Abstract
Purpose of Review
Lung cancer is the leading cause of cancer deaths in the USA. Computed tomography (CT) offers the potential for early detection by screening asymptomatic high-risk patients. We aimed to review the benefits and potential harms of lung cancer screening, discuss the logistics of a screening program, and provide insight from our own experience.
Recent Findings
The National Lung Screening Trial (NLST), a large population-based study, has demonstrated mortality benefit from screening, but relatively few eligible patients currently participate. An effective screening program requires input and cooperation from multiple stakeholders. Effort should be made to actively engage patients in the process including a thorough discussion of benefits and possible harms. At our institution, this approach has resulted in a rapidly growing and sustainable program.
Summary
Lung cancer screening has proven mortality benefit in high-risk patients but is underutilized. Developing and growing a screening program is a complex process requiring coordination among multiple specialties with a focus on patient autonomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Screening high-risk patients for lung cancer with low-dose chest computed tomography (LDCT) has demonstrated a significant mortality benefit in a large randomized clinical trial, the National Lung Screening Trial (NLST), published in 2011. Subsequently, in 2013, the US Preventative Services Task Force (USPSTF) issued a grade B recommendation for annual lung cancer screening (LCS) with LDCT, and provisions for reimbursement by the Centers for Medicaid and Medicare Services (CMS) followed shortly thereafter. Despite these developments, screening is considerably underutilized in the USA with less than 4% of eligible patients participating as of 2016 [1, 2]. Broader implementation of LCS presents a major public health opportunity with the potential to prevent thousands of lung cancer deaths each year.
An effective LCS program should strive to provide care comparable to centers that participated in the NLST. While imaging plays a central role in screening, implementation requires a multidisciplinary effort that begins and ends outside the radiology department. Input from multiple stakeholders is essential including pulmonology, thoracic surgery, and primary care.
At the University of Cincinnati, our multidisciplinary approach to LCS has given rise to a thriving clinical practice that currently screens about 120 patients per month and continues to grow rapidly. Since inception in 2012, our program has detected over 50 confirmed lung cancers, and the majority of these patients have successfully undergone surgical resection.
In this article, we discuss current evidence in support of LCS, critical elements required to implement an effective LCS program, and insights gleaned from our own institutional experience. Potential harms including radiation exposure and over-diagnosis are also reviewed.
Why Screen for Lung Cancer?
Despite falling rates of smoking in the USA, lung cancer remains the leading cancer killer among both men and women [3]. In 2018 alone, lung cancer accounted for an estimated 150,000 deaths nationwide representing 25% of all cancer-related mortality.
Prognosis and survival rates for non-small cell lung cancer (NSCLC) are generally poor but depend significantly on initial staging. For stage IA disease, surgical excision is often curative, and 5-year survival exceeds 80%. Conversely, those who present with stage IVB disease are not surgical candidates and have a 2-year survival of only 10%. Since most lung cancers are clinically silent until advanced stages, early detection among asymptomatic individuals presents the possibility of drastically decreasing mortality.
Lung cancer screening was initially trialed in the 1970s with chest x-ray (CXR) and sputum cytology surveillance, but no mortality benefit was demonstrated [4]. Advances in multi-detector computed tomography (CT), however, have now made rapid high-resolution imaging of the chest feasible, providing a more sensitive screening modality.
The National Lung Screening Trial (NLST), funded by the National Cancer Institute, set out to determine if screening with LDCT could reduce lung cancer mortality [5••]. More than 53,000 participants were enrolled. Eligibility criteria included an age between 55 and 74, at least a 30-pack-year smoking history, and either currently smoking or quit within the past 15 years. Subjects were randomly assigned to annual screening with either LDCT or CXR over a 2-year period for a total of three exams and followed for an average of 6.5 years. The radiologist’s interpretation and recommendations were reported to the participant and his/her healthcare provider, but no standardized algorithm for the management of positive screening exams was mandated.
Results from the NLST were reported in 2011, demonstrating a 20% relative reduction in lung cancer mortality in the CT group versus the CXR group. More recently, similar results were demonstrated in Europe through the NELSON trial where over 15,000 patients from Belgium and the Netherlands were randomized to low-dose screening CT or usual care [6].
Since over 8 million Americans meet the NLST eligibility criteria [7], the implications for public health are significant: CT screening has the potential to prevent an estimated 12,000 deaths per year, and US public health agencies have taken necessary steps to make screening accessible. The USPSTF issued a grade B recommendation in 2013, mandating coverage of LCS for those with private insurance. CMS followed suit in 2015 with reimbursement provisions. All insured Americans who meet eligibility criteria can now be screened without incurring out-of-pocket costs for an annual CT (although additional costs may be incurred for subsequent imaging and other tests if the screening exam is positive).
Implementation: A Multidisciplinary Approach
The American College of Radiology (ACR) in collaboration with Society of Thoracic Radiology has provided practice parameters [8] while the American College of Chest Physicians and American Thoracic Society have issued policy statements [9] which provide a framework for lung cancer screening programs.
Establishing a successful LCS program requires a step-wise approach with input and communication among multiple specialties. While imaging plays a central role, the screening process begins and ends outside the radiology department. Critical elements of an effective program include a Program Coordinator (PC), usually a registered nurse, who is involved in nearly every step of the process and facilitates timely communication and cooperation between the specialists, referring physician, and patient. A multidisciplinary tumor board is also essential, providing a venue for specialists to discuss complex cases and arrive at a consensus for management.
At the University of Cincinnati, our LCS program was founded in 2012 by a small group of physicians representing thoracic surgery, pulmonology, radiology, and oncology. One of the greatest initial hurdles was to justify the hiring of a full-time employee (the PC) to hospital administrators, since direct revenue from the LCS exams was not expected to cover this cost. Ultimately, the expense was supported on the basis of the secondary income that would inevitably follow, as every new diagnosis of lung cancer has the potential to generate substantial revenue for multiple specialties within the hospital system. This anticipated benefit proved correct since more than 90% of screening patients with confirmed lung cancers have chosen to remain within the UC Health network for treatment. As a result, the program achieved financial sustainability within the first year.
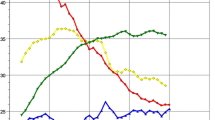
Patient education and recruitment are generally the greatest challenges for any LCS program, and our efforts in this area have led to robust and sustained growth (Fig. 1). In our experience, outreach to primary care providers is the single-most effective method of increasing patient volume. An estimated 42% of primary care physicians are not aware of the USPSTF guidelines for lung cancer screening [10], and unlike other preventative services such as mammography or hemoglobin A1c screenings, assessment for LCS eligibility is not currently considered a quality measure. Other patient recruitment initiatives at our institution include radio advertising and participation in community health events such as employer health fairs. One of our team members has also highlighted the benefit of LCS on local TV networks.
Once a potential screening patient has been identified, the next step in our program is for the patient or primary care physician to contact the screening Program Coordinator by telephone. Eligibility must first be verified. For private insurance, asymptomatic patients with at least a 30-pack-year smoking history between the ages of 55 and 80 are eligible, provided they are a current smoker or have quit in the past 15 years. For Medicare, criteria are similar but the age range is 55 to 77. We allow patients who exceed the respective age limits or those who have quit smoking more than 15 years ago to participate in screening, but payment under those circumstances is out-of-pocket. Medical comorbidities that might preclude therapy are discussed with the patient, but we do not exclude patients from screening on the basis of existing medical conditions.
Medicare mandates a “counseling and shared decision making visit” prior to screening. To fulfill this requirement, a visit with a physician or qualified non-physician practitioner must cover multiple elements of LCS including eligibility criteria, the benefits and harms of screening, impact of comorbidities on the ability to undergo further diagnosis and treatment, and counseling on the importance of smoking cessation. While this visit is ordinarily performed by a primary care provider, in our experience, the Program Coordinator is in many cases better equipped to answer specific patient questions or address concerns, so our Program Coordinator performs a separate shared decision-making discussion via telephone with all patients (Medicare, private insurance, and self-pay) prior to screening. To further engage the patient in the process, we also ask that he or she sign a written consent form that reviews the benefits and potential harms. Once these steps are complete, the patient is scheduled for a screening exam.
The LCS exam is similar to a standard non-contrast CT chest with a few important differences. First, the CT order is placed by one of our thoracic surgeons or pulmonologists participating in the LCS program (rather than the referring provider) to ensure that positive exams are systematically flagged for follow-up. To assist the radiologist and CT technologist with proper protocolling, we have created a specific order for lung cancer screening in our electronic medical record system (EMR) which is separate from that of a non-contrast CT chest. Second, a low-dose CT technique is utilized in which the x-ray tube current (mA) is fixed at a relatively low level, and the pitch (table distance traveled per gantry rotation) is increased, resulting in a significant reduction in radiation dose (Table 1). The major tradeoff of the low-dose technique is reduced signal yielding noisier images, particularly in the mediastinum and upper abdomen; however, since the lung parenchyma is an inherently high-contrast tissue, this reduction in image quality has minimal impact on the detection of pulmonary nodules. Third, the CT technologist instructs the patient to cough vigorously immediately before scanning, helping to clear mucus secretions in the airway which can mimic endobronchial lesions.
Medicare requires that LCS exams be interpreted by board eligible/certified radiologists who have read at least 300 chest CTs over the past 3 years. At our institution, these studies are read exclusively by dedicated thoracic subspecialists. Other requirements include submission of data for each exam (patient identifier, smoking history, radiation dose, etc.) to the American College of Radiology (ACR) registry to generate data for benchmarking.
Findings should be reported with a standardized lung nodule identification, classification, and reporting system. Structured reporting and the Lung-RADS system developed by the American College of Radiology (ACR) provide for consistent, clear, and concise communication of results. Lung-RADS is modeled after the popular BI-RADS system for mammography, providing a standardized classification for lung nodules based on their size, stability, and other imaging characteristics (Table 2). Normal exams, those with clearly benign nodules, and small nodules that have a high probability (> 99%) of being benign are classified as Lung-RADS 1 and 2 respectively, and continued annual screening is recommended. Nodules which are likely benign (< 5% probability of being malignant) are assigned Lung-RADS 3 and are usually amenable to 6-month follow-up. Nodules with solid components greater than 8 mm which are new, growing, or present on a baseline exam are classified as Lung-RADS category 4A, 4B, and 4X depending on the presence of suspicious features. Exams that require outside imaging for comparison or are incomplete for other reasons should be classified as Lung-RADS 0.
A subtle but important feature of the Lung-RADS algorithm is that nodule suspicion is based on the probability of clinically active cancer. The potential for over-diagnosis of indolent neoplasms is addressed by classifying sub-solid nodules (which are unlikely to be clinically active) into Lung-RADS categories 2 and 3 [11•].
The radiologist should also report any incidental findings and appropriate recommendations for clinical follow-up. The Lung-RADS system provides an “S” modifier for findings other than lung cancer that the radiologist considers clinically significant. For example, an exam that is negative for lung cancer with an incidental finding of an esophageal mass would be categorized as 1S.
The “C” modifier is used if the patient has a personal history of lung cancer, while an “X” modifier is used for imaging findings that are highly suspicious for lung cancer.
In the case of a negative screening exam (categories 1 or 2), the Program Coordinator relays these results and any incidental findings to the primary care physician. The patient also receives a mailed letter explaining the result in layman’s terms.
For all exams where the radiologist believes further discussion would be beneficial including all Lung-RADS 4 exams, the radiologist alerts the Program Coordinator who then presents the case at our weekly thoracic tumor board. This multidisciplinary team predated our LCS program and was originally established as a venue to discuss complex thoracic oncology cases. Once the LCS program was created, review of positive screening exams became a natural extension of the board’s responsibilities. Tumor board members include the LCS Program Coordinator and physicians representing radiology, nuclear medicine, pulmonology, thoracic surgery, pathology, medical oncology, and radiation oncology.
Management of Lung-RADS 4 exams can be complex and often requires input from multiple specialties to plan the appropriate next step. The degree of suspicion for lung cancer, amenability of the nodule to biopsy, and other associated findings such as hilar lymphadenopathy are considered. In the majority of these cases, suspicion is relatively low (Lung-RADS 4A), and a 3-month follow-up CT is performed. Solid nodules that demonstrate greater than a 3-month stability have a very low probability of malignancy and are reclassified as Lung-RADS 2. When the degree of suspicion is higher (4B and 4X), the tumor board may recommend consultation with a thoracic surgeon and/or CT/PET to assess the metabolic activity of the nodule and assess for other hypermetabolic lesions such as lymph nodes that may be targets for biopsy. (Lung-RADS also lists CT chest with and without contrast as an option to further evaluate 4B and 4X lesions; however, in our experience, these exams are unlikely to change management.)
We rarely proceed to invasive procedures for tissue sampling without first obtaining a short-interval follow-up or CT/PET. This relatively conservative approach has resulted in fewer than 10 invasive procedures performed on patients in our screening population in which lung cancer was not confirmed, a rate much less than that reported in the NLST. The majority of these procedures were bronchoscopy performed for a suspected endobronchial lesion that turned out to be focal mucus secretions. As a result, we now almost always recommend a short-interval follow-up CT for possible endobronchial nodules prior to any intervention.
Tumor board consensus is communicated by the Program Coordinator to the referring physician and the patient. The radiologist dictates an addendum to the LCS report to document multidisciplinary recommendations. If the ensuing workup does confirm lung cancer, the case may be referred back to tumor board to discuss therapeutic options.
Additional Benefits of Screening
An interesting secondary finding in the NLST was a statistically significant relative reduction in all-cause mortality of 7% among the CT group versus radiography, an effect which cannot be attributed to lung cancer-specific mortality alone. While the underlying reasons for this reduction are not entirely clear, it is possible that detection of pathology other than lung cancer was a contributing factor. A typical CT of the chest images a substantial amount of extrapulmonary anatomy including the lower neck, mediastinum, breasts, upper abdomen, and musculoskeletal structures. The lung cancer screening CT is an opportunity to identify a myriad of possible incidental findings which may be clinically significant. (While often a benefit, this can also be considered a risk of screening given that some of these findings may not be clinically relevant but lead to additional testing and procedures.)
One example of a potential ancillary benefit is an evaluation for coronary artery disease. Calcium within the coronary arteries is easily visualized on non-contrast CT and serves as a reliable biomarker for the presence of atherosclerosis. Smokers who meet LCS criteria have a relative risk of death from coronary artery disease that is greater than 3 times that of the general population, and this risk can be partially mitigated by smoking cessation. Thus, recognition of coronary artery disease in these patients serves two purposes for the referring provider: not only can it direct improved medical management but it may also lead to a more meaningful conversation regarding the benefits of smoking cessation and other lifestyle modifications [12•].
Dedicated CT imaging for coronary artery calcium scoring has been utilized in clinical practice for over a decade. These exams typically rely on the Agatston method to calculate an objective score ranging from 0 to > 400. This score provides prognostic information for coronary artery events beyond that determined by the Framingham risk score [13]. Moreover, rapid progression of the calcium score is an independent risk factor for all-cause mortality [14].
The Agatston method is not currently feasible for LCS exams, as use of the required software is not reimbursed, and the method has not been specifically validated for CTs performed without EKG gating; however, a non-quantitative gestalt assessment of coronary artery calcification by the radiologist is likely a non-inferior technique [15]. For every LCS CT performed at our institution, coronary artery calcium is subjectively assessed by the radiologist on a four-level scale (none, mild, moderate, and severe). As referring physicians often read only the impression section of a radiology report [16], we reiterate the coronary calcium score in the impression for all LCS examinations.
Low-dose CT also allows for detection of asymptomatic thoracic aortic disease including aneurysm formation or aortic valve calcification. Almost 4% of cardiovascular deaths in the NLST were related to aortic aneurysm or dissection. While aortic caliber is influenced by multiple factors including gender and body surface area [17], an ascending aorta diameter greater than or equal to 4 cm is considered dilated and should be reported [18]. Saccular aneurysms are less common but considered higher risk and should be reported regardless of size.
Other possible benefits include the detection of extrapulmonary neoplasms. In the NLST, 0.39% of participants in the CT group had extrathoracic cancers that were diagnosed during screening with kidney, thyroid, and liver cancers being the most common [19].
Potential Harms
While the benefits of LCS are well-established, there are several potential harms that may arise from screening. These should be disclosed to the patient during the shared decision-making discussion, and such risks should be minimized to the extent possible.
All CT scans involve exposure to ionizing radiation, a known risk factor for multiple types of cancer. For screening CTs performed with low-dose technique, effective dose is typically in the range of 2–3 mSv, slightly less than the average annual radiation dose from natural sources. For perspective, a single dose of 1000 mSv (1Sv) is estimated to increase lifetime cancer risk by 5%. Latency for radiation-induced solid tumors ranges from 5 to 40 years [20] which in many cases would exceed the life expectancy of patients in the screening population. Therefore, a lung cancer screening CT likely poses a minimal but non-zero risk of inducing future malignancy.
Any cancer screening test must demonstrate high sensitivity to be effective, and false-positive tests are inevitable. In the NLST, a total of 18,146 positive CTs were reported, and lung cancer was confirmed in 649 cases. The overwhelming majority of these were due to benign pulmonary nodules such as intrapulmonary lymph nodes or granulomas which were shown to be stable on follow-up exams. In some instances, however, patients were subjected to invasive procedures and associated complications.
Lung biopsy options include surgical wedge resection, CT-guided FNA, and endobronchial approaches. These are generally considered more invasive than those performed in other cancer screening settings such as mammography or colonoscopy, with major possible complications including pneumothorax, severe hemorrhage, and air embolism [21]. Among patients in the NLST with positive CT findings, 1125 invasive procedures were performed with 245 reported complications, the majority of which were classified as intermediate or major severity. Nearly half of these biopsies (46%) did not confirm lung cancer.
It should be noted that the NLST did not dictate specific criteria for managing CT results, including when to recommend follow-up or tissue sampling; therefore, current management is likely much more conservative. In fact, retrospective application of Lung-RADS to the NLST population reduces the false-positive rate from 26.6 to 12.8% [22••].
False positives are of particular concern in geographic areas where endemic fungal infections are common. The University of Cincinnati is located within the Ohio River Valley, a region where up to 90% of the population has been exposed to histoplasmosis. Pulmonary histoplasmosis is generally asymptomatic in immunocompetent individuals, but the ensuing granulomatous response often produces small pulmonary nodules that can mimic early lung cancer on CT. Many of these nodules demonstrate benign characteristics such as central calcification, but some do not, increasing the probability of false-positive exams in our population.
In the early 2000s, our institution undertook a pilot study to determine if LCS was feasible under these circumstances. As expected, initial screening exams were positive at a rate significantly higher than the national average (28% of baseline screening exams would have been classified as Lung-RADS 3 or greater). However, in our experience, these initial false positives rarely lead to unnecessary invasive procedures. Granulomas typically show long-term stability or very slow growth in contrast to invasive lung cancer; thus, short-term follow-up can differentiate these entities and significantly curtail the number of benign biopsies. In the pilot study, a follow-up algorithm very similar to the current Lung-RADS guidelines was utilized for small nodules, and nodules greater than 8 mm were managed per tumor board consensus. Using this protocol, no benign biopsies were performed, and no cancers were missed. Our experience in the LCS program has been similar; after 7 years of screening, fewer than 10 invasive procedures have been performed as a result of LCS findings in which lung cancer was not confirmed.
The possible psychological impact of false-positive results on patients should also be acknowledged. While high-quality studies on this topic are sparse, current literature indicates that screening participants experience temporary distress after a positive or indeterminate result [23], but long-term psychologic outcomes for screened patients versus control are similar [24]. In our experience, proactive communication with the patient tends to mitigate the anxiety associated with a positive exam. Explanation of the typical steps that are required for further workup (short-interval follow-up, additional imaging, etc.) and emphasis on the fact that most positive results do not result in a cancer diagnosis should be included in the shared decision-making process.
Conclusion
Screening high-risk individuals for lung cancer offers the possibility to prevent thousands of deaths each year. Despite recent actions by US public health agencies to increase access, relatively few eligible patients currently undergo screening, and much of this potential remains untapped. Establishing and growing a lung cancer screening program requires cooperation among multiple stakeholders. Critical elements of a successful program include a Program Coordinator who is involved in nearly every step of the screening process, a venue for multidisciplinary decision-making, and an emphasis on patient autonomy.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pham D, Bhandari S, Oechsli M, Pinkston C, Kloecker G. Lung cancer screening rates: data from the lung cancer screening registry. J Clin Oncol. 2018;36(suppl abstract 6504):6504.
Jemal A, Fedewa SA. Lung Cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3(9):1278–81.
Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–64.
Marcus PM, Bergstralh EJ, Fagerstrom RM, Williams DE, Fontana R, Taylor WF, et al. Lung cancer mortality in the Mayo Lung Project: impact of extended follow-up. J Natl Cancer Inst. 2000;92(16):1308–16.
•• National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. This study reports the results of the National Lung Screening Trial demonstrating reduced mortality with screening low-dose CT.
De Koning H, Van Der Aalst C, Ten Haaf K, Oudkerk M. Effects of volume CT lung cancer screening: mortality results of the NELSON randomised-controlled population based trial. 2018 World Conference on Lung Cancer. Abstract PL02.05. Presented September 25, 2018.
Goulart BH, Bensink ME, Mummy DG, Ramsey SD. Lung cancer screening with low-dose computed tomography: costs, national expenditures, and cost-effectiveness. J Natl Compr Cancer Netw. 2012;10(2):267–75.
Kazerooni EA, Austin JH, Black WC, Dyer DS, Hazelton TR, Leung AN, McNitt-Gray MF, Munden RF, Pipavath S; American College of Radiology; Society of Thoracic Radiology. ACR-STR practice parameter for the performance and reporting of lung cancer screening thoracic computed tomography (CT): 2014 (Resolution 4). J Thorac Imaging 2014;29(5):310–316.
Wiener RS, Gould MK, Arenberg DA, Au DH, Fennig K, Lamb CR, Mazzone PJ, Midthun DE, Napoli M, Ost DE, Powell CA, Rivera MP, Slatore CG, Tanner NT, Vachani A, Wisnivesky JP, Yoon SH; ATS/ACCP Committee on Low-Dose CT Lung Cancer Screening in Clinical Practice. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med 2015;192(7):881–891.
Kanodra NM, Pope C, Halbert CH, Silvestri GA, Rice LJ, Tanner NT. Primary care provider and patient perspectives on lung cancer screening. A qualitative atudy. Ann Am Thorac Soc. 2016;13(11):1977–82.
• Godoy MCB, Odisio EGLC, Erasmus JJ, Chate RC, Dos Santos RS, Truong MT. Understanding lung-RADS 1.0: a case-based review. Semin Ultrasound CT MR. 2018;39(3):260–72. Review of Lung-RADS categories and recommendations with case examples.
• Ravenel JG, Nance JW. Coronary artery calcification in lung cancer screening. Transl Lung Cancer Res. 2018;7(3):361–7. Discusses the usefulness of coronary artery calcification scoring in the lung cancer screening population.
Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291(2):210–5.
Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3(12):1229–36.
Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, DeMello S, Desjardins SS, Munden RF1; NLST Study Team. Association of Coronary Artery Calcification and Mortality in the National Lung Screening Trial: a comparison of three scoring methods. Radiology. 2015;276(1):82–90.
Johnson EB. Methods for text mining in radiology. 2016 May. Retrieved from https://etd.ohiolink.edu/!etd.send_file?accession=case1459514073
Munden RF, Carter BW, Chiles C, MacMahon H, Black WC, Ko JP, et al. Managing incidental findings on thoracic CT: mediastinal and cardiovascular findings. A white paper of the ACR incidental findings committee. J Am Coll Radiol. 2018;15(8):1087–96.
Litmanovich D, Bankier AA, Cantin L, Raptopoulos V, Boiselle PM. CT and MRI in diseases of the aorta. AJR Am J Roentgenol. 2009;193(4):928–40.
Godoy MCB, White CS, Erasmus JJ, Wu CC, Truong MT, Munden RF, et al. Extrapulmonary neoplasms in lung cancer screening. Transl Lung Cancer Res. 2018;7(3):368–75.
Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. The essential physics of medical imaging. 2nd ed; 2002.
Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol. 2011;196(6):W678–82.
•• Pinsky PF, Gierada DS, Black W, Munden R, Nath H, Aberle D, et al. Performance of lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485–91. Demonstrates significant reduction in false-positive screens with Lung-RADS retrospectively applied to the NLST.
Brain K, Lifford KJ, Carter B, Burke O, McRonald F, Devaraj A, et al. Long-term psychosocial outcomes of low-dose CT screening: results of the UK Lung Cancer Screening randomised controlled trial. Thorax. 2016;71(11):996–1005.
Slatore CG, Sullivan DR, Pappas M, Humphrey LL. Patient-centered outcomes among lung cancer screening recipients with computed tomography: a systematic review. J Thorac Oncol. 2014;9(7):927–34.
Special Acknowledgment
The authors thank Mona Hemingway, BSN, RN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michael Burch, Sangita Kapur, and Sandra Starnes declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pulmonary Radiology
Rights and permissions
About this article
Cite this article
Burch, M., Kapur, S. & Starnes, S. Lung Cancer Screening: Insights from a Thriving Clinical Practice. Curr Pulmonol Rep 8, 96–103 (2019). https://doi.org/10.1007/s13665-019-00231-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13665-019-00231-0