolic events were reported during the study.
Conclusion
Although the phase I and phase II results for deucravacitinib display potential for its use as a novel psoriasis treatment, phase III results will be needed to confirm its safety profile. In phase II trial, the most common adverse effects were headache, nasopharyngitis, upper respiratory tract infection, nausea, acne, and diarrhea [27•], which is similar to the adverse effects seen in another TYK2 inhibitor, PF-06826647. While serious adverse side effects such as mycobacterial or viral infections, or hematological abnormalities may be a theoretical concern, the safety profile of deucravacitinib has looked very promising in phase II trials and if confirmed in phase III trials should allay any significant fears of major risks while the efficacy may hearken a new standard in oral psoriasis therapy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Langley R, Krueger G, Griffiths C. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64:ii18–23 This paper is a good starting point for approaching psoriasis that, while a bit dated, represents a good historical summary of psoriasis before the age of biologics.
Laws PM, Young HS. Current and emerging systemic treatment strategies for psoriasis. Drugs. 2012;72:1867–80.
Balogh EA, Bashyam AM, Ghamrawi RI, Feldman SR. Emerging systemic drugs in the treatment of plaque psoriasis. Expert Opin Emerg Drugs. 2020;25:89–100.
Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209–22.
Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278–85.
Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RGB, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37–49.
Naldi L, Raho G. Emerging drugs for psoriasis. Expert Opin Emerg Drugs. 2009;14:145–63.
Torres T, Filipe P. Small molecules in the treatment of psoriasis. Drug Dev Res. 2015;76:215–27.
• Vangipuram R, Alikhan A. Apremilast for the management of moderate to severe plaque psoriasis. Expert Rev Clin Pharmacol. 2017;10:349–60 This manuscript outlines the data supporting the use of apremilast, the only targeted small molecule drug for psoriasis currently on the market, in treating psoriasis.
Harrison DA. The /STAT pathway. Cold Spring Harb Perspect Biol. 2012. https://doi.org/10.1101/cshperspect.a011205.
• Villarino AV, Kanno Y, Ferdinand JR, O’Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol. 2015;194:21–7 This is an excellent manuscript summarizing the role of JAK/STAT signaling in multiple immune pathways leading to human disease processes.
Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21.
Lim CP, Cao X. Structure, function, and regulation of STAT proteins. Mol BioSyst. 2006;2:536–50.
Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–87.
Minegishi Y, Saito M, Morio T, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–55.
Kilic SS, Hacimustafaoglu M, Boisson-Dupuis S, Kreins AY, Grant AV, Abel L, et al. A patient with tyrosine kinase 2 deficiency without hyper-IgE syndrome. J Pediatr. 2012;160:1055–7.
• Kreins AY, Ciancanelli MJ, Okada S, et al. Human TYK2 deficiency: mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med. 2015;212:1641–62 This report shows the effects of TYK2 deficiency on people, illustrating the potential effects of blocking this pathway too strongly. While a small molecule drug would likely be able to stay within a therapeutic window to avoid these negative effects, it’s still important to recognize potential effects of inhibition of this pathway.
Dendrou CA, Cortes A, Shipman L, et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci Transl Med. 2016;8:363ra149.
Boisson-Dupuis S, Ramirez-Alejo N, Li Z, Patin E, Rao G, Kerner G, et al. Tuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant. Sci Immunol. 2018;3:eaau8714. https://doi.org/10.1126/sciimmunol.aau8714.
Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2, Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985–90.
Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–50.
Ishizaki M, Muromoto R, Akimoto T, Sekine Y, Kon S, Diwan M, et al. Tyk2 is a therapeutic target for psoriasis-like skin inflammation. Int Immunol. 2014;26:257–67.
Ishizaki M, Akimoto T, Muromoto R, Yokoyama M, Ohshiro Y, Sekine Y, et al. Involvement of tyrosine kinase-2 in both the IL-12/Th1 and IL-23/Th17 axes in vivo. J Immunol. 2011;187:181–9.
Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11:eaaw1736.
• Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019. https://doi.org/10.1021/acs.jmedchem.9b00444This paper outlines the development and basic properties of BMS-986165, now known as deucravacitinib.
Catlett I, Aras U, Liu Y, Bei D, Girgis I, Murthy B, et al. SAT0226 A first-in-human, study of BMS-986165, a selective, potent, allosteric small molecule inhibitor of tyrosine kinase 2. Ann Rheum Dis. 2017;76:859.
• Papp K, Gordon K, Thaçi D, Morita A, Gooderham M, Foley P, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313–21 This is the main study report of the phase 2 trial of deucravacitinib in treating psoriasis.
Forman SB, Pariser DM, Poulin Y, Vincent MS, Gilbert SA, Kieras EM, et al. TYK2/JAK1 inhibitor PF-06700841 in patients with plaque psoriasis: phase IIa, randomized, double-blind, placebo-controlled trial. J Invest Dermatol. 2020;140:2359–2370.e5. https://doi.org/10.1016/j.jid.2020.03.962.
Acknowledgments
The authors wish to thank Dr. Jeffrey Weinberg for reviewing their manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, L.S., Wu, J.J. & Han, G. Deucravacitinib for Psoriasis. Curr Derm Rep 10, 1–5 (2021). https://doi.org/10.1007/s13671-020-00326-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-020-00326-x