Abstract
Hematopoietic stem cell transplant, a life-saving therapeutic option for some patients with malignant and non-malignant disease, may be complicated by a variety of cutaneous and systemic sequelae. Dermatologists are an integral part of the multidisciplinary effort involved in the care of stem cell transplant patients, as skin tissue may be the initial, and/or only, site of graft-versus-host disease (GVHD). Consequently, prompt diagnosis and treatment of cutaneous eruptions in the early post-transplant period may contribute to a reduction in morbidity and mortality. An important confounding issue is the clinical and histopathologic overlap of features among common cutaneous eruptions in stem cell transplant patients, with particular difficulties associated with differentiating GVHD from both cutaneous reactions to drugs (CRDs) as well as viral exanthema, including viral reactivation. We review challenges in the initial diagnosis of cutaneous eruptions following hematopoietic stem cell transplantation and provide an update on approaches to the differential diagnosis for GVHD, CRDs, and viral exanthema.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cell transplantation is the definitive therapy for a variety of malignant and non-malignant hematologic diseases. Graft-versus-host disease (GVHD) is a common complication of allogeneic hematopoietic stem cell transplantation (HSCT) occurring in 30–70 % of patients and its prevalence continues to increase with increasing prevalence of HSCT [1, 2••]. Important skin complications after stem cell transplantation include GVHD, cutaneous reactions to drugs (CRDs), and viral exanthema, especially as may be related to viral reactivation. HSCT patients are a uniquely vulnerable patient population; their weakened immune system makes them more susceptible to infection and more likely to undergo polypharmacy treatment approaches that lead to increased risk for CRDs [3].
The differential diagnosis for morbilliform eruptions post-HSCT includes GVHD, CRDs, and viral exanthema, all of which might share indistinguishable clinical and histopathologic findings [4]. Evaluation of such patients by a dermatologist, particularly as part of a multidisciplinary team, is essential to the effort to decrease morbidity and mortality attributable to cutaneous eruptions following HSCT [5•]. The common scenario of trying to differentiate acute graft-versus-host disease (aGVHD) from drug eruptions and viral exanthema highlights the importance of dermatology consults and skilled dermatopathologists [6].
Acute Graft-Versus-Host Disease
GVHD is an immunologic reaction of immunocompetent donor immune cells against non-identical host cells targeting mainly the skin, gastrointestinal tract, and liver [7]. In 2014, the NIH Chronic GVHD Consensus Criteria revised the 2005 consensus criteria to clarify GVHD diagnosis and subcategories, differentiating acute and chronic GVHD based on clinical symptoms, and not on the time elapsed since transplant [2••]. Common cutaneous features/symptoms of aGVHD include erythema, maculopapular lesions, and pruritus with oral mucosal involvement manifested by gingivitis, mucositis, erythema, and pain. Although skin may sometimes be the only organ targeted, the gastrointestinal tract and liver are also commonly involved [8]. aGVHD severity is uniformly assessed as stage 1–4 in each target organ by measuring affected skin body surface area (BSA) in the skin, bilirubin and serum glutamic oxaloacetic transaminase (SGOT) levels in the liver, and diarrhea output in the gut (Table 1) [9, 10]. A composite score of skin, liver, and gut staging is used to determine the aGVHD grade from I to IV. Systemic symptoms such as fever, nausea, vomiting, diarrhea, weight loss, and elevations in liver enzymes and bilirubin levels are variably present [2••].
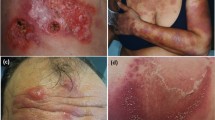
Cutaneous aGVHD is classically described as a sudden onset, symmetric, morbilliform eruption, predominantly involving the upper back, palms, soles, pinnae, and cheeks [2••, 8]. Acral involvement of the palms and soles as well as an erythematous to violaceous discoloration of the ears are highly suggestive of GVHD [7]. Severe GVHD might present with confluence of lesions and evolution to generalized erythroderma, bullae formation, a positive Nikolsky sign, and erosive mucosal changes [7, 8, 11•].
Skin eruption is recognized by the Glucksberg criteria and the 2005 NIH Consensus Conference as integral to the diagnosis of aGVHD; however, specific morphologic and anatomic features have not been systematically categorized [12••]. Given the clinical variability of cutaneous aGVHD, atypical presentations occur frequently and are important to differentiate [1]. Atypical morphologic presentations of aGVHD have included eczematous (craquele-like), psoriasiform, follicular, lichenoid, contact dermatitis-like, and pityriasis rubra pilaris-like [1]. Recent attempts have been made to elucidate the clinical morphology and primary anatomic sites for aGVHD. A retrospective review of 42 patients with aGVHD following HSCT described the morphology of skin aGVHD skin eruption as morbilliform in 55 %, patchy erythema in 38 %, confluent in 33 %, follicular accentuation in 29 %, purpuric/violaceous in 24 %, desquamative in 14 %, reticulated in 10 %, bullous in 5 %, and erythrodermic in 5 % [12••]. In this same study, favored anatomic sites for cutaneous eruptions included the trunk in 69 %, arms/legs in 67 %, face in 62 %, ears in 38 %, and palms in 38 %. A retrospective review of 22 patients with aGVHD by Byun et al. found that involvement of the face, especially in conjunction with the palms and soles, favors a diagnosis of aGVHD [3].
Histological criteria for grading cutaneous aGVHD include vacuolization of the basal epithelial layer (grade 1), keratinocyte apoptosis and satellitosis (grade 2), subepidermal clefting (grade 3), and epidermal separation (grade 4) [8]. Recent NIH guidelines found the minimum requirement for diagnosis of skin aGVHD to be apoptosis within the basilar or lower spinosum layers of the epidermis and recommend focusing on interpretation of vacuolar changes and apoptotic keratinocytes in cases of minor alteration [13•]. Dermatopathology is an important factor in the diagnostic workup for patients lacking clear and distinct clinical features of aGVHD [8, 11•, 13•].
The sensitivity and specificity of dermatopathology for aGVHD is unknown [1]. Although there is no pathognomonic histologic finding for aGVHD, the consensus criteria for histologic diagnosis of aGVHD were released by an NIH working group, recognizing the fact that making a definitive diagnosis is highly subject to histologic interpretation within a particular clinical context [5•, 11•, 13•]. It has been suggested that in order to improve the standardization and reproducibility of a histopathologic diagnosis of aGVHD, it is important to give careful attention to the presence of interface changes consistent with dermatitis as well as the presence of apoptotic keratinocytes in adnexal structures [11•]. Analysis of dermatopathology results was found to be most accurate for aGVHD with samples of sufficient size and taken from an anatomic site with a dense presence of sweat glands or hair follicles in order to best allow for evaluation of interface alteration [11•]. The diagnosis of aGVHD cannot be made in isolation, and the usefulness of correlating histological and clinical findings has been previously demonstrated with the percentage of correct diagnosis of aGVHD increasing from 33 to 80 % when dermatopathology is informed with clinical data [11•].
The utility of skin biopsies early post-HSCT has been challenged given the nonspecific lesional morphology, inconsistent correlation to disease severity, and evidence that clinical management may not greatly differ regardless of specific diagnosis [5•, 7, 14, 15]. In a retrospective study evaluating dermatopathology from 352 HSCT recipients, a discordance rate of 55 % between pre-biopsy and post-biopsy diagnosis was found, but biopsy results led to therapeutic management changes in only 16 % of patients [14]. Although clinical management was not altered in the majority of patients, this propensity for change from pre-biopsy to post-biopsy diagnosis suggests that the biopsy remains an important tool that serves to establish a definitive diagnosis in post-HSCT recipients with skin rash.
Cutaneous Reactions to Drugs
Post-transplantation, patients typically require drugs and biologic agents, several of which are associated with cutaneous reactions that may be difficult to differentiate from aGVHD [15]. CRDs are idiosyncratic drug or biologic agent which induced skin reactions (unrelated to dose or pharmacological action) that appear 7–10 days, or more, after initial exposure to the inciting agent, and typically appear more acutely with re-exposure [4]. The most common agents associated with CRDs post-HSCT are antibiotics, particularly penicillin moieties and sulfonamide moieties [16]. Typically, CRDs are described as morbilliform, exanthematous, and centrifugal rashes, beginning on the trunk and spreading peripherally to proximal extremities. Urticarial, bullous, and pustular subtypes are not as common; pruritus is typically present; in severe cases, confluent erythema may proceed to erythroderma and widespread exfoliation [3, 4, 16]. Severe rash may also be accompanied by constitutional and non-specific gastrointestinal symptoms that may mimic non-drug causes of HSCT rash [3, 4, 16].
Drug-induced hypersensitivity syndrome (DIHS) and drug rash with eosinophilia and systemic symptoms (DRESS) are multi-organ disorders that usually present as morbilliform or erythrodermic cutaneous features, peripheral eosinophilia, and hypogammaglobulinemia [17]. Classic histologic findings include perivascular lymphocytic infiltration with extravascular eosinophils, and rarely epidermal necrosis or degeneration of the basal layer [17]. Although both DIHS/DRESS and aGVHD share histologic findings of interface dermatitis, apoptotic keratinocytes, spongiosis, and vacuolar degeneration, such findings may be of greater severity in aGVHD, perhaps due to significantly higher ratio of regulatory FoxP3+ T cells/CD3+ T cells in DIHS/DRESS compared to aGVHD skin lesions [17].
Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS) are acute mucocutaneous eruptions that can present with extensive blistering and desquamation, which can be difficult to differentiate from aGVHD [18]. Reports have described stage IV aGVHD as both clinically and histologically mimicking TEN, making a definitive diagnosis difficult [3, 7]. Skin biopsy results can be indistinguishable among stage IV aGVHD and early TEN, with full thickness epidermal necrosis as a hallmark for both entities [18]. It has been suggested that aGVHD and TEN have a shared mechanism involving autoreactive CD8+ T cell activation and upregulation of interleukin-2 and interleukin-2 receptor that may account for similar clinical and histological findings in early TEN and stage IV aGVHD [7, 19].
Viral Exanthema
The immunocompromised state of HSCT patients and resultant impaired cell-mediated immunity frequently results in reactivation of human herpesvirus 6 (HHV-6), Epstein-Barr virus (EBV), cytomegalovirus (CMV), enteroviruses, adenoviruses, parvovirus-B19, and herpes simplex virus (HSV); such reactivation may manifest as skin eruptions similar to drug exanthema as well as aGVHD [4, 16, 20]. Common systemic components of viral reactivation include fever, gastrointestinal symptoms similar to those often found in aGVHD and some drug reactions, as well as myalgias and pneumonitis [21].
For many HSCT patients, viral reactivation is a difficult diagnosis due to non-specific cutaneous findings, atypical presentation, or a lack of classically associated symptoms [20]. Cutaneous presentation is most often a non-specific morbilliform rash [16, 20]. If present, findings of a petechial exanthema with fever, malaise, myalgia, cervical lymphadenopathy, and non-exudative pharyngitis all favor a diagnosis of CMV, while findings of fever, cervical lymphadenopathy, pharyngitis, periorbital edema, and palatal petechiae all favor EBV [20]. Dermatopathologic findings unique to viral infection, such as the presence of intranuclear inclusions surrounded by a halo in CMV, allows for diagnosis; however, most often, biopsy findings are non-specific and therefore cannot be differentiated from aGVHD and drug eruptions such that further testing to detect viruses such as polymerase chain reaction (PCR) may prove to be useful in obtaining a definitive diagnosis for viral exanthema [16, 20].
The relationship between viruses and aGVHD appears to be bidirectional and is an area of active exploration [20]. HHV-6 is associated with a morbilliform rash similar to that of aGVHD, and the virus has also been linked to the development of aGVHD [21, 22]. Kitamura et al. found that HHV-6 deoxyribonucleic acid (DNA) levels in peripheral blood correlated with development of a skin rash that was diagnosed as aGVHD [21]. Differentiating HHV-6 reactivation from aGVHD post-HSCT is complicated by the uncertain role that HHV-6 may play in the development of aGVHD [21, 22]. Due to difficulty in distinguishing between HHV-6-induced skin lesions and aGVHD, it has been reported that HHV-6 should be included in the differential diagnosis of aGVHD and that serum HHV-6 viral load should be ascertained [20, 21, 23]. HHV-6 reactivation is typically associated with non-specific systemic symptoms; however if present, pneumonitis or neurologic symptoms may allow for recognition and assist with diagnosis of viral infection with skin rash post-HSCT [23].
Herpes-associated erythema multiforme (HAEM) can clinically and histologically appear similar to aGVHD [24]. HAEM and aGVHD lesions were found to express HSV Pol protein antigen and DNA, but the possibility that the skin lesions diagnosed as aGVHD were actually virus-associated lesions in the HAEM family could not be excluded, highlighting the inability to easily differentiate aGVHD from viral exanthema [24]. In some patients, HAEM, which was misdiagnosed as aGVHD, was found to have lesional Pol expression with Pol levels that correlated to lesional severity, but not to the histopathologically diagnosed aGVHD grade [25]. These studies are supportive of the fact that viral exanthema remain particularly difficult to differentiate from aGVHD and underscores the importance of dermatology consultation as well as the need for further exploration to understand the relationship of these factors in HSCT patients with skin rash.
Differential Diagnosis
Common causes of morbilliform rash post-HSCT include aGVHD, CRDs, and viral exanthema, including reactivation, all of which invoke a challenge to correctly diagnose a post-HSCT skin rash given their overlapping clinical and histologic features and considering that aGVHD is a clinical diagnosis and often one of exclusion.
Clinical Approach
Clinical evaluation of post-HSCT skin lesions for prominent characteristics of aGVHD is recommended as an initial approach [4, 8]. It has been noted that the rash of aGVHD is characteristically accentuated in anatomical sites located on the face, palms, soles, pinnae, and cheeks [3, 8]. More distinct characteristics of aGVHD rash include violaceous discoloration of the pinnae and follicular prominence.
Extracutaneous clinical findings may be correlated to skin findings to aid in diagnosis. Diarrhea and hyperbilirubinemia are often associated with aGVHD, but may also be associated with other post-HSCT conditions including drug reactions and viral reactivations [3, 16, 18]. In a study comparing 22 patients with aGVHD and 17 patients with CRDs post-HSCT, combined diarrhea and hyperbillirubinemia were only present in the aGVHD cohort, leading to a conclusion that post-HSCT skin rashes present for more than 2 to 3 days in the absence of diarrhea or hyperbilirubinemia are less likely to be GVHD [3]. Moreover, although respiratory and neurologic findings may favor viral origin, any internal organ can be affected in association with CRDs, including the lungs, liver, kidney, pancreas, heart, and thyroid [4, 23].
Diagnostic Tests
Although dermatopathology is an important aspect of the differential diagnosis for cutaneous eruptions following HSCT, it is important to note that biopsies alone cannot consistently render a definitive diagnosis [11•, 13•]. The 2015 NIH Consensus guidelines noted that false-positive diagnosis of aGVHD might result from concurrent infections, drug eruptions, or inflammatory reactions unrelated to aGVHD [13•]. To diagnose aGVHD in equivocal cases, it is important to focus on vacuolar changes and apoptotic keratinocytes within the adnexal epithelia [11•, 13•]. It is useful to note that in a study comparing aGVHD and CRDs, aGVHD was found to lack spongiosis and have fewer dermal eosinophils [5•].
Quantitative eosinophil analysis has been proposed as a method for differentiating CRDs from aGVHD as it has been noted that there is a lower density of eosinophils in aGVHD compared to what is typically seen for CRDs [15]. An NIH consensus project concluded that the presence of tissue eosinophils demonstrated in the histological examination of skin cannot reliably diagnose drug hypersensitivity as eosinophils may also be present in skin affected by aGVHD [13•]. Moreover, relying on eosinophils to exclude aGVHD may result in an incorrect diagnosis and potentially life-threatening delays in treatment [13•, 15].
A viral exanthema in HSCT patients relies on evidence from viral culture, serologic testing, antigen detection, and nucleic acid detection via PCR in order to differentiate viral exanthema from aGVHD [20, 26]. For example, although histologic findings are often non-specific, a definitive diagnosis of CMV viral exanthema is made if enlarged endothelial cells with intranuclear inclusions surrounded by a halo are visible [16].
Serum biomarkers for the diagnosis of aGVHD are an important area of research interest. While there are many candidate biomarkers for the diagnosis of systemic aGVHD, the only biomarker specific for skin aGVHD is elafin [27, 28]. Paczesny et al. found that elafin may be helpful in diagnosis of skin aGVHD and that its sensitivity and specificity can be increased when combined with systemic aGVHD biomarkers [27]. However, a recent study by Bruggen et al. found that elafin could not differentiate between aGVHD and CRDs [29]. It remains to be seen if elafin or other aGVHD markers will be able to reliably differentiate aGVHD skin rash from other causes of post-HSCT cutaneous eruptions.
Conclusions
A better understanding of aGVHD skin eruption morphology and anatomic distribution is expected to allow for better differentiation of similar lesions caused by CRDs and viral exanthema, but systematic investigations are lacking. Given that aGVHD is diagnosed clinically and that there are limitations to the utility of skin biopsies for patients presenting with a morbilliform rash early after HSCT, there has been debate regarding the utility of skin biopsy. Skin biopsy was recommended by only 62 % of a group of dermatopathologists, dermatologists, transplant-physicians, and transplant-pathologists convened for a consensus regarding aGVHD and the role of skin biopsy in forming a diagnosis [30]. They concluded that skin biopsies are most important in cases of atypical clinical features or for exclusion of other non-aGVHD diagnoses. Taking all findings into consideration, diagnosis of a morbilliform skin eruption post-HSCT requires correlation of clinical findings with dermatopathologic and laboratory findings to help differentiate aGVHD, CRDs, and viral exanthema.
Abbreviations
- GVHD:
-
Graft-versus-host disease
- HSCT:
-
Hematopoietic stem cell transplantation
- aGVHD:
-
Acute graft-versus-host disease
- BSA:
-
Body surface area
- SGOT:
-
Serum glutamic oxaloacetic transaminase
- CRDs:
-
Cutaneous reactions to drugs
- DIHS:
-
Drug-induced hypersensitivity syndrome
- DRESS:
-
Drug rash with eosinophilia and systemic symptoms
- TEN:
-
Toxic epidermal necrolysis
- SJS:
-
Stevens-Johnson syndrome
- HHV-6:
-
Human herpesvirus-6
- EBV:
-
Epstein-Barr virus
- CMV:
-
Cytomegalovirus
- HSV:
-
Herpes simplex virus
- PCR:
-
Polymerase chain reaction
- DNA:
-
Deoxyribonucleic acid
- HAEM:
-
Herpes-associated erythema multiforme
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cornejo CM, Kim EJ, Rosenbach M, Micheletti RG. Atypical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2015;72(4):690–5. doi:10.1016/j.jaad.2014.12.022.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401. doi:10.1016/j.bbmt.2014.12.001. This paper contains clinically relevant updates.
Byun HJ, Yang JI, Kim BK, Cho KH. Clinical differentiation of acute cutaneous graft-versus-host disease from drug hypersensitivity reactions. J Am Acad Dermatol. 2011;65(4):726–32. doi:10.1016/j.jaad.2010.07.042.
Bircher AJ, Scherer HK. Drug hypersensitivity reactions during hematopoietic stem cell transplantation. Curr Probl Dermatol. 2012;43:150–64. doi:10.1159/000335483.
Lehman JS, Gibson LE, El-Azhary RA, Chavan RN, Hashmi SK, Lohse CM, et al. Acute cutaneous graft-vs.-host disease compared to drug hypersensitivity reaction with vacuolar interface changes: a blinded study of microscopic and immunohistochemical features. J Cutan Pathol. 2015;42(1):39–45. doi:10.1111/cup.12427. This paper contains clinically relevant updates.
McMahon P, Goddard D, Frieden IJ. Pediatric dermatology inpatient consultations: a retrospective study of 427 cases. J Am Acad Dermatol. 2013;68(6):926–31. doi:10.1016/j.jaad.2012.12.949.
Correia O, Delgado L, Barbosa IL, Domingues JC, Azevedo R, Vaz CP, et al. CD8+ lymphocytes in the blister fluid of severe acute cutaneous graft-versus-host disease: further similarities with toxic epidermal necrolysis. Dermatology. 2001;203(3):212–6.
Hausermann P, Walter RB, Halter J, Biedermann BC, Tichelli A, Itin P, et al. Cutaneous graft-versus-host disease: a guide for the dermatologist. Dermatology. 2008;216(4):287–304. doi:10.1159/000113941.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–8.
Ziemer M, Haeusermann P, Janin A, Massi D, Ziepert M, Wolff D, et al. Histopathological diagnosis of graft-versus-host disease of the skin: an interobserver comparison. J Eur Acad Dermatol Venereol. 2014;28(7):915–24. doi:10.1111/jdv.12215. This paper contains clinically relevant updates.
Bach D, Damstetter E, West D, Mehta J, Cotliar J. Clinical features of acute cutaneous graft-versus-host disease following allogeneic hematopoietic stem cell transplant biology of blood and marrow transplantation. Salt Lake City, UT: Elsevier; 2013. This paper contains clinically relevant updates.
Shulman HM, Cardona DM, Greenson JK, Hingorani S, Horn T, Huber E, et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transplant. 2015;21(4):589–603. doi:10.1016/j.bbmt.2014.12.031. This paper contains clinically relevant updates.
Paun O, Phillips T, Fu P, Novoa R, Honda K, Lu Q et al. Cutaneous complications in hematopoietic cell transplant recipients: impact of biopsy on patient management. 2013 ASCO Annual Meeting2013.
Marra DE, McKee PH, Nghiem P. Tissue eosinophils and the perils of using skin biopsy specimens to distinguish between drug hypersensitivity and cutaneous graft-versus-host disease. J Am Acad Dermatol. 2004;51(4):543–6.
Mays SR, Kunishige JH, Truong E, Kontoyiannis DP, Hymes SR. Approach to the morbilliform eruption in the hematopoietic transplant patient. Semin Cutaneous Med Surg. 2007;26(3):155–62. doi:10.1016/j.sder.2007.09.004.
Morito H, Ogawa K, Fukumoto T, Kobayashi N, Morii T, Kasai T, et al. Increased ratio of FoxP3+ regulatory T cells/CD3+ T cells in skin lesions in drug-induced hypersensitivity syndrome/drug rash with eosinophilia and systemic symptoms. Clin Exp Dermatol. 2014;39(3):284–91. doi:10.1111/ced.12246.
Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69(2):187. doi:10.1016/j.jaad.2013.05.002. e1-16; quiz 203-4.
Takatsuka H, Takemoto Y, Yamada S, Mori A, Wada H, Fujimori Y, et al. Similarity between eruptions induced by sulfhydryl drugs and acute cutaneous graft-versus-host disease after bone marrow transplantation. Hematology. 2002;7(1):55–7.
Tong LX, Worswick SD. Viral infections in acute graft-versus-host disease: a review of diagnostic and therapeutic approaches. J Am Acad Dermatol. 2015;72(4):696–702. doi:10.1016/j.jaad.2014.12.002.
Kitamura K, Asada H, Iida H, Fukumoto T, Kobayashi N, Niizeki H, et al. Relationship among human herpesvirus 6 reactivation, serum interleukin 10 levels, and rash/graft-versus-host disease after allogeneic stem cell transplantation. J Am Acad Dermatol. 2008;58(5):802–9. doi:10.1016/j.jaad.2008.01.005.
Yoshikawa T, Ihira M, Ohashi M, Suga S, Asano Y, Miyazaki H, et al. Correlation between HHV-6 infection and skin rash after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2001;28(1):77–81. doi:10.1038/sj.bmt.1703099.
Dulery R, Salleron J, Dewilde A, Rossignol J, Boyle EM, Gay J, et al. Early human herpesvirus type 6 reactivation after allogeneic stem cell transplantation: a large-scale clinical study. Biol Blood Marrow Transplant. 2012;18(7):1080–9. doi:10.1016/j.bbmt.2011.12.579.
Akpek G, Joseph R, Gunay C, Kessler II, Shvartsbeyn M, Bhatnagar B, et al. Frequent detection of herpes simplex virus antigen in skin and peripheral blood CD34+ mononuclear cells from patients with graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19(4):529–37. doi:10.1016/j.bbmt.2012.12.021.
Joseph R, Shvartsbeyn M, Gunay C, Akpek G, Aurelian L. Acute skin graft-versus-host disease with molecular features mimicking herpes simplex virus-associated erythema multiforme: report of three cases. Dermatology. 2014;228(2):125–9. doi:10.1159/000355182.
Lin R, Liu Q. Diagnosis and treatment of viral diseases in recipients of allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2013;6:94. doi:10.1186/1756-8722-6-94.
Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2(13):13ra2. doi:10.1126/scitranslmed.3000406.
Levine JE, Paczesny S, Sarantopoulos S. Clinical applications for biomarkers of acute and chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(1 Suppl):S116–24. doi:10.1016/j.bbmt.2011.10.019.
Bruggen MC, Petzelbauer P, Greinix H, Contassot E, Jankovic D, French L, et al. Epidermal elafin expression is an indicator of poor prognosis in cutaneous graft-versus-host disease. J Investig Dermatol. 2015;135(4):999–1006. doi:10.1038/jid.2014.489.
Hillen U, Hausermann P, Massi D, Janin A, Wolff D, Lawitschka A, et al. Consensus on performing skin biopsies, laboratory workup, evaluation of tissue samples and reporting of the results in patients with suspected cutaneous graft-versus-host disease. J Eur Acad Dermatol Venereol. 2015;29(5):948–54. doi:10.1111/jdv.12737.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ahuva D. Cices, Chantelle Carneiro, Sara Majewski, Gary Tran, Dr. Amanda Champlain , Dr. Dennis P West, Dr. Jonathan A Cotliar, and Dr. Beatrice Nardone declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Cutaneous Drug Reactions
Rights and permissions
About this article
Cite this article
Cices, A.D., Carneiro, C., Majewski, S. et al. Differentiating Skin Rash After Stem Cell Transplantation: Graft Versus Host Disease, Cutaneous Reactions to Drugs and Viral Exanthema. Curr Derm Rep 5, 12–17 (2016). https://doi.org/10.1007/s13671-016-0126-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13671-016-0126-9