Abstract
Purpose of Review
Tissue characterization using imaging is important to understand the pathophysiology of atherosclerosis and subsequent outcomes. In this review, the clinical utility of virtual histology intravascular ultrasound (VH-IVUS), which provides a quantitative and more objective evaluation of tissue characterization compared with grayscale IVUS, will be summarized.
Recent Findings
Patient clinical characteristics, including coronary risk factors and medications, are associated with lesion morphology, especially plaque vulnerability. Different levels of vulnerability cause different clinical presentations and associated long-term outcomes. For example, in the first natural history study (Providing Regional Observations to Study Predictors of Events in the Coronary Tree [PROSPECT]), we showed that large plaque burden, small lumen area, and presence of thin-cap fibroatheroma were associated with subsequent outcomes at 3-year follow-up.
Summary
VH-IVUS has contributed significantly to our understanding of atherosclerosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tissue characterization is important in order to understand the pathophysiology and treatment efficacy of coronary artery disease (CAD); however, reproducible or automatic tissue characterization using grayscale intravascular ultrasound (IVUS) is difficult. Because grayscale IVUS uses only the amplitude of reflected ultrasound (power) from each region of interest, the accuracy to evaluate lipid-rich plaque (a key tissue type to predict future events) is limited. Virtual histology (VH) IVUS has been developed to overcome these limitations [1•].
Using not only amplitude, but also frequency information, VH-IVUS can categorize tissue into four types with acceptable accuracy and reproducibility: necrotic core (NC, red), dense calcium (DC, white), fibrous tissue (dark green), or fibrofatty (light green) (Fig. 1). Based on ex vivo validation of 20 MHz phased array IVUS and using pathology as a gold standard in 94 plaques of 51 coronary arteries, the predictive accuracy is reported as 93.5–96.7% for each tissue type [2]. The predictive accuracy using 45 MHz rotational IVUS is reported to be 85.8–90.8%; this improves to 93.0–96.8% after eliminating regions of interest behind calcium because most ultrasound is reflected at the surface of calcium and the information behind calcium is limited [3]. Hartmann et al. [4] showed reproducibility of the measured volume of each tissue component; relative differences were 0.22–1.12% for intra-observer using the same pullback, 0.85–2.66% for intra-observer using repeated pullbacks, and 1.58–3.85% for inter-observer using the same pullback.
Virtual histology plaque phenotype. a Necrotic core is shown as red, dense calcium is shown as white, fibrofatty is shown as light green, and fibrous tissue is shown as dark green. b Virtual histology (VH) thin-cap fibroatheroma (TCFA) is defined as a fibroatheroma (confluent necrotic core >10%) abutting to the lumen >30° (white double headed arrow) in ≥3 consecutive frames (~1.5 mm in length). If there is a visible fibrous cap (white arrow) on the top of NC, a fibroatheroma is defined as thick-cap fibroatheroma. Pathological intimal thickening is defined as a mixture of fibrofatty plaque, spotty necrotic core, and fibrous tissue. A fibrotic plaque contains mainly fibrous tissue, and a fibrocalcific plaque contains mainly fibrous tissue and dense calcium
In the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study, we developed 5 VH-IVUS plaque phenotypes similar to pathology [5••, 6•, 7] (Fig. 1). Thin-cap fibroatheroma (TCFA) was defined as a plaque having >10% of confluent NC that abutted to the lumen with >30° of circumference in ≥3 consecutive slices. Because of the limited pixel resolution (150–200 μm) of VH-IVUS, it was not possible to detect fibrous cap thickness <65 μm—the pathologic definition of a thin fibrous cap; therefore, the “diagnosis” of VH-TCFA was inferred and presumably included fibroatheromas having a fibrous cap thickness of 65~200 μm. If there was a visible fibrous cap on top of the NC, the plaque was defined as a thick-cap fibroatheroma. If the plaque had a mixture of fibrofatty, spotty NC, and fibrous tissue, it was defined as pathological intimal thickening. Plaque with mainly fibrous tissue was defined as fibrotic plaque, and plaque with mainly DC and fibrous tissue was defined as fibrocalcific plaque. The intra-observer and inter-observer variability yielded good concordance for lesion phenotype (ҡ = 0.90 and 0.87, respectively).
VH-IVUS does not have an algorithm for thrombus. Intramural thrombus is usually colored as fibrous tissue or fibrofatty, and the presence of thrombus reduces the accuracy of VH-IVUS [8].
Clinical Presentation and VH-IVUS Lesion Morphology
Using the largest cohort of 3-vessel non-culprit VH-IVUS lesion analysis from PROSPECT and both culprit and non-culprit lesions from the Assessment of Dual AntiPlatelet Therapy with Drug-Eluting Stents (ADAPT-DES) study, we reported the association between patient clinical characteristics and VH-IVUS lesion morphology.
When we compared culprit lesion morphology among patients who presented with ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation acute coronary syndromes (NSTE-ACS), and stable CAD, STEMI lesions had a higher prevalence of VH-TCFA and plaque rupture along with greater plaque burden and positive remodeling compared with NSTE-ACS or stable CAD lesions [9]. In addition, the relation between clinical presentation and underlying plaque morphology was correlated to patient age and sex [10, 11]. In patients <65 years of age (the median age of this cohort), lesions in men with STEMI or NSTE-ACS had more VH-TCFA and plaque rupture than in women; however, in patients ≥65 years of age, there was no sex-related difference. Patients statin naïve before admission more often presented as ACS and had more VH-TCFA and NC versus those taking statins before admission, suggesting that statin therapy affects lesion morphology and subsequent clinical presentation [12].
We also evaluated anatomical characteristics that were associated with plaque rupture in non-culprit lesions. Overall, 74 ruptured fibroatheromas and 2396 non-ruptured fibroatheromas were identified in non-culprit vessels [13]. The majority of fibroatheromas (73.6%) were located within 40 mm of the ostium, and the vessel area and plaque burden progressively decreased from proximal to distal fibroatheroma location. Shorter distance from the ostium to the maximum NC site, larger plaque burden and vessel area, and right coronary artery location were positively associated with non-culprit plaque rupture. On the other hand, presence of calcium was negatively associated with non-culprit plaque rupture.
Risk Factors and VH-IVUS Morphology
Renal insufficiency affects plaque morphology [14, 15]. Patients with worse renal function have greater amounts of calcium or calcified thick-cap fibroatheroma compared to those with normal renal function in both culprit and non-culprit lesions, indicating a systemic rather than a local effect.
The effect of smoking on plaque morphology has been related to age and severity of atherosclerosis. In patients ≤65 years of age (but not in patients >65 years of age), smoking (current or former) has a vascular constrictive effect (ie, smaller vessel size) that contributes to luminal stenosis severity in non-culprit lesions. On the other hand, smokers >65 years of age have more plaque with greater plaque instability (plaque rupture) in both culprit and non-culprit lesions [16, 17].
High on-treatment platelet reactivity has also been correlated to clinical risk factors and presentation (older age, more diabetes and renal insufficiency, more ACS) [18]. High on-treatment platelet reactivity is associated with increased culprit lesion atherosclerotic burden and adverse plaque morphology (more fibroatheromas) [19].
Chronic Total Occlusion
Fifty CTO lesions after guidewire crossing were evaluated by VH-IVUS. Fibroatheromas were observed in 84% of CTO segments. This prevalence was similar to lesions proximal to the CTO [20].
In-Stent Restenosis
Kang et al. [21] reported VH-IVUS analysis in 117 lesions with angiographic in-stent restenosis and >50% neointimal hyperplasia. Percentages of NC and DC within the in-stent neointima increased over time, suggesting the development of neoatherosclerosis. In a separate cohort of patients who had both VH-IVUS and optical coherence tomography, a large NC was correlated with the presence of neointimal TCFA by optical coherence tomography [22].
Cardiac Transplantation
Two independent groups reported that both NC and DC increased over the time along with an increase in intimal area in patients with cardiac transplant of various duration (1 month to 15 years) [23, 24]. Although VH-IVUS was validated in de novo coronary artery lesions and not in transplant vasculopathy, the results of these findings were consistent with pathological reports or other imaging studies.
Prediction of Distal Embolization
A meta-analysis including 1697 patients, 17% of whom developed distal embolization during stent implantation, showed that the amount of NC by VH-IVUS (absolute amount of NC or amount of NC relative to plaque burden) and the presence of VH-TCFA were significantly greater in the embolization group compared to the non-embolization group [25].
Serial Changes in Tissue Composition After Lipid Lowering Therapy
Hong et al. [26] randomized 100 statin-naïve stable patients to either 20 mg of simvastatin or 10 mg of rosuvastatin; angiographically non-significant lesions were evaluated with VH-IVUS at baseline and 1-year follow-up. Although there were no differences between groups, the amount of NC significantly decreased in the rosuvastatin-treated group from baseline to follow-up (15.5 to 13.0 mm3, p = 0.02) but not in the simvastatin-treated group (15.8 to 14.4 mm3, p = 0.22). Subsequently, Park et al. [27] randomized patients 2:1 to high-dose (40 mg) versus low-dose (10 mg) rosuvastatin in patients who had a VH-IVUS fibroatheroma without physiologic ischemia. Although there was no difference between groups, analysis of the overall cohort showed that %NC decreased significantly from baseline to 12 months (21.3 ± 6.8 to 18.0 ± 7.5%, p < 0.001), and the prevalence of VH-TCFA also decreased from 54.7 to 29.3% (p < 0.001). In the Study of Coronary Atheroma by intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin (SATURN), which compared the effect of 40 mg of rosuvastatin versus 80 mg of atorvastatin, volumetric change of NC was negatively correlated with time-weighted average (on-treatment) levels of serum high-density lipoprotein cholesterol (r = −0.27) and positively correlated with C-reactive protein (r = 0.25). The prevalence of fibroatheroma increased from baseline (33.1%) to follow-up (44.3%) [28]. The Integrated Biomarker and Imaging Study-2 (IBIS-2) study evaluated the efficacy of darapladib (an oral Lp-PLA2 inhibitor) compared to placebo using VH-IVUS and showed that at 12 months there was a significant difference in NC between groups (−5.2 mm3, p = 0.01) without a change in total atheroma volume (p = 0.95) [29].
Dynamic Evolution of Plaque Phenotype
We reported the evolution of plaque phenotype in 216 non-culprit lesions in 99 patients (77% stable CAD) from baseline to 12-month follow-up [30]. Among 20 baseline VH-TCFAs, 15 (75%) became non-TCFAs (13 thick-cap fibroatheromas and 2 fibrotic plaques), and only 5 remained VH-TCFAs. Conversely, 12 new VH-TCFAs appeared, and the overall prevalence of VH-TCFA was similar between baseline and 12-month follow-up.
In patients presenting with STEMI from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial, the frequency of non-culprit VH-TCFA increased from 41% at baseline to 54% at 13-month follow-up [31]. In lesions classified as VH-TCFA, the minimum lumen area (MLA) decreased from 8.1 mm2 (7.4–8.8) at baseline to 7.8 mm2 (7.2–8.4) at follow-up along with an increase in NC.
Prediction of Future Events
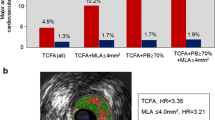
In PROSPECT, there was an 11.6% non-culprit lesion event rate at 3-year follow-up [5••]. Using pre-specified criteria including VH-IVUS plaque phenotype, the presence of VH-TCFA (hazard ratio [HR] 3.21, 95% confidence interval [CI] 1.77–6.36, p < 0.001), MLA ≤4 mm2 (HR 3.21, 95% CI 1.61–6.42, p = 0.001), and plaque burden ≥70% (HR 5.03, 95% CI 2.51–10.11, p < 0.001) were independent predictors of subsequent non-culprit lesion-related major adverse cardiac events (MACE). These results were confirmed in two similar studies. In VH-IVUS in Vulnerable Atherosclerosis (VIVA) and the European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis - Intravascular Ultrasound Study (ATHEROREMO-IVUS), VH-TCFA and large plaque burden (>70%) were associated with non-culprit lesion-related MACE [32,33].
We have reported various post-hoc analyses from PROSPECT. First, we hypothesized that a severe stenosis had a worse outcome compared with a less severe stenosis because of more vulnerable or advanced lesion morphology [34]. When we divided non-culprit lesions into quartiles according to the angiographic diameter stenosis, there were increases in the prevalence of lesions with VH-TCFA, MLA ≤4 mm2, plaque burden ≥70%, plaque rupture, and more NC from mild to severe stenosis along with a greater prevalence of VH-TCFAs having multiple NCs (presumably multiple healed plaque ruptures). Second, we evaluated calcified nodules defined as an irregular and protruding calcium with a convex luminal surface by grayscale IVUS [35]. Patients with calcified nodule were older and had more plaque volume and more VH-TCFA compared to those without. Third, we evaluated the negative predictive value of VH plaque phenotype [36]. When we compared patients without any fibroatheroma versus those with at least one fibroatheroma, 3-year MACE was 3.7 versus 12.9%, p = 0.03, respectively. After adjusting other morphological factors, absence of fibroatheroma was a significant predictor of a lower 3-year rate of non-culprit MACE (HR 0.23, 95%CI 0.06–0.95, p = 0.04). Fourth, we assessed remodeling and reported three different patterns: Negative remodeling (non-culprit remodeling index [vessel area at the MLA divided by reference vessel area] <0.88), intermediate remodeling (remodeling index 0.88–1.00), and positive remodeling (remodeling index >1.0) [37]. Non-culprit lesion-related MACE occurred more often in both the negative and positive remodeling lesions compared with intermediate remodeling lesions. Negative remodeling lesions had the smallest MLA, positive remodeling lesions had the largest plaque burden, and VH-TCFA with multiple NCs (presumably more advanced NC) were most common in negatively remodeling lesions. Lastly, untreated, non-culprit plaque ruptures seen in 14.1% of patients did not impact subsequent MACE [38].
Conclusions
By VH-IVUS, there is a clear and consistent association between clinical characteristics/medications and lesion morphology that predicts subsequent outcomes.
Abbreviations
- ACS:
-
Acute coronary syndromes
- CAD:
-
Coronary artery disease
- CTO:
-
Chronic total occlusion
- DC:
-
Dense calcium
- IVUS:
-
Intravascular ultrasound
- MACE:
-
Major adverse cardiac event
- MLA:
-
Minimum lumen area
- NC:
-
Necrotic core
- NSTE:
-
Non-ST-segment elevation
- STEMI:
-
ST-segment elevation myocardial infarction
- TCFA:
-
Thin-cap fibroatheroma
- VH:
-
Virtual histology
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Nair A, Kuban BD, Tuzcu EM, et al. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–6. This is the first paper to show the quantitative assessment of tissue characterization by VH-IVUS.
Nair A, Margolis MP, Kuban BD, et al. Automated coronary plaque characterization with intravascular ultrasound backscatter: ex vivo validation. EuroIntervention. 2007;3:113–20.
Campos CM, Fedewa RJ, Gracía-Gracía HM, et al. Ex vivo validation of 45MHz intravascular ultrasound backscatter tissue characterization. Eur Heart J Cardiovasc Imaging. 2015;16:1112–9.
Hartmann M, Mattern ES, Huisman J, et al. Reproducibility of volumetric intravascular ultrasound radiofrequency-based analysis of coronary plaque composition in vivo. Int J Cardiovasc Imaging. 2009;25:13–23.
•• Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. This is the first prospective natural history of atherosclerosis study to show the importance of plaque burden, lumen narrowing, and thin-cap fibroatheroma by VH-IVUS to predict future events.
• Maehara A, Cristea E, Mintz GS, et al. Definitions and methodology for the grayscale and radiofrequency intravascular ultrasound and coronary angiographic analyses. JACC Cardiovasc Imaging. 2012;5:S1–9. This describes (in detail) the definitions and methodology of VH-IVUS.
García-García HM, Mintz GS, Lerman A, et al. Tissue characterization using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009;5:177–89.
Nasu K, Tsuchikane E, Katoh O, et al. Impact of intramural thrombus in coronary arteries on the accuracy of tissue characterization by in vivo intravascular ultrasound radiofrequency data analysis. Am J Cardiol. 2008;101:1079–83.
Dong L, Mintz GS, Witzenbichler B, et al. Comparison of plaque characteristics in narrowings with ST-elevation myocardial infraction (STEMI), non-STEMI/unstable angina pectoris and stable coronary artery disease (from the ADAPT-DES IVUS substudy). Am J Cardiol. 2015;115:860–6.
Wang L, Mintz GS, Witzenbichler B, et al. Differences in underlying culprit lesion morphology between men and women: an IVUS analysis from the ADAPT-DES study. JACC Cardiovasc Imaging. 2016;9:498–9.
Qian J, Maehara A, Mintz GS, et al. Impact of gender and age on in vivo virtual histology-intravascular ultrasound imaging plaque characterization (from the global virtual histology intravascular ultrasound [VH-IVUS] registry). Am J Cardiol. 2009;103:1210–4.
Kadohira T, Mintz GS, Souza CF, et al. Impact of chronic statin therapy on clinical presentation and underlying lesion morphology in patients undergoing percutaneous intervention: an ADAPT-DES IVUS substudy. Coron Artery Dis. 2017;28:218–24.
Zheng B, Mintz GS, McPherson JA, et al. Predictors of plaque rupture within nonculprit fibroatheromas in patients with acute coronary syndromes. JACC Cardiovasc Imaging. 2015;8:1180–7.
Baber U, Stone GW, Weisz G, et al. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S53–61.
Chin CY, Mintz GS, Saito S, et al. Relation between renal function and coronary plaque morphology (from the assessment of dual antiplatelet therapy with drug-eluting stents virtual histology-intravascular ultrasound substudy). Am J Cardiol. 2017;119:217–24.
Kang SJ, Mintz GS, Weisz G, et al. Age-related effects of smoking on coronary artery disease assessed by gray scale and virtual histology intravascular ultrasound. Am J Cardiol. 2015;115:1056–62.
Kang SJ, Mintz GS, Witzenbichler B, et al. Age-related effects of smoking on culprit lesion plaque vulnerability as assessed by grayscale and virtual histology-intravascular ultrasound. Coron Artery Dis. 2015;26:476–83.
Kirtane AJ, Parikh PB, Stuckey TD, et al. Is there an ideal level of platelet P2Y12-receptor inhibition in patients undergoing percutaneous coronary intervention?: “window” analysis from the ADAPT-DES study (assessment of dual antiplatelet therapy with drug-eluting stents). JACC Cardiovasc Interv. 2015;8:1978–87.
Yun KH, Mintz GS, Witzenbichler B, et al. Relationship between platelet reactivity and culprit lesion morphology. An assessment from the ADAPT-DES intravascular ultrasound substudy. JACC Cardiovasc Imaging. 2016;9:849–54.
Guo J, Maehara A, Mintz GS, et al. A virtual histology intravascular ultrasound analysis of coronary chronic total occlusions. Catheter Cardiovasc Interv. 2013;81:464–70.
Kang SJ, Mintz GS, Park DW, et al. Tissue characterization of in-stent neointima using intravascular ultrasound radiofrequency data analysis. Am J Cardiol. 2010;106:1561–5.
Kang SJ, Mintz GS, Akasaka T, et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation. 2011;123:2954–63.
König A, Kilian E, Sohn HY, et al. Assessment and characterization of time-related differences in plaque composition by intravascular ultrasound-derived radiofrequency analysis in heart transplant recipients. J Heart Lung Transplant. 2008;27:302–9.
Hernandez JM, de Prada JA, Burgos V, et al. Virtual histology intravascular ultrasound assessment of cardiac allograft vasculopathy from 1 to 20 years after heart transplantation. J Heart Lung Transplant. 2009;28:156–62.
Jang JS, Jin HY, Seo JS, et al. Meta-analysis of plaque composition by intravascular ultrasound and its relation to distal embolization after percutaneous coronary intervention. Am J Cardiol. 2013;111:968–72.
Hong MK, Park DW, Lee CW, et al. Effects of statin treatments on coronary plaques assessed by volumetric virtual histology intravascular ultrasound analysis. JACC Cardiovasc Interv. 2009;2:679–88.
Park SJ, Kang SJ, Ahn JM, et al. Effect of statin treatment on modifying plaque composition: a double-blind, randomized study. J Am Coll Cardiol. 2016;67:1772–83.
Puri R, Libby P, Nissen SE, et al. Long-term effect of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur H J Cardiovasc Imaging. 2014;15:380–8.
Serruys PW, Gracía-Gracía HM, Buszman P, et al. Effect of the direct lipoprotein-associated phospholipase A2 inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–82.
Kubo T, Maehara A, Mintz GS, et al. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol. 2010;55:1590–7.
Zhao Z, Witzenbichler B, Mintz GS, et al. Dynamic nature of nonculprit coronary artery lesion morphology in STEMI. A serial IVUS analysis from the HORIZONS-AMI trial. JACC Cardiovasc Imaging. 2013;6:86–95.
Calvert PA, Obaid DR, O’Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease. The VIVA (VH-IVUS in vulnerable atherosclerosis) study. JACC Cardiovasc Imaging. 2011;4:894–901.
Cheng JM, Gracía-Gracía HM, de Boer SP, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur Heart J. 2014;35:639–47.
Yun KH, Mintz GS, Farhat N, et al. Relation between angiographic lesion severity, vulnerable plaque morphology and futures adverse cardiac events (from the providing regional observations to study predictors of events in the coronary tree study). Am J Cardiol. 2012;110:471–7.
Xu Y, Mintz GS, Tam A, et al. Prevalence, distribution, predictors, and outcomes of patients with calcified nodules in native coronary arteries. A 3-vessel intravascular ultrasound analysis from providing regional observations to study predictors of events in the coronary tree (PROSPECT). Circulation. 2012;126:537–45.
Dohi T, Mintz GS, McPherson JA, et al. Non-fibroatheroma lesion phenotype and long-term clinical outcomes. A substudy analysis from the PROSPECT study. JACC Cardiovasc Img. 2013;6:908–16.
Inaba S, Mintz GS, Farhat NZ, et al. Impact positive and negative lesion site remodeling on clinical outcomes. Insights from PROSPECT. JACC Cardiovasc Imaging. 2014;7:70–8.
Xie Y, Mintz GS, Yang J, et al. Clinical outcome of nonculprit plaque ruptures in patients with acute coronary syndrome in the PROSPECT study. JACC Cardiovasc Imaging. 2014;7:397–405.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Akiko Maehara reports grants and personal fees from Boston Scientific Corporation, grants and personal fees from St Jude Medical, and personal fees from OCT Medical Imaging Inc., outside the submitted work. Gary S. Mintz reports grants and personal fees from Boston Scientific Corporation, grants and personal fees from Volcano Corporation, personal fees from ACIST, and grants from St Jude Medical, outside the submitted work.
Human and Animal Rights and informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Maehara, A., Mintz, G.S. Clinical Utility of Virtual Histology Intravascular Ultrasound. Curr Cardiovasc Imaging Rep 10, 29 (2017). https://doi.org/10.1007/s12410-017-9426-0
Published:
DOI: https://doi.org/10.1007/s12410-017-9426-0