Abstract
Purpose of Review
Contralateral prophylactic mastectomy (CPM) at the time of unilateral breast cancer surgery is increasing, though still controversial in BRCA(−) patients. We review the relevant literature regarding CPM and specifically abnormal imaging/biopsies in patients following unilateral mastectomy (UM) versus CPM and present results from our own retrospective chart review.
Recent Findings
A large cohort study by van la Parra et al. examined the incidence/risk of breast biopsy at follow-up, finding the 5-year estimated biopsy rate to be the lowest for UM compared to breast-conserving surgery. Our retrospective series, similar to others, did not find a significant difference in imaging/biopsies between UM and CPM groups.
Summary
Current literature regarding imaging/biopsies after CPM is sparse. Recent publications suggest that the incidence of abnormal imaging or biopsy is not reduced in women undergoing bilateral mastectomies for treatment of unilateral breast cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Unilateral mastectomy (UM) is a common procedure for the treatment of breast cancer, and is performed to treat approximately half of non-metastatic breast cancer cases [1]. Although breast conserving treatment, consisting of lumpectomy followed by radiation, is the preferred surgical approach for early breast cancer, mastectomy is often employed due to extent of disease in the breast, nipple involvement, inability to receive radiation, or patient preference [2]. The risk of contralateral cancer in patients undergoing ipsilateral mastectomy (and without a genetic mutation conferring higher risk) is 0.5–1.0% per year [3,4,5]. Contralateral prophylactic mastectomy (CPM) has been shown to be protective against new contralateral cancer in women with a BRCA mutation or significant family history and has been shown to be cost-effective for BRCA mutation carriers with unilateral cancer [6, 7]. However, overall survival benefit in those with increased familial or genetic risk (FGR) and protection against contralateral cancers in those without increased FGR is controversial.
Patients may elect to have bilateral mastectomy for many reasons, such as “peace of mind”, the perception that they are receiving more aggressive treatment, or even a desire for greater symmetry. For patients without genetic mutations conferring an increased risk of breast cancer, the benefits of removing a normal breast are nebulous from the perspective of recurrence, overall survival, and costs, which encompass not only financial expense, but extend to time and quality of life. Enmeshed with this line of thought is the discontinuation of screening mammography and the perceived decrease in subsequent evaluations of the chest wall or breast reconstruction after bilateral mastectomy. The objective of this paper is to review the existing literature surrounding the use of follow-up imaging and biopsy in women treated for breast cancer and undergoing prophylactic mastectomy, as well as to summarize the results of a single-institution case series evaluating this population compared to a matched group of women treated with unilateral mastectomy and followed with surveillance mammography.
Surveillance Imaging and Breast Biopsies After Breast Cancer Treatment
After initial breast cancer treatment, the American Society of Clinical Oncology recommends a careful history and physical every 3–6 months for the first 3 years, followed by every 6–12 months for the next 2 years, and then annually. Women with breast conserving therapy are recommended to have their first mammogram within 6 months after radiation therapy and every 6–12 months thereafter [8]. No screening imaging is indicated for women treated with mastectomy (for the treated breast), and any follow-up imaging is prompted by abnormal physical exam findings. In women with an intact contralateral breast after unilateral mastectomy, screening with yearly mammograms and breast exam should continue as normal for that breast.
False positive mammograms are a risk associated with regular screening. One large prospective cohort study found that after 10 years of annual screening for a woman who starts screening at age 40, the cumulative probability of a recall mammogram was 61.3%. The 10-year cumulative probability of biopsy was 7.0% for the same group [9]. Given this risk, a potential benefit of choosing CPM would be the elimination of regular mammography screening and any subsequent imaging or biopsies that result, making CPM a desirable option for women with the goal of avoiding false-positive imaging and resultant biopsies.
Women undergoing breast conservation, not surprisingly, are subjected to more follow up biopsies than breast cancer patients undergoing mastectomy as their primary surgical treatment. A large cohort study by van la Parra et al. published in 2018 examined the incidence and risk of breast biopsy at follow-up after breast cancer treatment, in addition to subsequent breast cancer treatment [10••]. The cohort included 121,879 patients with either Medicare (66 or older) or commercial insurance (64 or younger), diagnosed with stage I–III breast cancer between 2000 and 2011. Exclusions include those with simultaneous cancer diagnosis within 12 months of breast cancer diagnosis and those with a history of breast cancer and bilateral mastectomy. Median follow-up was 3.7 years for the commercial insurance cohort and 5.8 years for the Medicare cohort. When comparing the management options of treatment with breast conserving surgery (BCS) alone, BCS with whole breast irradiation (WBI), BCS with brachytherapy, and unilateral mastectomy, the 5-year estimated biopsy rate was the lowest for UM in both cohorts.
In this large cohort, the UM group had a 5-year estimated breast biopsy rate of 10.4% in the commercial insurance group (estimated by cumulative incidence) and 7.7% in the Medicare group as estimated by log-rank test (survival status not reported). Compared to BCS plus WBI as the reference, UM had a significantly decreased risk of biopsy, with adjusted hazard ratios (HR) of 0.60 (95% CI 0.56–0.64) and 0.53 (95% CI 0.50–0.55) for the commercial and Medicare cohorts, respectively. BCS plus brachytherapy had adjusted HRs of 1.53 (95% CI 1.38–1.70) and 1.76 (95% CI 1.63–1.91) for the commercial and Medicare cohorts, respectively, while BCS alone had adjusted HRs of 1.43 (95% CI 1.28–1.60) and 0.80 (95% CI 0.74–0.86) for the same.
The unilateral mastectomy group was used to estimate the contralateral biopsy rate, with the assumption that after UM, all biopsies would be in the contralateral side. This group served as a convenience sample to estimate ipsilateral biopsy rates in the other cohorts after subtracting the assumed contralateral rate. This study, not surprisingly, does demonstrate that initial UM seems to decrease biopsy incidence on follow-up compared to breast conserving therapy. The end goal of this study was determining general biopsy rate after breast cancer treatment, and BM patients were excluded. Though the UM group was used to estimate the contralateral biopsy rate, this study is limited by lack of reporting of laterality in the databases. Clearly, this is not perfect methodology and underscores the striking lack of data regarding this particular topic.
The real question of additional imaging and biopsy after mastectomy is one that has not been addressed in large cohorts. A separate retrospective single institution review by Ahn et al. published in 2018 compared UM versus bilateral mastectomy (BM) cases to determine imaging and biopsy rates after mastectomy only [11•]. The study included women treated for either invasive breast carcinoma or DCIS at that institution, including those diagnosed with bilateral breast cancer. Any contralateral imaging for UM patients at diagnosis was excluded due to screening being the standard of care. Thus, there was no data collected for imaging or biopsies in the non-operated side for UM patients. Data were analyzed for 185 UM and 200 BM cases between 2009 and 2015 with a mean follow-up of 30 months. In the BM group, 19.5% (39/200) patients had bilateral breast cancer. The BM group was significantly younger and more likely to be premenopausal (age of 49 versus 57, and 115 premenopausal versus 54 premenopausal). Tumor size was significantly smaller for the BM group (9.5 mm versus 17 mm). Nineteen patients total (5%) tested positive for a BRCA mutation, and all of these patients received a BM.
In this study, 10% (19/185) of UM patients underwent imaging for abnormal physical exam findings compared to 15.5% (31/200) of BM patients. Of the BM patients requiring imaging, 76% were on the ipsilateral cancer side (31 images total, including 5 studies for patients who were treated for bilateral cancer that were counted as ipsilateral). Six percent (11/185) and 8% (16/200) of UM and BM patients, respectively, required biopsy. Eleven of the biopsies were on the ipsilateral side (including one bilateral cancer patient). Two UM patients had a malignant diagnosis on biopsy (malignancy rate 1% overall after UM). Three BM patients had subsequent ipsilateral malignancy (malignancy rate of 1.5% after BM). Of the 19 BM patients positive for a BRCA mutation, 3 had imaging and 2 had biopsies with benign findings.
Thus, this study did not find a significant difference between the amount of imaging and biopsies between the groups, despite the removal of an unaffected breast, for over 80% of the bilateral mastectomy group. This study demonstrates that those undergoing UM and BM had no significant difference in imaging/biopsies despite complete removal of an unaffected breast. As a goal of this study was to quantify imaging/biopsies after mastectomy, contralateral findings for UM patients are not included, so quantification of findings in the retained breast was not performed. Despite this omission, Ahn’s study demonstrates that the need for imaging and biopsy are not eliminated after mastectomy. However, the fact that this study excludes imaging and biopsies on the contralateral side for UM patients means that no direct comparison between imaging/biopsy needs after CPM versus in the unoperated breast of a UM patient can be performed.
Given that women cite a decrease in follow-up imaging as a reason for pursuing prophylactic mastectomy, our own interest expanded this further to evaluate the use of expected imaging follow-up for retained unaffected breasts compared to imaging and biopsy performed at the site of a prior mastectomy. We performed a single-institution retrospective review of a matched series of women undergoing mastectomy with immediate reconstruction and evaluated abnormal follow-up breast imaging, biopsies, and subsequent breast cancers in patients receiving CPM versus unilateral mastectomy with surveillance to discern any differences in the frequency of these events between the groups. Because one of our primary endpoints was evaluation of contralateral breast cancer risk, the cohort was selected to have a minimum follow-up period of 5 years. In general, breast cancer patients at our institution are followed at least yearly by their oncologic surgeon for 5 years or longer. Given that there are multiple options of breast reconstructive procedures with varying levels of complexity and risk, we elected to match patients by age, stage, and type of reconstruction to control for technical and age-related differences.
Institutional Case Series: Methods and Results
An IRB approved, retrospective, case-controlled, single-institution chart review of breast cancer patients receiving mastectomy and immediate reconstruction from January 1990 to May 2013 was performed. Cases were matched 1:1 by reconstruction type and age (± 5 years) at the time of surgery to limit procedure and age-related confounding variables. Patients with delayed mastectomy, delayed reconstruction, or bilateral cancer diagnosis at surgery were excluded. Staging, pathology, genetics, diagnostic imaging, surgical, clinical, and outcome data were collected. Due to the small numbers, descriptive statistics were utilized to summarize our findings.
Results
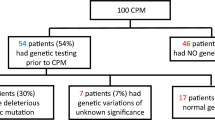
Forty-five UM cases were matched to bilateral mastectomy (BM) by age and immediate breast reconstruction procedure; all patients had a diagnosis of unilateral breast cancer at the time of mastectomy, and all patients had surgery at an NCCN-designated Comprehensive Cancer Center with a fellowship-trained breast surgeon as well as a board certified plastic surgeon. Mean age (n = 90) was 52.2 years (range 21.5–74.9) with mean follow-up time of 7.1 years (range 0.2–19.8). Mean BMI for the entire study population was 25.3 (range 18.3–42.7). There was no significant difference between UM and BM with regard to BMI, pathologic stage, follow-up time, distant recurrence, or survival. Genetic status was available for 31.1% (28/90) of cases; 5/28 (17.9%) women were BRCA(+) and had BM. A majority of patients had stage 1 (n = 37) breast cancer at presentation; 88/90 (97.7%) were alive at last follow-up. Demographics and clinical data for the two groups are illustrated in Table 1.
Biopsies and Imaging
Six UM and 10 BM patients had abnormal follow-up breast imaging (Table 2). Of these, 5 UM and 5 BM patients had abnormal imaging contralateral to the original cancer. Six UM and 9 BM patients had breast/chest wall biopsies after abnormal imaging, with 5 and 4 contralateral biopsies after 4.3 years (range 7.0–0.6) and 4.2 years (range 7.3–1.1), respectively. Pathology results are shown in Fig. 1. One UM patient developed contralateral cancer; 4 BM patients had local recurrence in the ipsilateral side of the prior cancer. There were no contralateral breast cancers in prophylactic mastectomy sites. Two UM and 5 BM patients had distant recurrences.
In our study, 22% of BM patients had new abnormal imaging and 20% had biopsies, while 13.3% of UM patients had abnormal imaging and biopsies. Note that we included contralateral abnormal imaging/biopsies for UM patients, but excluded any normal surveillance imaging results for the contralateral breast. Our data suggests that removal and reconstruction of an unaffected breast does not decrease the potential for subsequent breast imaging and breast biopsies. In fact, biopsies from the prophylactic breast showed pathologies likely driven by postsurgical changes.
Discussion
Contralateral Breast Cancer Risk
The estimated risk of contralateral breast cancer (CBC) given a patient’s genetic and family history plays an important role in determining the utility of CPM. Familial risk, not to be underestimated, can confer risk similar to that of BRCA mutation carriers. The WECARE study by Reiner et al., a case control study comparing matched CBC cases to unilateral controls younger than 55, characterizes the risk of CBC according to family history and age of diagnosis in patients testing negative for BRCA mutations [12]. The update of this study (2018) with the completion of WECARE II gave updated risk estimates for patients with varied family histories and included a larger sample size (1521 cases and 2212 controls) [13••]. This study also included a subset of women testing negative for BRCA1, BRCA2, ATM, CHEK2*1100delC, and PALB2 (130 cases and 93 controls) for comparison.
The updated study reports women without a first- or second-degree family history have a 10year CBC risk of 4.3% (95% CI 4.1–4.5). Those with any first degree history had a 10 year risk of 8.1% (95% CI 6.7–9.8), while those with only a second degree history had a 10 year risk of 6.0% (95% CI 4.9–7.4). When the first degree relative was older than 40 at diagnosis or only had a unilateral breast cancer history, 10 year risks were 7.5% (95% CI 6.1–9.1) and 7.4 (95% CI 6.1–9.1), respectively. When the first-degree relative was younger than 40 at diagnosis, had bilateral breast cancer history, or both, the 10-year risks were much higher, at 13.5% (95% CI 8.8–20.8), 14.1% (95% CI 9.5–20.7), and 36.3% (95% CI 14.5–90.5) respectively. The group testing negative for deleterious mutations had comparable results. This study shows that CBC risk is heavily dependent on family history, even after excluding deleterious mutation carriers. Most notably, those with a first-degree relative diagnosed younger than 40, or with bilateral breast cancer, had 10-year risks similar to a WECARE analysis of BRCA mutation carriers, reported at 20.5% and 15.9% for BRCA1 and BRCA2, respectively [14].
Survival and Mortality
When determining the value of CPM for a patient, one must consider whether significant benefits can be demonstrated for a similarly risk-matched population; age and inherited breast cancer risk are the most highly scrutinized characteristics. An early retrospective study by Herrinton et al. reviewed women diagnosed with unilateral breast cancer between 1979 and 1999 (1072 had CPM versus 317 without) and showed that CPM was protective against contralateral breast cancer (0.5% with CPM vs. 2.7% without) and reduced mortality (8.1% with CPM vs. 11.7% without), although genetic risk was not considered for separate analysis, as genetic testing did not exist prior to 1996 [15].
A recent retrospective study by Wong et al. evaluated 496,488 women diagnosed with unilateral breast cancer between 1998 and 2012 (59.6% had breast conserving surgery, 33.4% UM, and 7.0% CPM). In this large population, CPM conferred no improvement in breast cancer specific survival or overall survival when compared to breast conserving surgery [16••]. The difference in outcome between these studies could be due to the populations having different overall characteristics or treatment-related differences due to the timeframes of the studies (the efficacy of adjuvant treatment has increased in later years). A recent meta-analysis including 14 studies analyzing the effectiveness of CPM after unilateral breast cancer found that CPM decreases metachronous contralateral breast cancer in patients with increased familial or genetic risk, but, paradoxically, does not improve overall survival or breast cancer specific mortality in this group. This study also found that those without increased familial or genetic risk who had undergone CPM had improved overall survival and breast cancer specific mortality despite no absolute reduction in metachronous contralateral breast cancer risk [6]. Selection bias was identified as a possible explanation of there being no absolute reduction in new contralateral breast cancers but an improvement in overall survival with CPM, as CPM patients may be more likely to have general characteristics contributing to longevity, such as younger age, adequate health insurance, or non- invasive histology [17,18,19]. In our age and procedure-matched series, only 1 of 45 patients developed a contralateral breast cancer (2%) after 5 or more years of follow-up. This finding is consistent with the existing literature with regard to development of contralateral breast cancers among breast cancer survivors without increased genetic risk.
Conclusion
In our age-matched and procedure-matched population, CPM did not reduce contralateral imaging or biopsies, and the additional biopsies in BM patients may in fact be directly related to physical findings after prophylactic mastectomy and reconstruction. The removal of the healthy breast tissue increases the possibility of scarring, fat necrosis, and other irregularities that may result in abnormal imaging and biopsies, while potentially not providing any benefit in cost or survival. Since a patient may undergo CPM for “peace of mind”, the increase in abnormal imaging and biopsies could be very distressing. Comparisons to other studies in this area can be difficult due to different goals, methods, and exclusions, and the research in this area remains sparse. Given the complication risks associated with CPM, along with the lack of decrease in abnormal imaging and biopsies demonstrated in this study, we strongly recommend that patients be adequately counseled regarding the benefits and risks associated with CPM versus UM.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McGuire KP, Santillan AA, Kaur P, Meade T, Parbhoo J, Mathias M, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16(10):2682–90. https://doi.org/10.1245/s10434-009-0635-x.
National Accreditation Program for Breast Centers.: American College of Surgeons; 2014.
Chaudary MA, Millis RR, Hoskins EO, Halder M, Bulbrook RD, Cuzick J, et al. Bilateral primary breast cancer: a prospective study of disease incidence. Br J Surg. 1984;71(9):711–4. https://doi.org/10.1002/bjs.1800710924.
Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16(2):441–52. https://doi.org/10.1200/JCO.1998.16.2.441.
Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. https://doi.org/10.1016/S0140-6736(99)05036-9.
Fayanju OM, Stoll CR, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg. 2014;260(6):1000–10. https://doi.org/10.1097/SLA.0000000000000769.
Zendejas B, Moriarty JP, O'Byrne J, Degnim AC, Farley DR, Boughey JC. Cost-effectiveness of contralateral prophylactic mastectomy versus routine surveillance in patients with unilateral breast cancer. J Clin Oncol. 2011;29(22):2993–3000. https://doi.org/10.1200/JCO.2011.35.6956.
Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(7):961–5. https://doi.org/10.1200/JCO.2012.45.9859.
Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155(8):481–92. https://doi.org/10.7326/0003-4819-155-8-201110180-00004.
•• van la Parra RFD, Liao K, Smith BD, Yang WT, Leung JWT, Giordano SH, et al. Incidence and outcome of breast biopsy procedures during follow-up after treatment for breast cancer. JAMA Surg. 2018;153(6):559–68. https://doi.org/10.1001/jamasurg.2017.5572This large study estimates the breast biopsy rate after various breast-conserving therapies or mastectomy in Medicare and commercial insurance cohorts. Unilateral mastectomy patients had lower estimated biopsy rates than those treated with breast conserving therapy in both cohorts.
• Ahn S, Elnekaveh B, Schmidt H, Weltz C, Pisapati K, Port E. Defining the need for imaging and biopsy after mastectomy. Ann Surg Oncol. 2018;25(13):3843–8. https://doi.org/10.1245/s10434-018-6735-8This retrospective review determined the rates of imaging and biopsy in unilateral mastectomy and bilateral mastectomy groups at a single institution, excluding imaging and biopsy on the non-operated side in unilateral mastectomy patients. No significant difference was seen between the groups.
Reiner AS, John EM, Brooks JD, Lynch CF, Bernstein L, Mellemkjaer L, et al. Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the Women's Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol. 2013;31(4):433–9. https://doi.org/10.1200/JCO.2012.43.2013.
•• Reiner AS, Sisti J, John EM, Lynch CF, Brooks JD, Mellemkjaer L, et al. Breast cancer family history and contralateral breast cancer risk in young women: an update from the Women's Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol. 2018;36(15):1513–20. This WECARE study, a case-control study comparing those with contralateral breast cancer cases with unilateral controls, characterizes the risk of contralateral breast cancer for women with specific family histories. https://doi.org/10.1200/JCO.2017.77.3424.
Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 2010;28(14):2404–10. https://doi.org/10.1200/JCO.2009.24.2495.
Herrinton LJ, Barlow WE, Yu O, Geiger AM, Elmore JG, Barton MB, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol. 2005;23(19):4275–86. https://doi.org/10.1200/JCO.2005.10.080.
•• Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann Surg. 2017;265(3):581–9. This SEER database study evaluates women treated for unilateral breast cancer with either breast conserving therapy, unilateral mastectomy, or contralateral prophylactic mastectomy (CPM) between 1998 and 2012. They found no improvement in breast cancer specific or overall survival in the CPM group, while interestingly finding a threefold increase in CPM rate over the study period. https://doi.org/10.1097/SLA.0000000000001698.
King TA, Sakr R, Patil S, Gurevich I, Stempel M, Sampson M, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158–64. https://doi.org/10.1200/JCO.2010.29.4041.
McLaughlin CC, Lillquist PP, Edge SB. Surveillance of prophylactic mastectomy: trends in use from 1995 through 2005. Cancer. 2009;115(23):5404–12. https://doi.org/10.1002/cncr.24623.
Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998-2007. Ann Surg Oncol. 2010;17(10):2554–62. https://doi.org/10.1245/s10434-010-1091-3.
Author information
Authors and Affiliations
Contributions
Steven DeBiase was involved in data acquisition, analysis, writing, and edits. Weihong Sun was involved in data acquisition, interpretation, revision, and final review. David Boulware was involved in statistical analysis and review. Christine Laronga was involved in data acquisition, interpretation, revision, and final review. Marie Catherine Lee was involved in study design, data collection, review of results, and revision and editing of manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hot Topics in Breast Cancer
Rights and permissions
About this article
Cite this article
DeBiase, S.R., Sun, W., Boulware, D. et al. Busting the Contralateral Prophylactic Mastectomy Myth: Incidence of Follow-up Imaging and Biopsy After Risk-Reductive Breast Surgery. Curr Breast Cancer Rep 11, 347–352 (2019). https://doi.org/10.1007/s12609-019-00338-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-019-00338-y