Abstract
This retrospective study aimed to verify the short-term and long-term outcomes of elderly patients who underwent gastric resection for gastric cancer and to compare the results between younger and elderly patients. 222 Patients, who underwent gastrectomy between January 2005 and December 2014, were divided into 2 groups: ≤ 75 years old (group A) and > 75 years (group B). The groups were homogeneous except for more advanced pathological stage (p = 0.011) and higher number of comorbidities in group B (p < 0.001) and a higher rate of neoadjuvant or adjuvant complementary therapy in group A (p = 0.029 and p < 0.001). Perioperative morbidity rates were 38.7 and 65.5% (p = 0.001), and mortality rates were 2.5 and 7.9% (p = ns), respectively. The independent negative prognostic factors for morbidity were age older than 75 years [odds ratio (OR) 2.7], multiple organ resection (OR 2.4), and male gender (OR 1.8). The 36-month survival rates were 76.1% and 42.1% (p = 0.002) and disease-free survival rates were 85% and 76.3% (p = 0.017), respectively. Surgical indications should not be limited by age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past 60 years, a progressive increase in the mean age of the population has been registered, although the global incidence of gastric cancer (GC) is decreasing in the general population, an increase of elderly patients with GC has been registered [1]. In western countries, among whites, the incidence of GC per 100,000 person-years decreased significantly from 2.6 [95% confidence interval (CI), 2.4–2.8] to 2.0 (95% CI, 1.9–2.1) for those 40–59 years of age and from 19.8 (95% CI, 19.0–20.6) to 12.8 (95% CI, 12.5–13.1) for those 60–84 years of age [2]. In Italy, according to data obtained by the National Institute of Statistics (ISTAT–AIRTUM Working Group) in 2014, the 5-year global overall survival (OS) for GC was similar for males and females (34.0 vs 36.0%), with a prevalence of 74,000 cases, and evenly distributed between males and females (55.0 vs 45.0%), representing 3.1% of all people affected by a malignant disease. Among them 680 per 100,000 are 75 years or older, which is twice the number of GC patients between 60 and 74 years and nine-times the number of those between 45 and 59 years old [3]. GC represents the second cause of death from malignant disease in the world, with its peak incidence in the sixth decade of life. Different analysis demonstrated that elderly patients have a higher incidence of well-differentiated cancer localized in the middle or distal third compared to younger patients [4]. The therapeutic approach for elderly patients affected by resectable GC is not univocal. Radical surgery with extended lymphadenectomy could improve disease-free survival and quality of life compared to a medical approach for elderly individuals; at the same time, it could be responsible for high morbidity and mortality rates due to comorbidities that are often present in this group of patients [5, 6].
We decided to analyze our experience in the past decade by comparing the results obtained from a group of patients 75 years or younger with those of a group of patients older than 75 years who underwent gastric resections or total gastrectomies for cancer with the aim of evaluating perioperative data in terms of intraoperative, postoperative, and oncological results.
Materials and methods
This retrospective analysis involved consecutive patients who underwent gastrectomy for GC at a single institution (Humanitas Research Hospital—Rozzano, Milano) from January 2005 to December 2014. Patients were divided into 2 groups according to age: group A 75 years or younger and group B older than 75 years.
Inclusion criteria were age older than 18 years, diagnosis of resectable GC and surgery with curative intent. Patients with EGJ cancers and concomitant malignancies were excluded.
Preoperative staging was performed using endoscopy, computed tomography (CT) scan, and positron emission tomography (PET) when necessary. Patients were evaluated by a multidisciplinary team and the indication for neoadjuvant or adjuvant treatment was based on patient and tumor characteristics. The multimodal approach changed during the years according to the evolution of European Society for Medical Oncology (ESMO) guidelines (multidisciplinary team: surgeons, oncologists, radiotherapists, endoscopists and radiologists).
During subtotal gastrectomy, the resected nodes included 1, 3, 4, 5, 6, 7, 8a, and 9 for D1 + lymph node dissection and 11p and 12a for D2 dissection. During total gastrectomy, D1 + lymph node dissection included 1, 2, 3, 4, 5, 6, 7, 8, 9, and 11p extended to 11d; 12 was included for D2 modified dissection.
D1 and D3 lymph node dissections were limited to selected cases. D1 + lymph node dissection was indicated for cT1/2 and N0/+ GC. D2 was performed for all other clinical stages.
Postoperative morbidity and mortality risks were evaluated considering the ASA and P-POSSUM score. Laparotomic or laparoscopic approach was determined according to clinical stage and presence of comorbidities. Patients affected by cancer of the distal third underwent subtotal gastrectomy, whereas for cancer of the middle third, the indication depended on the histological type. Total gastrectomy was performed in the case of diffuse cancers and for cancers of the proximal third. Pathological staging was performed according to 7th edition of the American Joint Committee on Cancer (AJCC). Severity of postoperative complications was defined using the Clavien-Dindo (CD) classification [7]. After surgery, patients were scheduled for instrumental and clinical follow-up according to pathological stage.
Statistical analysis
When appropriate, data were described as number and percentage or mean and standard deviation or median and range. For qualitative data, the differences between the two groups were analyzed with the Chi-square test using the Fisher correction when appropriate; quantitative data were analyzed with the nonparametric Wilcoxon test.
OS and disease-free survival were calculated from the date of surgery and last follow-up available. Survival curves were estimated using the Kaplan–Meier test. Prognostic elements were evaluated using the Cox regression model; independent factors with p < 0.1 were considered for Cox multivariable regression analysis. Specific survival according to cause was estimated with the competitive events method; death was the event and noncancer-related deaths were the competitive event. All analyses were performed using Stata 13.
Results
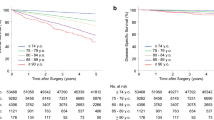
Two hundred twenty-two patients who underwent total or subtotal gastrectomy for GC from January 2004 to December 2014 were included in the analysis, 159 patients were 75 years or younger (71.6%, group A) and 63 patients were older than 75 years (28.4%, group B). The median age was 63 ± 10 years in group A and 80 ± 3 years in group B. The two groups were similar for gender, preoperative body mass index (BMI), tumor site, histology, and clinical stages. In group B, more comorbidities were observed, with a median of two diseases for each patient rather than a median of one for group A (p < 0.001). For both groups, cardiovascular and metabolic comorbidities were the most frequently represented. In group B, the prevalence of cardiovascular disease was 71.4% compared to 41.5% for group A (p < 0.001). ASA score and the risk of postoperative morbidity and mortality, calculated using P-POSSUM, were significantly higher for group B than for group A (p < 0.001). Clinico-pathological and surgical features are presented in Table 1. Overall postoperative in-hospital mortality was 4.1%; it was 2.5% for group A and 7.9% for group B. Overall postoperative morbidity was 44.1; 38.7% of patients in group A and 65.5% in group B (p = 0.001) presented postoperative complications. A comparison of the different grades of complication severity for the two groups showed similar results. To evaluate the accuracy of the P-POSSUM score for postoperative morbidity and mortality, we stratified the patients in three different groups according to postoperative complications and mortality. In-hospital mortality was 2.2% for the group of patients with P-POSSUM scores < 3%, 6.6% in case of P-POSSUM scores between 3 and 10%, and 19.2% in case of P-POSSUM scores > 10% (p = 0.005). Cardiovascular complications in group B were the most frequent adverse events we observed (17.4%), significantly more frequent than those for group A (3.1%) (p < 0.001). There were no differences in both groups in terms of anastomotic leak (7.2% overall and 6.3% and 9.5% in group A and group B, respectively). During univariate analysis age older than 75 years, number of comorbidities, male gender, multiple organ resections, and intraoperative blood transfusions were negative prognostic factors for the development of postoperative complications. The independent negative prognostic factors for death during hospitalization were: age > 75 years (OR 2.8), multiorgan resection (OR 2.8), surgical re-intervention (OR 2.5) and number of comorbidities (OR 1.5). Preoperative oncologic treatment did not significantly influence the postoperative course (p = 0.234). In group A, adjuvant treatment was indicated for 50.9% of patients, whereas for group B it was indicated for 23.8% (p < 0.001). The median follow-up was 32.8 months (range 0.1–120.3). Three-year OS rates were 73.9 and 33.1 months for group A and group B, respectively. Three-year overall cancer-related survival was 76.1 months for group A and 42.7 months for group B (p = 0.002). During follow-up, deaths that were not cancer-related occurred for 2.5% of group A and 12.7% of group B (p = 0.005). The 12- and 36-month disease-free survival rates were 85 and 73.8% for group A and 74.5 and 51.9% for group B (p = 0.017) Fig. 1. Data related to disease-free survival and overall specific survival obtained by univariate and multivariate analyses are reported in Tables 2, 3. Pathological stage was the most important element for OS. The risk was 3.6-times higher for stage II, 6.5-times higher for stage III, and 17-times higher for stage IV (p < 0.001). After comparing the two groups according to pathological stage, differences were observed for OS for stage II. OS for group B was significantly lower than that for group A (p = 0.019) Fig. 2.
Discussion
An increase in the number of patients who can potentially benefit from surgery has been determined by the longer life expectancy of the general population; consequently, a higher rate of GC has been detected in elderly patients. Scientific literature does not provide a unanimous way to define the old population; usually, 65 years is the cut-off. In past decades, the cut-off has increased gradually and further categories have been created: young-old (65–74 years old), old–old (75–84 years old), and oldest-old (> 85 years old) [8]. The United Nations uses 80 years to define old people. In Italy, the latest data from the National Institute for Statistics showed that the median life expectancy is 82.3 years (78.9 for males and 84.8 for females).
Considering the Italian life expectancy, we decided to use 75 years as the age to define old patients in our study. The pool obtained accounts for 28.4% of the total and properly fits the rates published in the scientific literature so far [9]. Age alone cannot be considered a negative prognostic factor influencing short-term and long-term outcomes. A retrospective analysis involving 7781 patients evidenced the prognostic nutritional index (PNI), comorbidities, old age, male sex, and combined resections as independent prognostic factors for postoperative morbidity in GC patients [10]. Sarcopenia or Charlson Comorbidity Index (CCI) 2 was identified as an independent risk factor for postoperative complications of elderly patients with GC who were candidates for surgery [11]. These data are similar to our multivariate analysis results indicating that age older than 75 years, male sex, and multiple organ resections are negative prognostic factors for development of postoperative complications. In a retrospective analysis, it was evidenced that performance status and low BMI were negative prognostic factors for the development of postoperative morbidities, particularly medical ones, and that comorbidities were negative prognostic factors for long-term outcomes [12]. In our study, the comorbidities evaluation was performed using ASA and P-POSSUM risk scores to allow for a comparison between the estimated morbidity and mortality and the actual observed. Our results show that mortality and morbidity (estimated with P-POSSUM) for group B patients were significantly higher (p < 0.001). Furthermore, it is possible to highlight an optimal correlation between estimated and observed results, as demonstrated in the current literature [13]. Other authors demonstrated the validity of this score in a prospective evaluation; in the case of high risk, a reduction in mortality has been demonstrated through modification of the surgery variable, such as changing the extent of lymphadenectomy [14]. We have not modified lymphadenectomy for the old patients group. This issue is still debated and the literature reports different results. A randomized control trial focused on D1 versus D2 lymphadenectomy performed for 711 patients evidenced that patients older than 70 years and treated with D2 dissection had significantly higher morbidity and hospital mortality and significantly shorter survival compared with patients younger than 70 years [15]. Brenkman compared data from 2387 patients younger than 75 years and 1377 patients older than 75 years who underwent different degrees of lymphadenectomy during GC (The Netherlands Cancer Registry of patients who underwent curative gastrectomy). The results demonstrated no differences in terms of postoperative morbidity and OS related to the number of nodes retrieved from the two groups of patients. Considering the retrospective nature of this study and the presence of many confounding factors, such as comorbidities, the authors suggest performing less extensive lymphadenectomy for selected cases (low performance status or high CCI) [16]. In our experience, D2 lymphadenectomy for elderly patients seems feasible without a significant increase in morbidity and mortality. Our results evidenced that medical complications were more frequent in group B. These data indicate the importance of frailty and comorbidities for old patients, particularly cardiovascular ones. Katsunobu compared elderly patients with a control group and evidenced no statistically significant differences in terms of postoperative complications between elderly and nonelderly patients after gastric surgery [17]. Saif presented a review of similar results from western and eastern studies evidencing no differences in postoperative morbidity results for elderly patients compared to younger patients [18]. An analysis of anastomotic leaks in our groups of patients did not indicate differences. A retrospective analysis conducted involving 3632 patients evidenced that, according to multivariate analysis, age older than 65 years and malnourishment were adverse risk factors for the development of postoperative leaks in GC [19]. In-hospital mortality results did not evidence differences between the two groups, although a higher number of deaths occurred in group B, but without statistical significance. Yang retrospectively evaluated 824 Korean patients who underwent surgery for GC (558 patients 60–64 years; 198 patients 74–79 years; 68 patients 80 years or older). The results evidenced significantly higher in-hospital mortality for those older than 80 years; however, another study concluded that age older than 80 years is a negative prognostic factor for postoperative complications and mortality. These data probably emerged because of the distribution of the patients in three categories; ASA scores were significantly higher for the older group (age older than 80 years). These data evidenced that age was associated with comorbidities and postoperative morbidity and mortality for elderly patients [20]. Some indications exist in the literature regarding the different biological evolution of tumors regarding macroscopic appearance and histotype in an old population compared with those in a young population. A retrospective cohort study of patients from the Kaiser Permanente Northern California (KPNC) cancer registry evidenced that in GC patients without EGJ tumors, the incidence of diffuse/mixed-type cancers was significantly higher for younger patients, and moderate differentiated cancers were observed in the old patients. Except for patients younger than 40 years, the OS trend was shorter according to age [21]. Tural retrospectively compared two groups of patients younger than and older than 70 years, and the results showed no differences for tumor sites in the stomach (cardia/non-cardia), tumor histology, perineural invasion (PNI), lymphovascular invasion, or tumor stage [22].
In our experience, statistically significative differences between the two groups have not been found when analyzing pathologic features; however, we found more advanced stages (p = 0.011) and a greater incidence of node metastasis in group B patients (p = 0.0169). These data were probably derived from a significantly lower number of patients who underwent neoadjuvant treatment in group B (p = 0.029). Becker analyzed 480 GC patients who underwent neoadjuvant treatment; there was a complete response of 21.2% and subtotal tumor regression of 10%. Tumor regression and lymph node regression were independent prognostic factors for survival in this group of patients [23]. Furthermore, it must be carefully evaluated whether old patients should undergo integrated treatment because the side effects of the medicines used might worsen the general conditions of these patients [24]. No differences have been found regarding residual tumors (R 1–2) in the two groups. Results of our analysis regarding survival showed a worse OS for group B, which had a median OS after 3 years that was significantly lower than that of group A (p = 0.002). In our study, univariate analysis showed that age was a negative prognostic factor for survival. This variable in the multivariate analysis lost all significance because the most important ones were pathological stage and complications after surgery for OS; pathological stage and comorbidities were the most important variables for disease-specific survival. Sakurai published a retrospective analysis of 461 patients that demonstrated that the elderly group had worse OS and disease-specific survival than the control group, particularly stage II and stage III patients. These differences were related to a significantly lower number of patients treated with adjuvant therapy [25].
As previously shown by our results, group B had a higher number of deaths not related to GC during follow-up (p = 0.005). Competitive analysis of risk was performed to evaluate the disease-specific survival. Results obtained with this analysis showed that there were statistically significant differences only for stage II patients in groups A and B (p = 0.019). This evidence can be associated with a significantly lower percentage of patients treated with adjuvant therapy according to the data obtained from the literature. Regarding the oncologic results, the long-term results showed faster disease relapse for group B that for group A (p = 0.017). This was related to worse pathologic stage for group B compared with group A and the use of more complementary treatments for young patients than for elderly ones.
The main limit of the present study was its retrospective nature, although the data were recorded prospectively. The main bias of the study involved the selection of elderly patients who we determined to be fit for surgery and the lack of data for patients who we decided should not undergo surgery. This group of elderly patients does not represent all those affected by GC and theoretically suitable for surgery. All patients considered were discussed by a multidisciplinary team and the same participants were involved during the period of the study. Therefore, selection bias should be reduced by determining a better way to define treatment for young and elderly patients.
Conclusions
GC is an aggressive disease that is best treated with surgery. Elderly patients are fragile and often present many associated diseases at the time of diagnosis that influence the multidisciplinary team who choose the best treatment for each patient. Comorbidities for elderly patients impact the short-term and long-term outcomes and can limit perioperative and surgical treatments. Patients affected by GC should be constantly discussed in the multidisciplinary setting to guarantee that the best treatment is tailored to each patient. Considering age alone limits the therapeutic options we have to treat this type of cancer.
References
Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F et al (2014) Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 50:1330–1344
Anderson WF, Rabkin CS (2010) Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 303:1723–1728
AIRTUM Working Group (2014) Prevalence and cure of cancer in Italy. Epidemiol Prev 38(6 Suppl 1):1–144
Kim DY, Joo JK, Ryu SY, Park YK, Kim YJ, Kim SK (2005) Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol 11:22–26
Ueno D, Matsumoto H, Hirai T (2017) Prognostic factors for gastrectomy in elderly patients with gastric cancer. World J Surg Oncol 15:59
Rausei S, Ruspi L, Rosa F, Morgagni P, Marrelli D, Cossu A et al (2016) Extended lymphadenectomy in elderly and/or highly co-morbid gastric cancer patients: a retrospective multicenter study. Eur J Surg Oncol 42:1881–1889
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Crews DE, Zavotka S (2006) Aging, disability, and frailty: implications for universal design. J Physiol Anthropol 25:113–118
Orsenigo E, Tomajer V, Staudacher C (2007) Impact of age on postoperative outcomes in 1118 gastric cancer patients undergoing surgical treatment. Gastric Cancer 10:39–44
Lee JY, Kim HI, Kim CB (2016) Clinical significance of the prognostic nutritional index for predicting short- and long-term surgical outcomes after gastrectomy: a retrospective analysis of 7781 gastric cancer patients. Med 95(18):e3539
Zhou CJ, Zhang FM, Chen XX (2017) Sarcopenia: a new predictor of postoperative complications for elderly gastric cancer patients who underwent radical gastrectomy. J Surg Res 211:137–146
Kazuhito M, Hideto I, Keiichi F (2013) Postoperative complications and survival after gastric cancer surgery in patients older than 80 years of age. J Gastrointest Surg 17:2067–2073
Fang Y, Wu C, Chen Z (2014) Perioperative mortality and morbidity prediction using POSSUM, P-POSSUM and APACHE II in Chinese gastric cancer patients: surgical method is a key independent factor affecting prognosis. Int J Clin Oncol. 19:74–80
Lamb P, Sivashanmugam T, White M, Irving M, Wayman J, Raimes S et al (2008) Gastric cancer surgery–a balance of risk and radicality. Ann R Coll Surg Engl 90:235–242
Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I et al (2004) Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 22:2069–2077
Brenkman HJF, Goense L, van Hillegersberg R (2017) A high lymph node yield is associated with prolonged survival in elderly patients undergoing curative gastrectomy for cancer: a Dutch population-based cohort study. Ann Surg Oncol 24(8):2213–2223
Katsunobu S, Kazuya G, Kosei H (2015) The outcome of surgical treatment for elderly patients with gastric carcinoma. J Surg Oncol 111:848–854
Saif MW, Makrilia N, Syrigos K (2010) Gastric cancer in the elderly: an overview. Eur J Surg Oncol 36:709–717
Tu RH, Lin JX, Huang CM (2017) Development of a nomogram for predicting the risk of anastomotic leakage after a gastrectomy for gastric cancer. Eur J Surg Oncol 43:485–492
Yang Jun-Young, Hyuk-Joon Lee, Han-Kwang Yang (2017) Short- and long-term outcomes after gastrectomy in elderly gastric cancer patients. Ann Surg Oncol 24:469–477
Kim MS, Kim S (2016) Outcome of gastric cancer surgery in elderly patients. J Gastric Cancer 16:254–259
Tural D, Selçukbiricik F, Büyüküna E (2012) A comparison of patient characteristics, prognosis, treatment modalities, and survival according to age group in gastric cancer patients. World J Surg Oncol 10:234
Becker K, Langer R, Hofler H (2011) Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 253:934–939
Balducci L (2015) Systemic treatment of gastric and esophageal adenocarcinoma in elderly patients. J Gastrointest Oncol 6:75–78
Sakurai K, Maguruma K, Hirakawa K (2015) The outcome of surgical treatment for elderly patients with gastric carcinoma. J Surg Oncol 111:848–854
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest or financial support.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Additional information
The article is part of topical collection on Gastric Cancer Surgery.
Rights and permissions
About this article
Cite this article
de Pascale, S., Belotti, D., Celotti, A. et al. Prognostic factors for short-term and long-term outcomes of gastric cancer surgery for elderly patients: 10 years of experience at a single tertiary care center. Updates Surg 70, 265–271 (2018). https://doi.org/10.1007/s13304-018-0548-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-018-0548-y