Abstract
Hormonal contraceptives may influence the immunological and microbiological milieu of the vagina and alter the risk of acquisition of sexually transmitted infections (STIs) and human immunodeficiency virus (HIV). Most studies demonstrate more normal vaginal flora and less bacterial vaginosis in hormonal contraceptive users compared to non-users, suggesting that contraceptive-induced alteration in vaginal microbiota is an unlikely mechanism for increased risk of STI/HIV acquisition. Measured impacts of hormonal contraceptive use on the presence and activity of vaginal immune cells and vaginal cytokine secretion varies depending on the experimental model, progestogen used, contraceptive delivery method, and length of use of the method, limiting cohesive conclusions. Further study is needed to evaluate the effects of specific progestogens, delivery methods, and long-term use of contraceptives, particularly intrauterine devices and implants, on innate and adaptive immune cells and function in order to ultimately understand impacts on susceptibility to sexually transmitted infections including HIV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
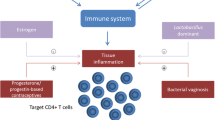
Endogenous, as well as exogenous progestogens and estrogens in the form of contraceptives, may influence the immunological and microbiological milieu of the vagina in a way that alters the risk of acquisition of sexually transmitted infections (STIs) [1–3]. While it has been known for at least 30 years that female reproductive hormones can alter immune function [4], more recently collective evidence from observational studies suggests that some progestin-containing contraceptives, particularly depot medroxyprogesterone acetate (DMPA), may increase acquisition risk of HIV [5–8]. A recent randomized controlled trial of South African women using injectable contraceptives (DMPA or norethisterone enanthate (NET-EN)) demonstrated significantly increased risk of HIV acquisition among women using DMPA compared to those using NET-EN (hazard ratio 1.53, 95 % CI 1.12–2.08; p = 0.007) [9•]. Are there differential impacts of these two injectable progestins that are responsible for differential HIV acquisition risk in similar women? In response to these concerns and questions, research interest focused on understanding various impacts of hormonal contraceptive use on vaginal and systemic immune function has intensified.
Hypotheses for biologic mechanisms that plausibly link increased risk of acquiring HIV/STIs and hormonal contraceptive use include changes in innate or adaptive immune function, alterations in protective or pathologic vaginal flora, changes in the mucosal barrier of the vagina, direct hormonal effects on STI pathogens, changes in number or density of HIV target cells present in the genital tract, and alterations in function, presence, and/or expression of HIV co-receptors on immune cells in the genital tract. One long-standing observation appears consistent with the hypothesis that alterations in local and/or systemic immune populations may influence HIV acquisition risk: coexisting STIs, particularly ulcerative STIs, have been well documented to increase HIV acquisition risk [5, 10, 11]. Unfortunately, efforts to reduce risk by treating STIs have largely failed to reduce HIV incidence rates. Our goal is to review recent publications and current research gaps relating to hormonal contraception and STI/HIV risk, with a particular focus on the microbiologic and immunologic environment of the lower female genital tract. A deeper understanding of this topic is critically important to inform contraceptive recommendations for all women, especially those living in high HIV prevalence areas, and to guide future contraceptive and microbicide research and development.
What Effects Do Contraceptives Have on Vaginal Flora?
Mucosal surfaces of the gastrointestinal and reproductive systems support their own ecosystems of microorganisms. These “microbiomes” have been shown to affect both host health and disease susceptibility. Vaginal microorganisms have increasingly been implicated in adverse reproductive tract outcomes, including susceptibility to and transmission of STIs, risk of preterm birth [12], and implantation failure with assisted reproduction [13]. Women with vaginal flora dominated by Lactobacillus species, particularly Lactobacillus crispatus, have been shown to have decreased incidence of HIV, human papillomavirus (HPV), and trichomonas infection [14] as compared to women with bacterial vaginosis (BV), a polymicrobial anaerobic vaginal flora typically with low prevalence of Lactobacilli and elevated vaginal pH [15]. Historically, symptomatic BV has been primarily attributed to Gardnerella vaginalis, but molecular technologies, such as 16s gene microarray, have identified a host of other bacteria associated with dysbiotic, or imbalanced, vaginal microbial states including Atopobium vaginae, Lactotrichia amnionii, Megasphaera, and newly described members of the Clostridiales sometimes referred to as BV-associated bacteria (BVAB) [15–17].
Lactic acid is produced by Lactobacilli through glycogen metabolism [18] and has been shown to protect against pathogenic vaginal organisms by acidification of the vagina [16, 19]. High estrogenic states, including pregnancy, are associated with increased presence of vaginal glycogen and thus increased lactic acid production [14], while low estrogenic states such as menopause are associated with lower vaginal glycogen levels and decreased Lactobacilli [20]. While some studies suggest that vaginal flora in an individual are stable over time [21], most published data suggest that shifts between flora considered normal and flora considered intermediate or abnormal occur frequently, even from day to day [22, 23]. Given the frequent shifts in endogenous hormones and flora throughout the menstrual cycle, contraceptive hormone use would likely need to cause dramatic shifts in vaginal microbiome to impact STI/HIV acquisition risk.
Most studies demonstrate decreased prevalence of BV as well as more successful remission of BV after treatment in women using contraceptive hormones compared to those not using hormonal contraceptives [6, 24–27]. Women using DMPA have decreased BV incidence when compared to women using no contraception [6, 28]. Mitchell, et al., further showed that women using DMPA had a reduction in colonization with Lactobacilli compared to their baseline without any difference in incidence of BV over 2 years [29]. Although the IUD in general has not been found to increase risk of BV when compared to no contraception [30], it may increase risk in individuals with irregular bleeding [31] or appear to increase risk when compared to oral contraceptives and condoms, which are known to be protective [25]. The aforementioned studies do not specify or separate hormonal versus non-hormonal IUDs. A recent study focusing solely on levonorgestrel (LNG) IUD users showed no significant change in vaginal flora within 12 weeks of LNG-IUD placement [32]. Understanding long-term impacts of hormonal IUD and contraceptive implant use on vaginal flora is a key research gap.
The impact of monthly contraceptive vaginal rings, such as NuvaRing®, on vaginal microbiota is of particular interest given the interest in developing both vaginal microbicide and dual-use (microbicide and contraceptive) vaginal rings. Studies investigating monthly contraceptive vaginal rings on vaginal microbiota suggest an increase in lactobacilli colonization over time without a change in BV incidence [33, 34]. Longer-duration (90-day and 365-day) vaginal rings are also in development, and there is keen interest in understanding if long-term use of rings will have any significant impact on the vaginal microbiome, particularly since a study of vaginal rings done in non-human primates show that biofilms develop on rings during long-term use [35]. Huang, et al., showed that use of an ethinyl estradiol/Nestorone® contraceptive ring designed for use for 1 year had no significant effect on either Lactobacillus colonization or BV incidence over 1 year. There was a significant increase in low levels of anaerobic Gram-negative rods in participants using these contraceptive rings for 1 year; however, the authors concluded that was unlikely to be clinically significant [36].
Several recent meta-analyses that have addressed the influence of hormonal contraceptive use on BV have similarly noted protection from BV with use of hormonal contraceptives [37•, 38•]. In one meta-analysis, the three studies determined to have the highest quality demonstrated 10–20 % reduction in BV in combined oral contraceptive pill (COC) users and 18–30 % reduction in DMPA users compared to non-hormonal contraceptive users, and all of the studies included in this meta-analysis showed either statistically significant decrease or no difference in BV prevalence in hormonal contraceptive users when compared to non-hormonal contraceptive users [37•]. Similarly, a meta-analysis including 55 studies showed approximately 25 % reduction in both incident and prevalent BV in hormonal contraceptive users compared with non-users, irrespective of whether the method of hormonal contraception was progestin-only or combined estrogen-progestin [38•]. In summary, the available body of evidence strongly suggests that use of hormonal contraception either does not alter or decreases BV incidence. Furthermore, since having BV has been associated with increased risk of HIV acquisition, it is therefore unlikely that alterations in vaginal microbiota affected by hormonal contraceptive use are responsible for changes in susceptibility to STIs/HIV.
What Effects Do Contraceptive Hormones Have on the Vaginal Mucosal Immune Environment?
Sex steroid hormones influence immune function [39, 40], theoretically to facilitate reproduction, including fertilization and subsequent fetal development, without immune interference [41]. Hypotheses such as the “window of vulnerability” [42] propose that hormones suppress normally protective immune activities during mid-cycle through the luteal phase of menses to allow fertilization and to prevent immune destruction of a new blastocyst. Immune function is dynamic throughout the menstrual cycle, with changes demonstrated in toll-like receptors (TLRs), inflammatory transcription factors such as NF-κB, cytokines, and secretion of antimicrobial proteins [1, 42]. Immune changes that increase immune tolerance may also increase vulnerability to STIs. For HIV transmission in particular, this relationship is likely complex, since elements of the immune system may either protect or enhance susceptibility to infection, given that local immune cells are both immune sentinels and targets for HIV infection. Biologically plausible mechanisms that could explain reproductive hormone-dependent increase in HIV susceptibility include alterations in the integrity or structure of the vaginal epithelium, changes in innate immune components in cervicovaginal fluid, increased density of target cells, increased expression of viral co-receptors CCR5 and/or CXCR4 on immune target cells, increased activation of immune cells, changes in secreted cytokines and chemokines, and changes in a myriad of secreted proteins with innate anti-viral activity.
DMPA is often used in laboratory animal models to render animals more susceptible to human STI pathogens including Chlamydia trachomatis [43], herpes simplex virus (HSV) [44], and HIV [45]. Significant thinning of the vaginal epithelium was observed in several of these animal models following administration of DMPA [46], which led to hypotheses that structural changes effected by DMPA were responsible for altering HIV susceptibility. In distinct contrast to animal models, mucosal thinning has not been observed in multiple human studies of both short-term and long-term DMPA use [47–51]. While other alterations in the integrity of the vaginal mucosal barrier could theoretically increase risk, no differences in epithelial tight junctions and adherin proteins have been demonstrated following DMPA injection [47].
Properties or components of cervicovaginal fluid (CVF) may provide inherent protection from vaginal infection with HIV [52, 53]. Recent work examining CVF in pre- and post-menopausal women showed that both postmenopausal women and premenopausal women using DMPA had significantly less viscous CVF than normally cycling premenopausal women in either phase of menses and than women using either LNG-IUDs or COCs. Increased CVF viscosity appears to inhibit movement of HIV virions in vitro, which suggests that increased viscosity may confer some protection from infection [54, 55]. Women using DMPA also had decreased CVF glycosylation by GlcNAc and terminal galactose [53], carbohydrate structures that, as components of glycoproteins, have been linked to antibody-dependent cellular cytotoxicity [56]. In this study, no changes were observed in a variety of other carbohydrate binding proteins examined by high-throughput lectin microarrays. Observed changes in viscosity and glycosylation patterns in these studies were subtle and were confounded by the presence of BV [52, 57]. Innate antiviral activity of CVF against HIV, HSV-1 and HSV-2 was not affected by use of COCs, LNG-IUD, or DMPA, but was significantly lower in postmenopausal women [58]. A myriad of other CVF-associated molecules have been evaluated, particularly in women with BV compared with those with normal flora, in search of a biomarker that may help link the higher HIV/STI acquisition risk with BV [59, 60].
One of the largest efforts to evaluate the impact of contraceptive methods on cytokines and innate immune protective molecules in CVF was a nested case control study comparing vaginal biomarkers of 199 users of DMPA, COCs, or no hormonal contraceptives who acquired HIV during a longitudinal study to 633 controls who remained HIV negative [61•]. Higher RANTES levels and lower secretory leukocyte protease inhibitor (SLPI) levels in cervical secretions were associated with higher risk of HIV seroconversion. RANTES, an acronym for regulated on activation, normal T cell expressed and secreted, is a secreted peptide released by T cells that may competitively block the entry of HIV to a target cell by binding the HIV co-receptor and also attracts HIV target cells to mucosal surfaces [62], and SLPI is a protein with inherent anti-HIV activity that is associated with human mucosal surfaces [63]. RANTES has been significantly increased in DMPA users in several in vivo studies [61•, 64]. Conversely, RANTES mRNA was decreased in an in vitro model using medroxyprogesterone acetate (MPA) [65], highlighting that there can be inconsistencies between in vitro and in vivo models. Other studies evaluating vaginal cytokine and secreted antimicrobial peptides, including SLPI, in DMPA users compared to non-DMPA users have shown mixed results, likely due to both the non-specific nature of these markers of inflammation and the comparison group encompassing ‘non-DMPA users’ who may have been using a variety of other progestins for contraception [64, 66, 67].
In vitro and in vivo studies addressing hormone-dependent changes in number and co-receptor expression of target cells as well as secreted antimicrobial molecules published over the past 2 years are summarized in Table 1. Notably, studies are variable in contraceptive methods evaluated, outcome measures, and study methodology. The most relevant study methodologies are likely those that evaluate cells that are freshly harvested from the female genital tract with minimal manipulation that may itself impact the viability and/or surface protein receptor expression patterns. Due to the specific concern for DMPA use and increased susceptibility to HIV, DMPA has been the most commonly studied contraceptive to date. Some studies have examined other hormonal contraceptives, including NET-EN, COCs, and LNG-IUD. One recent study showed an increased density of target cells and HIV co-receptors 12 weeks after DMPA injection [47], while another found decreased density of T cells but stable proportion of T cells and dendritic cells that are CCR5+ after 12 months of DMPA use [29], suggesting that the short-term effects may differ from long-term effects. Both LNG and copper IUDs were associated with decreased CCR5 co-receptor expression in endocervical CD4+ T cells and endometrial CD8+ T cells, indicating that hormonally independent mechanisms may also have impacts on upper genital tract immune cells in the case of IUDs [69].
Contraceptive hormones may alter the function of circulating systemic immune cells. Studies tend to show an association between hormonal contraceptives and decreased secretion of pro-inflammatory cytokines, including the antiviral IFN-α, TNF-α, and IFN-γ, from peripheral blood mononuclear cells (PBMCs) [66, 68, 72]. PBMCs incubated in vitro with MPA but not estradiol (E2) or progesterone (P4) showed increased CXCR4 and CCR5 co-receptor expression [72]. The relevance of data on circulating immune cells to the immune activity of the genital tract is unclear but certainly important for control of and susceptibility to systemic infection.
Do the Various Contraceptive Progestins Affect the Immune System and Vaginal Flora Differently?
Fortunately, a great diversity of contraceptive hormones and delivery methods exist, providing women a variety of methods from which to choose. Although much is known about the impact of these contraceptives on fertility, little is known about other non-contraceptive impacts, including impacts on genital tract immune cells and function and on vaginal microbiota. Most published studies that have compared the effects of contraceptives on the genital tract have compared various contraceptive methods that differ significantly with respect to both delivery method and hormone content (e.g., contraceptive injections containing medroxyprogesterone vs. implant containing etonogestrel or levonorgestrel), making interpretation challenging. There are currently no published studies comparing the vaginal microbiological effects of progestins contained in different oral contraceptives, so it is unknown if different formulations of COCs cause differential effects. Further, there are no published studies that examine vaginal microbiota differences when the same hormone is delivered through different delivery systems (e.g., levonorgestrel contained in oral contraceptives vs. subcutaneous implants vs. IUDs).
MPA may behave quite differently from the other contraceptive progestins with respect to non-contraceptive effects, and this may be due to the binding properties of MPA to the immunosuppressive glucocorticoid receptor [65, 73]. In cervical cell lines, IL-6, IL-8, and the anti-HIV RANTES molecule are constitutively expressed, and this expression is suppressed by MPA binding to the glucocorticoid receptor, unlike NET or endogenous progesterone (P4), neither of which suppress RANTES. This suppression is likely mediated by recruitment of the glucocorticoid receptor to the IL-6 and IL-8 gene promoter region, similar to the effect of dexamethasone [65]. Additionally, MPA but not NET or P4 enhance peripheral CD4+ T cell apoptosis, likely also via the glucocorticoid receptor [73]. In summary, in vitro and in vivo studies have demonstrated some differences in effect of MPA compared to other contraceptive hormones and have proposed a mechanism for this difference; however, very little is known about non-contraceptive effects of other specific exogenous hormones on the lower genital tract. Future research is needed that specifically categorizes evaluated contraceptives by progestin type and by local concentration of progestins to evaluate alterations in immune cells, cell surface receptors, and secreted molecules.
Conclusions
There is clear evidence that use of hormonal contraceptives, primarily COCs and DMPA, confers a protective effect against abnormal vaginal flora. Future research is needed to evaluate potential changes to the vaginal flora with use of contraceptive implants and LNG-IUDs, and with long-term use of all contraceptive methods. While studies focusing on the effects of contraceptive use on the vaginal innate and adaptive immune environment continue to yield more information, consistent conclusions cannot yet be made because of lack of contraceptive progestin specificity along with widely varying experimental models, contraceptive delivery methods, and methodologic approaches. No conclusive evidence, either behavioral or biological, yet explains the observed approximately 1.5- to twofold increased risk of HIV acquisition seen in women using DMPA in large observational studies. A large randomized controlled trial (ECHO) in which women will be randomized to use DMPA, LNG contraceptive implants, or copper IUDs for contraception has recently opened to enrollment with the goal of understanding if there is real increased risk of HIV acquisition with DMPA use [74]. If the evidence continues to indicate increased risk of HIV/STI acquisition in for women using DMPA, further research will need to be directed toward elucidating the mechanism (s) for increasing risk, evaluating the comparable safety of alternative highly effective long-acting contraceptive methods, and increasing the acceptability of these alternative options in regions where use is low. Further, efforts should continue toward developing dual-use technologies where HIV and pregnancy prevention can be combined. While addressing family planning needs and enabling protection from sexually transmitted infections are paramount for all women’s health, these needs are particularly urgent in areas with high HIV prevalence, many of which also have high rates of unintended pregnancy and, therefore, a great need for safe contraception.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015;15(4):217–30. doi:10.1038/nri3819.
Murphy K, Irvin SC, Herold BC. Research gaps in defining the biological link between HIV risk and hormonal contraception. Am J Reprod Immunol. 2014;72(2):228–35. doi:10.1111/aji.12209.
Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38(1):13–22.
Sonnex C. Influence of ovarian hormones on urogenital infection. Sex Transm Infect. 1998;74(1):11–9.
Martin Jr HL, Nyange PM, Richardson BA, Lavreys L, Mandaliya K, Jackson DJ, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178(4):1053–9.
Baeten JM, Nyange PM, Richardson BA, Lavreys L, Chohan B, Martin Jr HL, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185(2):380–5. doi:10.1067/mob.2001.115862.
Polis CB, Phillips SJ, Curtis KM, Westreich DJ, Steyn PS, Raymond E, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90(4):360–90. doi:10.1016/j.contraception.2014.07.009.
Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women’s risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015;15(2):181–9. doi:10.1016/S1473-3099(14)71052-7.
Noguchi LM, Richardson BA, Baeten JM, Hillier SL, Balkus JE, Chirenje ZM, et al. Risk of HIV-1 acquisition among women who use different types of injectable progestin contraception in South Africa: a prospective cohort study. Lancet HIV. 2015;2(7):e279–87. doi:10.1016/S2352-3018(15)00058-2. Demonstrated lower risk of HIV-1 acquisition in women using NET-EN than DMPA.
Morrison CS, Chen PL, Kwok C, Richardson BA, Chipato T, Mugerwa R, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24(11):1778–81. doi:10.1097/QAD.0b013e32833a2537.
Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):305–10. doi:10.1097/COH.0b013e32833a8844.
Witkin SS. The vaginal microbiome, vaginal anti-microbial defence mechanisms and the clinical challenge of reducing infection-related preterm birth. BJOG : Int J Obstet Gynaecol. 2015;122(2):213–8. doi:10.1111/1471-0528.13115.
Sirota I, Zarek SM, Segars JH. Potential influence of the microbiome on infertility and assisted reproductive technology. Semin Reprod Med. 2014;32(1):35–42. doi:10.1055/s-0033-1361821.
van de Wijgert JH, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One. 2014;9(8), e105998. doi:10.1371/journal.pone.0105998.
Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011;121(12):4610–7. doi:10.1172/JCI57172.
Cone RA. Vaginal microbiota and sexually transmitted infections that may influence transmission of cell-associated HIV. J Infect Dis. 2014;210 Suppl 3:S616–21. doi:10.1093/infdis/jiu459.
Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res : J Lab Clin Med. 2012;160(4):267–82. doi:10.1016/j.trsl.2012.02.008.
Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16(9):1809–13.
Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun. 1999;67(10):5170–5.
Mirmonsef P, Modur S, Burgad D, Gilbert D, Golub ET, French AL, et al. Exploratory comparison of vaginal glycogen and Lactobacillus levels in premenopausal and postmenopausal women. Menopause. 2015;22(7):702–9. doi:10.1097/GME.0000000000000397.
Jespers V, Menten J, Smet H, Poradosu S, Abdellati S, Verhelst R, et al. Quantification of bacterial species of the vaginal microbiome in different groups of women, using nucleic acid amplification tests. BMC Microbiol. 2012;12:83. doi:10.1186/1471-2180-12-83.
Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4), e10197. doi:10.1371/journal.pone.0010197.
Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis. 2014;210(11):1723–33. doi:10.1093/infdis/jiu330.
Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777–86. doi:10.1093/cid/cis1030.
Calzolari E, Masciangelo R, Milite V, Verteramo R. Bacterial vaginosis and contraceptive methods. Int J Gynecol Obstet. 2000;70(3):341–6. doi:10.1016/s0020-7292(00)00217-4.
Rifkin SB, Smith MR, Brotman RM, Gindi RM, Erbelding EJ. Hormonal contraception and risk of bacterial vaginosis diagnosis in an observational study of women attending STD clinics in Baltimore. MD Contracept. 2009;80(1):63–7. doi:10.1016/j.contraception.2009.01.008.
Holzman C, Leventhal JM, Qiu H, Jones NM, Wang J, Grp BVS. Factors linked to bacterial vaginosis in nonpregnant women. Am J Public Health. 2001;91(10):1664–70. doi:10.2105/ajph.91.10.1664.
Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex Transm Dis. 2007;34(12):954–9. doi:10.1097/OLQ.0b013e31811ed0e4.
Mitchell CM, McLemore L, Westerberg K, Astronomo R, Smythe K, Gardella C, et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis. 2014;210(4):651–5. doi:10.1093/infdis/jiu176.
Shoubnikova M, Hellberg D, Nilsson S, Mardh PA. Contraceptive use in women with bacterial vaginosis. Contraception. 1997;55(6):355–8. doi:10.1016/s0010-7824(97)00044-9.
Madden T, Grentzer JM, Secura GM, Allsworth JE, Peipert JF. Risk of bacterial vaginosis in users of the intrauterine device: a longitudinal study. Sex Transm Dis. 2012;39(3):217–22. doi:10.1097/OLQ.0b013e31823e68fe.
Jacobson JC, Turok DK, Dermish AI, Nygaard IE, Settles ML. Vaginal microbiome changes with levonorgestrel intrauterine system placement. Contraception. 2014;90(2):130–5. doi:10.1016/j.contraception.2014.04.006.
Veres S, Miller L, Burington B. A comparison between the vaginal ring and oral contraceptives. Obstet Gynecol. 2004;104(3):555–63. doi:10.1097/01.AOG.0000136082.59644.13.
De Seta F, Restaino S, De Santo D, Stabile G, Banco R, Busetti M, et al. Effects of hormonal contraception on vaginal flora. Contraception. 2012;86(5):526–9. doi:10.1016/j.contraception.2012.02.012.
Gunawardana M, Moss JA, Smith TJ, Kennedy S, Kopin E, Nguyen C, et al. Microbial biofilms on the surface of intravaginal rings worn in non-human primates. J Med Microbiol. 2011;60(Pt 6):828–37. doi:10.1099/jmm.0.028225-0.
Huang Y, Merkatz RB, Hillier SL, Roberts K, Blithe DL, Sitruk-Ware R, et al. Effects of a one year reusable contraceptive vaginal ring on vaginal microflora and the risk of vaginal infection: an open-label prospective evaluation. PLoS One. 2015;10(8), e0134460. doi:10.1371/journal.pone.0134460.
van de Wijgert JH, Verwijs MC, Turner AN, Morrison CS. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS. 2013;27(13):2141–53. doi:10.1097/QAD.0b013e32836290b6. This meta-analysis of 36 studies estimated a 10–20% reduction in BV in combined oral contraceptive uses and 18–30% reduction in BV in DMPA users.
Vodstrcil LA, Hocking JS, Law M, Walker S, Tabrizi SN, Fairley CK, et al. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PLoS One. 2013;8(9):e73055. doi:10.1371/journal.pone.0073055. This meta-analysis of 55 studies estimated a 25% reduction in incident and prevalent bacterial vaginosis in hormonal contraceptive users compared to non-users.
Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunol Res. 2005;31(2):91–106. doi:10.1385/IR:31:2:091.
Tan IJ, Peeva E, Zandman-Goddard G. Hormonal modulation of the immune system—a spotlight on the role of progestogens. Autoimmun Rev. 2015;14(6):536–42. doi:10.1016/j.autrev.2015.02.004.
Guleria I, Sayegh MH. Maternal acceptance of the fetus: true human tolerance. J Immunol. 2007;178(6):3345–51.
Rodriguez-Garcia M, Patel MV, Wira CR. Innate and adaptive anti-HIV immune responses in the female reproductive tract. J Reprod Immunol. 2013;97(1):74–84. doi:10.1016/j.jri.2012.10.010.
Tuffrey M, Taylorrobinson D. Progesterone as a key factor in the development of a mouse model for genital-tract infection with chlamydia-trachomatis. Fems Microbiol Lett. 1981;12(2):111–5.
Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes-simplex virus type-2. Lab Invest. 1994;70(3):369–80.
Abel K, Rourke T, Lu D, Bost K, McChesney MB, Miller CJ. Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of depo-provera before intravaginal challenge with simian immunodeficiency virus mac239. J Infect Dis. 2004;190(9):1697–705. doi:10.1086/424600.
Hild-Petito S, Veazey RS, Larner JM, Reel JR, Blye RP. Effects of two progestin-only contraceptives, Depo-Provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. Aids Res Hum Retrovir. 1998;14:S125–30.
Chandra N, Thurman AR, Anderson S, Cunningham TD, Yousefieh N, Mauck C, et al. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. Aids Res Hum Retrovir. 2013;29(3):592–601. doi:10.1089/aid.2012.0271.
Bahamondes L, Trevisan M, Andrade L, Marchi NM, Castro S, Diaz J, et al. The effect upon the human vaginal histology of the long-term use of the injectable contraceptive Depo-Provera. Contraception. 2000;62(1):23–7.
Bahamondes MV, Castro S, Marchi NM, Marcovici M, Andrade LA, Fernandes A, et al. Human vaginal histology in long-term users of the injectable contraceptive depot-medroxyprogesterone acetate. Contraception. 2014;90(2):117–22. doi:10.1016/j.contraception.2014.01.024.
Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60(1):15–24.
Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96(3):431–9.
Chappell CA, Rohan LC, Moncla BJ, Wang L, Meyn LA, Bunge K, et al. The effects of reproductive hormones on the physical properties of cervicovaginal fluid. Am J Obstet Gynecol. 2014;211(3):226 e1–7. doi:10.1016/j.ajog.2014.03.041.
Wang L, Koppolu S, Chappell C, Moncla BJ, Hillier SL, Mahal LK. Studying the effects of reproductive hormones and bacterial vaginosis on the glycome of lavage samples from the cervicovaginal cavity. PLoS One. 2015;10(5), e0127021. doi:10.1371/journal.pone.0127021.
Shukair SA, Allen SA, Cianci GC, Stieh DJ, Anderson MR, Baig SM, et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013;6(2):427–34. doi:10.1038/mi.2012.87.
Boukari H, Brichacek B, Stratton P, Mahoney SF, Lifson JD, Margolis L, et al. Movements of HIV-virions in human cervical mucus. Biomacromolecules. 2009;10(9):2482–8. doi:10.1021/bm900344q.
Zou G, Ochiai H, Huang W, Yang Q, Li C, Wang LX. Chemoenzymatic synthesis and Fcgamma receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcgammaIIIa receptor. J Am Chem Soc. 2011;133(46):18975–91. doi:10.1021/ja208390n.
Moncla BJ, Chappell CA, Mahal LK, Debo BM, Meyn LA, Hillier SL. Impact of bacterial vaginosis, as assessed by nugent criteria and hormonal status on glycosidases and lectin binding in cervicovaginal lavage samples. PLoS One. 2015;10(5), e0127091. doi:10.1371/journal.pone.0127091.
Chappell CA, Isaacs CE, Xu W, Meyn LA, Uranker K, Dezzutti CS, et al. The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol. 2015;213(2):204 e1–6. doi:10.1016/j.ajog.2015.03.045.
Masson L, Mlisana K, Little F, Werner L, Mkhize NN, Ronacher K, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect. 2014;90(8):580–7. doi:10.1136/sextrans-2014-051601.
Thurman AR, Kimble T, Herold B, Mesquita PM, Fichorova RN, Dawood HY, et al. Bacterial vaginosis and subclinical markers of genital tract inflammation and mucosal immunity. Aids Res Hum Retrovir. 2015. doi:10.1089/aid.2015.0006.
Morrison C, Fichorova RN, Mauck C, Chen PL, Kwok C, Chipato T, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr. 2014;66(2):109–17. doi:10.1097/QAI.0000000000000103. A nested case control study of hormonal contraceptive users showed that higher cervicovaginal fluid RANTES levels and lower SLPI levels were associated with HIV seroconversion, and that higher cervicovaginal RANTES levels were seen in DMPA users.
Caux C, Ait-Yahia S, Chemin K, de Bouteiller O, Dieu-Nosjean MC, Homey B, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22(4):345–69.
Wahl SM, McNeely TB, Janoff EN, Shugars D, Worley P, Tucker C, et al. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis. 1997;3 Suppl 1:S64–9.
Deese J, Masson L, Miller W, Cohen M, Morrison C, Wang M, et al. Injectable progestin-only contraception is associated with increased levels of pro-inflammatory cytokines in the female genital tract. Am J Reprod Immunol. 2015;74(4):357–67. doi:10.1111/aji.12415.
Govender Y, Avenant C, Verhoog NJ, Ray RM, Grantham NJ, Africander D, et al. The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PLoS One. 2014;9(5), e96497. doi:10.1371/journal.pone.0096497.
Michel KG, Huijbregts RP, Gleason JL, Richter HE, Hel Z. Effect of hormonal contraception on the function of plasmacytoid dendritic cells and distribution of immune cell populations in the female reproductive tract. J Acquir Immune Defic Syndr. 2015;68(5):511–8. doi:10.1097/QAI.0000000000000531.
Guthrie BL, Introini A, Roxby AC, Choi RY, Bosire R, Lohman-Payne B, et al. Depot medroxyprogesterone acetate use is associated with elevated innate immune effector molecules in cervicovaginal secretions of HIV-1-uninfected women. J Acquir Immune Defic Syndr. 2015;69(1):1–10. doi:10.1097/QAI.0000000000000533.
Huijbregts RPH, Helton ES, Michel KG, Sabbaj S, Richter HE, Goepfert PA, et al. Hormonal contraception and HIV-1 Infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154(3):1282–95. doi:10.1210/en.2012-1850.
Achilles SL, Creinin MD, Stoner KA, Chen BA, Meyn L, Hillier SL. Changes in genital tract immune cell populations after initiation of intrauterine contraception. Am J Obstet Gynecol. 2014;211(5):489 e1–9. doi:10.1016/j.ajog.2014.05.016.
Coleman JS, Mwachari C, Balkus J, Sanguli L, Muliro A, Agnew K, et al. Effect of the levonorgestrel intrauterine device on genital HIV-1 RNA shedding among HIV-1-infected women not taking antiretroviral therapy in Nairobi. Kenya J Acquir Immune Defic Syndr. 2013;63(2):245–8. doi:10.1097/QAI.0b013e31828decf8.
Ngcapu S, Masson L, Sibeko S, Werner L, McKinnon LR, Mlisana K, et al. Lower concentrations of chemotactic cytokines and soluble innate factors in the lower female genital tract associated with the use of injectable hormonal contraceptive. J Reprod Immunol. 2015;110:14–21. doi:10.1016/j.jri.2015.03.007.
Huijbregts RP, Michel KG, Hel Z. Effect of progestins on immunity: medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception. 2014;90(2):123–9. doi:10.1016/j.contraception.2014.02.006.
Tomasicchio M, Avenant C, Du Toit A, Ray RM, Hapgood JP. The progestin-only contraceptive medroxyprogesterone acetate, but not norethisterone acetate, enhances HIV-1 Vpr-mediated apoptosis in human CD4+ T cells through the glucocorticoid receptor. PLoS One. 2013;8(5), e62895. doi:10.1371/journal.pone.0062895.
Cates W. The Evidence for Contraceptive Options and HIV Outcomes Trial (ECHO). Clinicaltrials.gov. 2015. https://clinicaltrials.gov/ct2/show/NCT02550067. Accessed 26 Oct 2015.
Acknowledgments
This study was supported by the US National Institutes of Health/National Institute of Allergy and Infectious Diseases grant support (NIH R01-AI102835)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jessica Tarleton declares that she has no competing interests.
Lisa Haddad declares personal fees for working as a consultant on a medical advisory panel for Pfizer Pharmaceutical.
Sharon L. Achilles declares personal fees for consultant work for Merck Sharp & Dohme Corp.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Family Planning
Rights and permissions
About this article
Cite this article
Tarleton, J., Haddad, L. & Achilles, S.L. Hormonal Contraceptive Effects on the Vaginal Milieu: Microbiota and Immunity. Curr Obstet Gynecol Rep 5, 20–29 (2016). https://doi.org/10.1007/s13669-016-0142-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-016-0142-6