Abstract
Purpose of Reveiw
To compile knowledge of expected intravascular imaging findings in coronary venous and arterial bypass grafts, both at baseline and in diseased states in order to improve understanding of the pathology demonstrated in failing grafts. Also, to learn how intravascular imaging could be used to guide necessary graft interventions and to consider whether imaging practices in grafts could become formalized or potentially influence outcomes of graft interventions, which are notoriously poor.
Recent Findings
Disease in saphenous vein grafts has features overlapping with pathology in native vessels, but can also involve unique findings which have been described in studies and can be delineated well with intravascular imaging. The latter has also been used successfully to examine findings in internal mammary as well as radial artery grafts, particularly in the peri-operative period. Previously reported cases, as well as a case example here, show the feasibility and potential utility of these imaging modalities within grafts to guide coronary intervention.
Summary
Intravascular imaging within bypass grafts is feasible and expected findings can be recognized and understood with review of existing data and with clinical experience gained in individual operator practice. Future directions could explore guidelines for imaging-guided intervention within bypass grafts and could examine whether outcomes could be improved with intravascular imaging guidance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although it is unfortunately under-adopted, the use of intracoronary imaging with intravascular ultrasound (IVUS) and optical coherence tomography (OCT) to optimize stenting results in percutaneous coronary intervention (PCI) in native coronaries represents the evidenced-based standard of care and has been associated with improved patient outcomes. While it is preferable to intervene on native coronary lesions, bypass graft intervention continues to be confronted by interventional cardiologists, particularly in acute infarct settings where alternative complex PCI options may not be immediately feasible. Knowledge of the appearance by IC imaging of normal native coronary structure and pathology is becoming more widespread and though imaging-based guidelines for optimal stent deployment by IVUS or OCT criteria exist in that realm, the use of IC imaging to guide bypass graft intervention seems to be less prevalent in practice and the expected imaging findings and standards for intervention inside grafts appear to be more of a gray area. Moreover, it remains unknown whether factors such as imaging-based optimal stent apposition within grafts translate into longer term graft stent patency or improvement in patient outcomes. Anticipated imaging findings in bypass grafts and potential applications of IC imaging in grafts are reviewed here.
Is There Risk in Advancing Imaging Catheters Within Grafts?
As with advancement of any intravascular equipment, there are theoretical risks with IC imaging catheter advancement and contrast injections within bypass grafts. Most notably, caution would apply to use in the left internal mammary artery (LIMA) graft. It is probably ill-advised to advocate for routine IC imaging of the LIMA graft, particularly in cases of significant tortuosity, as the LIMA graft typically supplies a large portion of the myocardium and devastating consequences can occur with iatrogenic vessel dissection or induction of severe spasm. If imaging of a LIMA is to be undertaken with OCT, it would be key to avoid contrast injection with a dampened pressure waveform and to be cautious with forceful injections. Rough advancement of stiffer conventional IVUS catheters through any tortuous segments of a LIMA should likely be avoided, weighing the risk–benefit ratio for potential complications. Saphenous vein grafts (SVGs) are more forgiving in accommodating imaging catheters, but there are some considerations: culprit lesions in SVG are known to often have a high burden of associated thrombus and higher risk of distal embolization and no-reflow relative to native coronaries. Excessive catheter manipulation or contrast injections for imaging could therefore theoretically lead to higher risk of distal clot embolization, particularly in the absence of the use of distal embolic protection devices. Radial grafts are sometimes smaller in caliber and prone to spasm-IC imaging catheter advancement at these sites could potentially aggravate graft spasm, necessitating the usual interventions for the same. Overall, IC imaging in bypass grafts should be approached carefully in order to minimize potential complications.
IVUS or OCT in Grafts?
Many of the original reports of intravascular imaging in bypass grafts had their basis in IVUS imaging [1, 2]. IVUS in those early studies was able to identify features such as early neointimal hyperplasia in SVG and is still used to help characterize graft pathology in current practice. IVUS has a relative niche in chronic total occlusion PCI as well, where it may help identify graft anastomotic sites in distal vessels and wire positioning after retrograde crossing via bypass grafts with avoidance of contrast injections. However, while there are no head-to-head outcomes-based comparisons of IVUS vs. OCT in bypass graft intervention, it would appear that many of the characteristics known to bypass graft lesion pathology might be better delineated with the higher resolution of OCT vs. IVUS. When Adlam et al. performed elective angiography (n = 42) with OCT (n = 16) and IVUS (n = 21) in patent SVG in asymptomatic patients 3 years after surgery and compared their images, they found that OCT, but not IVUS, identified clear features of atherosclerosis, including circumferential fibrous neointima, thin-cap fibroatheroma, and adherent thrombus [3•]. The suggestion was that high-resolution imaging techniques such as OCT might have an advantage in understanding graft pathology. That being said, IVUS certainly has demonstrated important findings in graft failure previously [4, 5] and with the advent of the newer 60 MHz high definition IVUS platforms (also smaller/more flexible than conventional catheters) combined with the appeal of not needing contrast injection, IVUS holds potential for further growth in this area.
Saphenous Vein Grafts
Baseline-Expected Intracoronary Imaging Appearance
As is well known, both arteries and veins are composed of three layers: the intima, media, and adventitia, and the media and adventitial layers are much thicker in arteries vs. veins. As such, at baseline, a normal healthy vein is expected to be a thin-walled structure [1] with the intima formed by a single layer of squamous epithelium atop an elastic basement membrane with a thin middle layer of smooth muscle cells and an outermost layer of connective tissue elements [6]. In the early stages, OCT imaging of SVG has shown wall thickening with a monolayered appearance and without a visible external elastic membrane [7].
As time passes, IVUS studies have suggested that SVG undergo an “arterialization” process with intimal fibrous thickening, medial hypertrophy, and lipid deposition that creates an echolucent zone around the vessel or double-layered appearance that mimics the arterial external elastic membrane [7].
There are variable reports as to when this appearance can be expected on IC imaging of SVG, with some suggestion that these changes start to occur as early as within the first few weeks after implantation, generally stabilizing in healthy grafts after about 6 months [8].
Early Post-operative Expected Appearance and Findings in Early Failure
Very early vein graft thrombosis (within 1 month or less) happens up to about 15% of the time and is generally due to graft spasm or procedural issues [9]. Both IVUS and OCT-based imaging studies, some ex vivo, have reported imaging findings showing some of the technical injuries to vein grafts occurring, for instance, during the harvesting process. Dissection flaps can be visualized as well as ulceration, intimal injury, and post manipulation thrombus [10]. Canos et al. also reported IVUS findings in early SVG failure (< 1 year post-CABG) vs. later failure (> 1 year post-CABG) and noted the trouble at < 1 year was mostly proximal or ostial in nature, with focal lesions as a cause, but within grafts which tended to be smaller and to have more diffuse overall plaque burden identifiable by IVUS [5].
As vein grafts undergo fibroblast and smooth muscle cell accumulation in the intima with extracellular matrix deposition, the excessive cellular deposition leads to intimal layer expansion termed “neointima” or neointimal hyperplasia (NIH) and also media expansion. NIH is one of the best-known remodeling phenomena in SVG [8, 9].
Vein graft dimensions within 1 month of implantation are similar to those in grafts prior to implantation, with wall thickening accompanied by compensatory enlargement and preservation of luminal area [8]. By 6 months (and in some cases demonstrated as early as 3 weeks to 3 months post-op), however, significant wall thickening has typically occurred, even in angiographically normal grafts [8]. These changes are diffuse and concentric and are observed from the aortic root to the coronary anastomosis. Wall thickening does appear to reach a plateau after 6 months [8].
These early adaptive changes indeed pre-dispose grafts to accelerated atherosclerosis if and when plaque ultimately forms in these vessels.
Late Vein Graft Failure Findings
As noted above, further post-operatively, for instance beyond 6 months, NIH and the echolucent surrounding regions mimicking external elastic lamina appearance is expected. Unfortunately, however, NIH does pre-dispose to accelerated atherosclerosis and atheroma often becomes superimposed with onset of “vein graft disease.” Up to 50% of all vein grafts will be occluded out to 10 years post-operatively [8]. As atherosclerotic lesions progress, the vein, which can initially compensate for mild atherosclerosis, becomes limited by the dense neoadventitia and extensive collagen deposition which limits further vessel enlargement or adaptation to higher atherosclerotic disease burden and the lumen narrows [8]. Inflammation also plays a role and contributes to higher plaque volume and to a higher propensity to rupture/plaque vulnerability. Many individuals with susceptibility will develop occlusive atheroma within a year [8].
A small number of studies have reported imaging findings in these diseased SVGs:
Pregowski et al. reviewed pre-intervention IVUS images in SVG for plaque ruptures and were able to use the IVUS modality to detect 95 ruptured plaques which were compared with control lesions [4]. By IVUS, plaque ruptures were described as areas with a cavity communicating with the lumen and having an overlying residual fibrous cap fragment. IVUS-detected ruptured plaques often had angiographically complex morphology, with ulceration intimal flaps and sometimes aneurysmal segments and IVUS imaging was able to characterize these plaques and showed positive remodeling findings more often than control plaques (70% of the time) and showed eccentricity.
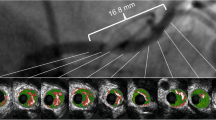
Adlam et al. performed elective angiography (n = 42) with OCT (n = 16) and IVUS (n = 21) assessment of patent SVG in asymptomatic patients 3 years after surgery [3•]. As such, this was SVG imaging outside of the acute coronary syndrome (ACS) setting. Fifty-five percent of SVGs were occluded [3•]. Typical findings in patent SVG were of the double-layered appearance of the vessel wall due to neointima formation with neointimal thickness varying both between patients and within different segments of the grafts (Fig. 1) [3•]. The patent grafts imaged had no significant stenotic disease—the implication was felt to be that there is a high rate of SVG failure by 3 years out from CABG, but that grafts which continued to be patent by that time seemed to have little stenotic disease or were “survivors” of sorts. The authors found that OCT, but not IVUS, was best capable of identifying features of atherosclerosis, including circumferential fibrous neointima, thin-cap fibroatheroma (Fig. 2), and adherent thrombus [3•]. OCT, but not IVUS, demonstrated findings consistent with thin-cap fibroatheroma (TCFA) in 6 of 16 SVGs and luminal adherent thrombus in 4 of 16 SVGs. It was proposed that those features could indicate vein grafts vulnerable to future occlusion and that OCT could be useful in characterizing mechanisms of graft failure/graft pathology.
Neointima in example SVG shown angiographically and with OCT and IVUS. Example images by optical coherence tomography (OCT) (A–D) and intravascular ultrasound (IVUS) (E–H) from saphenous vein grafts (SVGs) shown angiographically (I–L) for the same 4 patients. Typical findings were of a double-layered appearance of the vein graft vessel wall due to neointima formation within the original vein graft wall. Neointimal thickness varied both between patients and within different segments and sectors of the grafts. [3•]
Vein graft atherosclerosis. Areas of thin-cap fibroatheroma (TCFA) identified by OCT (A–D) characterized by a thin bright cap at the luminal surface of the SVG vessel wall with effacement of usual defined features deeper in the vessel wall. This OCT feature has been described in native coronary arteries and correlated histologically with the TCFA overlying a lipid-rich plaque and may be particularly associated with plaque of high macrophage content [2]. OCT features of TCFA were identified in 6 of 16 SVGs assessed with 3 SVGs extensively affected and 3 showing isolated plaques only. Examples affecting either local segments (A, D) or almost concentric involvement (B) are shown. IVUS images from the same segments of the same SVGs do not show a clear definable abnormality (E–H). Angiographically (I–L) SVGs shows only minor irregularity without significant stenotic disease and no specific features at the site of TCFA on OCT (arrows). [3•]
Davlouros et al. examined 28 SVG in 26 patients with ACS [11••]. Angiographically, these SVG lesions were generally complex (96.4%), with ulceration in 32.1% and thrombus in 21.4% [11••]. OCT disclosed a fibrofatty composition in all lesions, calcification in 32.1%, plaque rupture in 60.7%, and thrombus in 46.4% [11••]. Thrombus was progressively more frequent across groups of ACS severity (i.e., more prevalent in STEMI vs. UA) and a thin, fibrous cap was marginally more frequent in myocardial infarction patients [11••]. OCT features of friability were present in 67.9% of SVGs, not correlating with clinical presentation [11••]. As there is little information available on how to define IC findings within bypass grafts, it is worth reviewing how Davlouros et al. categorized their findings in more detail [11••]:
-
→ All cross-sections were divided into 4 quadrants. Lipid was semi-quantified as the number of involved quadrants on the cross-sectional OCT image with lipid-rich plaques being considered involvement of 2 or more quadrants in any plaque images.
-
→ Lipid pools were diffusely bordered, signal-poor regions, typically with overlying signal-rich bands, corresponding to fibrous caps.
-
→ Fibrous tissue appears homogenous with high reflectivity and low attenuation.
-
→ A cutoff point of 65 microns was used to distinguish a TCFA. TCFA appears as a thin, bright cap at the luminal surface with effacement of the usual defined features deeper in the vessel wall.
-
→ Calcifications were well-delineated, low-backscattering heterogeneous regions.
-
→ Plaque ruptures involved a cavity communicating with the lumen with an overlying residual fibrous cap fragment
-
→ Thrombi were either red or white as follows:
-
Red thrombi were high-backscattering protrusions with signal-free shadowing.
-
White thrombi were signal-rich, low-backscattering billowing projections protruding into the lumen.
-
-
→ Areas of degenerated, fragmented graft atheroma or “friable tissue” (noted in 67.9% of the SVG) were described as a layer of tissue in discontinuity with the vein wall, loosely attached to it. This was felt to be of interest as such significant tissue friability and fragmentation is not commonly seen in native coronaries.
Figures 3 and 4 show examples according to IVUS and OCT imaging of diseased SVG pathology including some of the unique features not commonly seen in native coronaries, as well as examples of acute plaque rupture [11••].
OCT of SVG lesions in a patient with an anterior STEMI. A Angiography of a degenerated SVG to right coronary artery, showing narrowing with plaque rupture (arrow). B–F OCT images at the site of the lesion. B, C The fibrous cap (B) and rupture (C) of the thin-cap fibroatheroma (60 um) with a large cavity underneath (arrows). D to F demonstrate signal-rich friable material loosely attached at the SVG wall (arrows). In all OCT frames, fibrofatty composition of the intima is evident. [11••]
OCT findings in SVGs from patients with ACS that are rarely demonstrated in native coronary arteries disease. A–D Arrows point to signal-free zones inside the walls of SVGs. Over these zones, signal-rich tissue is loosely adherent to the SVG wall. As such, images corresponded to areas of severe angiographic degeneration; they were considered an indication of SVG friable tissue. E Arrow points to a signal-free “micro-cavity,” which may represent neovascularization or tissue rupture. G1 to G3 show 3 successive OCT frames depicting tissue rupture with communication of a small cavity with the SVG lumen. In B and F, the wall of the SVG looks very thin with severe circumferential signal attenuation that is probably related to its tissue composition. We called this pattern “sun eclipse.” [11••]
Radial Artery Grafts at Baseline-Expected Intracoronary Imaging Appearance
Intracoronary imaging findings in radial artery (RA) grafts have actually been well outlined in intraoperative evaluations both in situ before harvest and ex vivo after harvest with OCT in particular, which has correlated well with histological section data [10, 12]. The optical reflectance properties of the normal RA show three distinct layers that correspond to the intima, media, and adventitia [10]. The external elastic membrane or boundary between the media and adventitia appears as a sharp line.
Failed Radial Artery Grafts
There is limited data on IC imaging findings in failed RA grafts; however, reports using IC imaging (OCT) have certainly detected some of the immediate post-harvesting manipulation injuries which can occur [10, 12]. Areas of intimal layer injury have been seen, and although less common in the RA vs. SVG, severe dissections have occasionally been detected after harvesting by OCT and have been correlated with histologic findings with locally increased tissue factor and increased potential for thrombogenicity (Fig. 5) [10]. Such injuries might be detectable via in situ IC imaging in early radial graft failure. Significant spasm has also been visualized in post-harvest RA as a loss of luminal area. Grafts with severe spasm and reduced luminal area could be prone to conduit failure.

Reproduced with permission from Brown et al. [10]
Intimal trauma associated with endoscopic harvest. Serial OCT imaging of bypass conduits before and after harvest provided the opportunity to evaluate the safety of novel techniques for vessel procurement such as endoscopic harvest. Unlike traditional open harvesting techniques, this method requires the creation of dissection planes around the vessel pedicle using blunt-tipped conical cannula. While this harvesting step is performed under endoscopic guidance, it creates tension at branch points that does not occur with the open method. This representative example of in situ OCT images obtained from a RA before (A) and after (B) blunt dissection illustrates a unique pattern of vessel injury to the intimal layer, localized within the Ostia of branch points (B, arrowhead). Although less common than trauma in the RA, severe dissections were occasionally imaged within the endoscopically harvested SV (C, E). This incidentally discovered finding was confirmed by comparison to histology (D, F) and was associated with increased local tissue factor activity, suggesting that this vessel segment was likely to be highly thrombogenic if used as a bypass graft.
Brown et al. have described radial arterial graft disease in detail (Fig. 6) [10]:
-
→ Fibrous plaque in RA has been described as a homogeneous region of signal-rich intensity that is eccentrically located on the interior of the vessel.
-
→ Concentric fibrotic neointimal thickening is seen as a bright inner band.
-
→ Fibrocalcific plaque appears as discrete areas of poor signal intensity with well-demarcated borders.
-
→ A fibroatheroma appears as shadowed areas capped by a bright overlying layer.
-
→ High attenuation of light within dense atheromatous tissue creates a shadowing artifact that limits penetration into the deeper media and adventitia layers.
-
→ Lipid-laden foam cells (macrophages) create bright localized reflections with spoke-like shadows on OCT imaging.

Reproduced with permission from Brown et al. [10]
Atherosclerotic plaques in the RA. Using histological sections that were registered to the areas of OCT images, we established that OCT was able to differentiate plaque morphology within RA conduits on a microscopic scale. A fibrous plaque (A, D) is demonstrated by a homogeneous region of signal-rich intensity that is eccentrically located on the interior of the vessel. The additional ability to discriminate the underlying media and adventitia layers illustrates that light penetration is effective in this area. In this example, the boundary between the bright intima and darker media is difficult to discern, but the external elastic membrane that demarcates the boundary between the media and adventitia appears as a sharp line. A fibrocalcific plaque (B) is recognized by discrete areas of poor signal intensity with well-demarcated borders that represent areas of intimal calcification. Here, the concentrically thickened intimal layer is seen as a bright inner band, consistent with the fibrotic neointimal tissue shown in the corresponding histological section (E). A fibroatheroma (C, F) appeared as shadowed areas capped by a bright overlying layer. High attenuation of light within dense atheromatous tissue creates a shadowing artifact that limits penetration into the deeper media and adventitia layers. Lipid-laden foam cells (macrophages), which create bright localized reflections with spoke-like shadows on OCT imaging, were identified by histological appearance (G) and CD68 IHC (H). Note the relatively low density of foam cells in the middle of the plaque and the corresponding region of preserved signal penetration in the OCT image (C).
Mammary Artery Grafts at Baseline-Expected Intracoronary Imaging Appearance
As outlined above in the case of the RA, at baseline, the LIMA or RIMA would be expected to have the usual standard IC imaging appearance for an arterial vessel.
Failed Mammary Artery Grafts—Mechanisms and Expected Intracoronary Imaging Findings
OCT study of the LIMA graft in situ 1 year post-CABG compared with non-harvested RIMA grafts has demonstrated that the grafts develop intimal thickening (65% increase) and non-significant medial thinning relative to the control RIMAs, but vasoactive testing in the limited study demonstrated preservation of the normal endothelium-derived vasodilation suggesting this mild intimal thickening is likely compensatory rather than truly pathologic in these vessels in contrast to the eventual maladaptive way it can behave in SVG once atherosclerosis is established [13]. In addition, select imaging examples of LIMA often show just this intimal thickening, with a lack of significant atherosclerosis [7]. OCT appearance of in situ RIMA and grafted LIMA is shown in Fig. 7 [13].

Reproduced with permission from Porto et al. [13]
Definition of the left internal thoracic artery (LITA) and right internal thoracic artery (RITA) segment used for quantitative coronary angiography (QCA) and frequency-domain-optical coherence tomography (FD-OCT) analysis. The figure shows the segment of LITA and RITA undergoing QCA and FD-OCT analysis. The analysis was targeted on the segment included between the ostium vessel (chosen as proximal reference) and the intersection point between the vessel itself and the superior margin of the second rib (chosen as distal reference). Minimal diameter, maximum diameter, mean diameter, and length of the segment analyzed are visualized in the left inferior part of each panel. As an example, 3 FD-OCT cross-sections (proximal, mid, and distal) of both LITA and RITA are visualized. D indicates distal reference; and P, proximal reference.
The exact nature of failed IMA graft lesions would have to be outlined on a case-by-case basis, as imaging examples at this stage are not available in large series. Of course, technical factors at the anastomotic site could potentially be seen with IC imaging, as would thrombus or atheroma, where it is present. As an example, a recent case report in an acutely failed RIMA graft demonstrated the use of OCT to detect the cause of a stenosis which was found to be a large intramural hematoma compressing the lumen at the anastomosis [14]. There was no flow limitation in that case and the patient was managed conservatively.
Post-Angioplasty Findings
IC imaging can be used to assess for stent expansion/apposition as in native vessel PCI. Also, as with native vessels, assessment in bypass grafts of peri-stent regions for intervention-related injury is possible and perhaps of increased relevance, especially in vein grafts, since while angioplasty is believed to injure vein grafts in a similar manner to arterial angioplasty, the resultant lesions can be more unstable given tissue friability and other factors [11••]. Dissection, intimal injury, and thrombus may be more prevalent or pronounced. Again, there is no significant outcomes-based data in the realm of using this IC imaging to guide graft intervention, so this is extrapolated from data in native coronaries. Furthermore, the predictive value for clinical and angiographic outcomes of data gathered with IC imaging during PCI on grafts in terms of, for instance, plaque composition in SVG, remains unclear.
Rates of restenosis within grafts treated with stents are high, and restenosis, especially in vein grafts, is frequently reported as early as 6 months post-procedure. In general, histologic examination of vein grafts that have been treated with angioplasty show intimal thickening with fibrocollagenous tissue, and fibrotic medial and adventitial layers, at both early and late (> 1 year) time points [6]. As is the case with native arteries, late restenotic lesions after angioplasty often show changes consistent with recurrent atherosclerosis. Tissue prolapse and areas of within-stent dissection have been shown protruding into SVG in case reports post-PCI. IVUS has also shown significant neointimal growth behind stents deployed in SVG at times. In repeated in-stent restenosis cases with multilayer stenting, there can therefore be deep layers of NIH behind the stents with a resultant relatively smaller lumen prone to ongoing restenosis.
Summary of Potential Applications (Table 1)
As was outlined above, both IVUS and perhaps incrementally so, OCT, can be used to characterize in situ pathology in graft lesions/failure.
As in native coronary intervention, IC imaging can be used to assess vessel size/stent size and lesion length and to optimize stent expansion. The correlation of stent apposition in graft intervention to clinical outcomes is unclear at this time. It can also assist in the assessment of peri-stent injury and the potential need for further intervention at stent borders.
IC imaging can also provide unique information about the characteristics of in-stent restenosis in grafts that could help guide further intervention strategies or clinical decision-making about revascularization options. Wolny et al. successfully used IVUS imaging in SVG to determine the mechanism of in-stent restenosis in SVG and found that the issue was predominantly biological with neoatherosclerosis but that earlier presentation did have some mechanical etiologies noted such as anastomotic issues or hinge-points lesions [15•].
With respect to the higher risk of no-reflow phenomenon after SVG stenting, the presence of mobile elements inside SVG (potentially at risk of embolization) has been described by IVUS and OCT and may help predict cases at particularly high risk for this phenomenon with intervention. In addition, the positive remodeling of SVG alone, observed by gray-scale IVUS, has been found to be a predictor of no-reflow phenomenon [8].
OCT has been reported as potentially useful in the cardiac surgical field as an intraoperative tool to select conduits for coronary artery bypass graft (CABG) surgery with the ability to identify both relevant pre-existing pathology and important post-harvesting vessel injury that could portend graft failure [10, 12]. In addition, it can help assess graft anastomotic sites and quality, distinctly examining the mid and distal segments of the anastomosis through to the native coronary when there are early peri-op issues in these regions and can clarify the underlying etiology and inform the treatment plan for the patient [10, 14].
When an angiographic culprit is not clear in ACS, combined with cardiac MRI which may implicate a grafted territory as culprit, IC imaging in grafts may help then confirm a culprit plaque with acute features such as thrombus and plaque rupture and guide intervention as was described in a case report by Xenogiannis et al. [16]. IC imaging can potentially help differentiate bends or kinks in grafts from atherosclerotic or thrombotic disease. In CTO PCI, IC imaging near graft anastomoses with native vessels can help clarify wire position and direct appropriate wire manipulations.
Case Example (Figs. 8, 9, 10, and 11)
Patient case example. Angiogram revealed a culprit critical stenosis within the saphenous vein graft-obtuse marginal (panels A and B, arrows) distal to previous stented segments (panel C, arrows) and close to the distal anastomosis. The native left circumflex was a complex chronic total occlusion (panel D, arrow)
Patient case example. OCT imaging was performed-the initial imaging attempt (panel A) was unsatisfactory as the catheter (asterisk) was near-occlusive within the severely diseased segment (arrow). Visualization was improved after initial balloon dilatation with improved vessel contrast opacification around the OCT catheter (panel B). Panels C and D are examples from the suboptimal initial imaging run prior to ballooning. Despite poor visualization, panel C shows at the distal lesion border, the double-layered type appearance typical of SVG after neointima formation within the vein wall with a less signal-rich “ring” noted (arrow) underlying an area of neointimal hyperplasia and signal-rich severe fibroatheromatous plaque. Panel D just proximal to the angiographic most severe lesion shows an area of friable tissue with a signal-rich layer almost separated from the underlying signal-poor region (arrow)
Patient case example. Imaging after initial ballooning to permit contrast opacification. The graft distal to the culprit lesion (panel A) showed neointimal hyperplasia with double-layer appearance (arrow) with no acute findings and the severe lesion site showed significant fibroatheromatous, signal-rich areas with severe luminal stenosis (panels B and C, asterisk), and more proximally, areas of intimal disruption and probable plaque rupture (panel D, arrows). Proximal to the severe angiographic lesion site, post-initial ballooning, Panels E and F show areas of possible dissection and severe tissue friability (arrows). Panel G shows further severe fibroatheromatous disease and panel H shows a comparatively less diseased proximal segment with more typical vein graft double-layer appearance (arrow)
Panels A shows the segment distal to the distal stent border with no evidence of dissection panels B and C show reasonable stent expansion within the stented segment. Panels D, E, and F show the areas of the previously stented proximal vein graft with imaging quality limited by blood swirl artifact (asterisk), but with reasonable distal expansion post-drug eluting balloon (D, arrows show stent struts) and reasonable apposition of old stents proximally (E, F). G shows the final angiographic result
A 72-year-old male with CABG over two decades prior presented with escalating anginal symptoms, and a nuclear perfusion study demonstrated inferolateral ischemia. He had prior stenting of his SVG to his obtuse marginal branch both 4 years and 2 years prior. Angiogram revealed a culprit critical stenosis within the SVG-OM distal to previous stented segments and close to the distal anastomosis (Fig. 8). There was also in-stent restenosis within the distal aspect of the previously stented segment in the proximal graft. The native left circumflex was a complex chronic total occlusion. OCT imaging was performed—the initial imaging attempt was unsatisfactory as the catheter was essentially occlusive within the severely diseased segment (Fig. 9). As such, initial balloon dilatation was performed followed by repeat OCT, after which better visualization was obtained (Fig. 10). The graft distal to the culprit lesion showed NIH and double-layered appearance as well as atherosclerosis, with no plaque rupture or acute findings. The severe angiographic lesion site itself demonstrated severe luminal narrowing with extensive fibroatheromatous disease and also some areas of suspected plaque rupture or cavitation. Interestingly, the segment above the severe lesion demonstrated areas of almost dissected or separated friable tissue-the friable tissue may have been disturbed by the initial balloon dilatation, but was also seen to a degree on the initial poor quality images prior to ballooning. This area would be planned for stent coverage too, along with a further area of fibroatheromatous luminal narrowing proximally. After further balloon dilation, stenting was performed to the target de novo lesion areas and drug-coated ballooning was performed to the area of in-stent restenosis after high-pressure non-compliant ballooning. A good final result was obtained at the newly stented segment with reasonable stent apposition by OCT (Fig. 11) and although the previously stented site remained poorly visualized due to blood swirl, the stent struts appeared reasonably well expanded there.
Conclusion
Although the longevity of PCI in bypass grafts is poor and treatment of native vessels is always preferable, PCI within bypass grafts continues to be something encountered by interventional cardiologists in dealing with revascularization in post-CABG patients, especially in acute settings. IC imaging with IVUS and OCT can help characterize the often very interesting and unique pathology underlying both de novo lesions in graft failure as well as in-stent restenosis. This can inform and guide interventions in these grafts. This would provide theoretical “optimization” of the PCI result, though this is a gray zone with extrapolation from data in native coronaries. The effect of IC-guided intervention in bypass grafts on stent patency and major adverse cardiac events remains unclear, as do the exact parameters which define an “optimal” result in these heavily diseased conduits. Experience and perhaps future study will help inform this area.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Willard JE, Netto D, Demian SE, Haagen DR, Brickner ME, Eichhorn EJ, et al. Intravascular ultrasound imaging of saphenous vein grafts in vitro: comparison with histologic and quantitative angiographic findings. J Am Coll Cardiol. 1992;19(4):759–64.
Kobayashi T, Makuuchi H, Naruse Y, Sato T, Fujiki T, Ninomiya M, et al. Assessment of saphenous vein graft wall characteristics with intravascular ultrasound imaging. Jpn J Thorac Cardiovasc Surg. 1998;46(8):701–6.
• Adlam D, Antoniades C, Lee R, Diesch J, Shirodaria C, Taggart D, et al. OCT characteristics of saphenous vein graft atherosclerosis. JACC Cardiovasc Imaging. 2011;4(7):807–9. Excellent imaging examples included and includes comparison images between the modalities of intravascular ultrasound and optical coherence tomography inside saphenous nein grafts in an asymptomatic post-bypass conhort.
Pregowski J, Tyczynski P, Mintz GS, Kim SW, Witkowski A, Waksman R, et al. Incidence and clinical correlates of ruptured plaques in saphenous vein grafts: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45(12):1974–9.
Canos DA, Mintz GS, Berzingi CO, Apple S, Kotani J, Pichard AD, et al. Clinical, angiographic, and intravascular ultrasound characteristics of early saphenous vein graft failure. J Am Coll Cardiol. 2004;44(1):53–6.
Collins MJ, Li X, Lv W, Yang C, Protack CD, Muto A, et al. Therapeutic strategies to combat neointimal hyperplasia in vascular grafts. Expert Rev Cardiovasc Ther. 2012;10(5):635–47.
Gonzalo N, Serruys PW, Piazza N, Regar E. Optical coherence tomography (OCT) in secondary revascularisation: stent and graft assessment. EuroIntervention. 2009;5(Suppl D):D93–100.
Murphy GJ, Angelini GD. Insights into the pathogenesis of vein graft disease: lessons from intravascular ultrasound. Cardiovasc Ultrasound. 2004;2:8
Wallitt EJ, Jevon M, Hornick PI. Therapeutics of vein graft intimal hyperplasia: 100 years on. Ann Thorac Surg. 2007;84(1):317–23.
Brown EN, Burris NS, Gu J, Kon ZN, Laird P, Kallam S, et al. Thinking inside the graft: applications of optical coherence tomography in coronary artery bypass grafting. J Biomed Opt. 2007;12(5):051704.
•• Davlouros P, Damelou A, Karantalis V, Xanthopoulou I, Mavronasiou E, Tsigkas G, et al. Evaluation of culprit saphenous vein graft lesions with optical coherence tomography in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2011;4(6):683–93. Among the larger and more detailed series describing intravascular imaging findings in failed saphenous vein grafts in the context of acute coronary syndromes. Includes excellent actual imaging examples cross-referenced with angiography and also highlights pathologic findings differing from those in native coronaries.
Brazio PS, Laird PC, Xu C, Gu J, Burris NS, Brown EN, et al. Harmonic scalpel versus electrocautery for harvest of radial artery conduits: reduced risk of spasm and intimal injury on optical coherence tomography. J Thorac Cardiovasc Surg. 2008;136(5):1302–8.
Porto I, Gaudino M, De Maria GL, Di Vito L, Vergallo R, Bruno P, et al. Long-term morphofunctional remodeling of internal thoracic artery grafts: a frequency-domain optical coherence tomography study. Circ Cardiovasc Interv. 2013;6(3):269–76.
Citarella A, Sheik, A.S., Chowdhary, S., Nwaejike, N. Evaluation of conduit in total arterial revascularization using optical coherence tomography (OCT) - a case report and literature review. Authorea. 2020.
• Wolny R, Mintz GS, Matsumura M, Ishida M, Fan Y, Fall KN, et al. Intravascular ultrasound assessment of in-stent restenosis in saphenous vein grafts. Am J Cardiol. 2019;123(7):1052–9. Highlights imaging-based findings/culprit pathology specifically in the context of in-stent restenosis within saphenous vein grafts.
Xenogiannis I, Lin D, Lesser JR, Hall AB, Cavalcante JL, Brilakis ES, et al. Finding the culprit: combining cardiac magnetic resonance imaging with optical coherence tomography. JACC Cardiovasc Interv. 2019;12(20):2106–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Allison Hall reports personal fees from Medtronic and personal fees from OpSens Medical, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Hall, A.B. Intracoronary Imaging for Bypass Graft Assessment and Intervention. Curr Cardiovasc Imaging Rep 14, 9 (2021). https://doi.org/10.1007/s12410-021-09559-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12410-021-09559-1