Abstract
Noncardiac incidental findings on cardiac CT are remarkably common and some of these may have a significant impact on patient management. Herein, we present a straightforward and cost-effective step-by-step approach for identifying and reporting noncardiac incidental findings. In Step 1, we discuss the ‘ABCDEFG’ search pattern for systematically reviewing noncardiac organ systems. The most prevalent and clinically significant incidental findings are highlighted with strategies for increasing their conspicuity. In Step 2, the importance of reviewing clinical history and prior imaging studies is discussed. In Step 3, we provide a classification scheme and follow-up recommendations for incidental findings based on their potential clinical significance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac computed tomography (CT) is an effective, noninvasive technique for imaging the heart and coronary arteries [1]. Raw data from cardiac CT includes substantial portions of the lungs, mediastinum, chest wall, and upper abdomen. Noncardiac incidental findings (IFs) on cardiac CT are remarkably common and some may have a significant impact on patient management. In a meta-analysis of over 15,800 cardiac CT exams from 19 studies, Flor et al [2] reported the mean pooled prevalence of noncardiac IFs was approximately 44 %. Clinically significant noncardiac IFs, defined as those requiring immediate therapy, intervention, additional imaging, or clinical follow-up, were identified in 16 % of cardiac CT exams. Although rare, incidental malignancies are also depicted in 0 %–1.2 % of exams [3]. With typical cardiac CT settings, only one-third of the total thoracic volume is displayed. However, when raw data is reconstructed at a maximum field-of-view (FOV), greater than 70 % of total thoracic volume will be displayed. Reconstructed FOV size directly impacts the detection of IFs, with most clinically significant IFs only identified on studies with maximized FOV reconstructions [4, 5]. Because noncardiac IFs are common and may alter management, it is in the best interest of the patient to have the noncardiac findings reviewed by a qualified imaging specialist.
Whether or not to search for noncardiac IFs on cardiac CT has been a topic of debate. Some critics argue that searching for IFs contributes to greater costs, increases diagnostic testing, and radiation dose, and increases patient and referring physician anxiety without proven health benefits [6, 7]. However, recent data suggest that downstream costs associated with noncardiac IFs on cardiac CT are modest. For example, in a study of 151 patients who underwent cardiac CT, Lee et al [7] reported the average direct cost of additional diagnostic workup was only $17 per patient screened and $438 per patient with imaging follow-up. Downstream testing may be further limited when prior studies are reviewed and written follow-up recommendations provided within the CT report. In addition, no study has demonstrated that IFs contribute to patient or referring physician anxiety.
Herein, we provide a comprehensive, cost-effective 3-step guide for identifying and reporting noncardiac IFs on cardiac CT. In Step 1, the reader should undertake a systematic review for noncardiac IFs using the “ABCDEFG” search pattern mnemonic: abdomen, bones, chest wall, diaphragm and pleura, esophagus and mediastinum, lung fields, and the great vessels and aorta. As the reader examines through these various organ systems, CT window display settings should be adjusted to facilitate detection of noncardiac pathology. In addition, structures may be reviewed in multiple imaging planes (including sagittal and coronal planes) to help localize pathology and clarify anatomy that is not clearly depicted in the axial plane. In Step 2, prior imaging studies and the patient’s clinical history should be clarified, as this information may influence the management of IFs. In Step 3, noncardiac IFs should be grouped into 1 of 4 categories based on their clinical significance, and explicit follow-up recommendations should be provided. While a comprehensive review of all potential noncardiac IFs is beyond the scope of this paper, the most prevalent and most clinically significant IFs will be discussed.
Step 1: Search for Noncardiac IFs Using the ABCDEFG Search Pattern
Abdomen
A limited portion of the upper abdomen is often visualized on cardiac CT, which may include portions of the liver, spleen, adrenal glands, and bowel. Excluding the presence of solid organ mass lesions is the most important task within the abdomen. Abdominal structures are best evaluated using soft tissue windows settings (center 40 HU, width 400 HU). The liver should also be evaluated using liver window settings (center 75 HU, width 150 HU) [8].
The simple liver cyst is one of the most common noncardiac IFs detected in the abdomen, occurring in roughly 5 % of the population [9] and found on 1.8 % of cardiac CT exams [10]. Simple liver cysts are rounded, fluid density lesions (-20 to +20 HU) with sharply defined margins and no internal enhancement (Fig. 1). They are benign and do not warrant follow-up imaging so long as their size measures less than 4 cm. For simple cysts larger than 4 cm, follow-up ultrasound can be recommended to document stability. To qualify as a simple cyst, the lesion should have no internal septations, no mural nodularity, and no wall calcifications.
Contrast-enhancing liver lesions are also common IFs, and may represent either a benign or malignant process. The most common benign enhancing liver lesion is the hepatic hemangioma, which is highly vascular and classically demonstrates peripheral nodular enhancement. Malignant liver lesions are most often metastases from a colon, breast, or lung cancer primary tumor. Features favoring a malignant etiology include multiple lesions with poorly defined margins, biliary ductal dilation, and portal vein thrombosis. Reviewing the patient’s clinical history and prior imaging studies is of upmost importance. If a hepatic lesion has been stable in size for over 2 years, it is unlikely to be malignant.
Hepatic steatosis, or fatty liver, is the most common chronic liver disease in the United States and is frequently associated with obesity and the metabolic syndrome. Fatty liver can be diagnosed on noncontrast CT when the attenuation of the liver is lower than that of the spleen [11]. Comparing differences in attenuation is less reliable on contrast-enhanced exams due to differences in enhancement patterns of the liver and spleen.
Adrenal masses are less common IFs on cardiac CT, with an estimated prevalence of 0.2 % [10]. Most are benign, and adrenal adenomas represent the most common adrenal mass. Adrenal adenomas are most commonly lipid-rich and can be diagnosed by nonenhanced CT if the mass has a density measuring less than 10 HU. For adrenal masses identified on contrast enhanced CT exams, comparison should be made with prior to imaging studies. If an adrenal mass has been stable for over 2 years, additional follow-up is typically unnecessary. If stability cannot be confirmed, follow-up CT or MRI can be performed to confirm a benign adenoma.
Bones
Bony structures of the thorax include the sternum, ribs, and thoracic spine. When images are reconstructed with a maximum FOV, the scapulae, clavicles, and humeral heads may also be depicted, depending on the z-axis coverage. The most important lesion to exclude is an osseous metastasis. Bones should be evaluated using bone window settings (center 500, width 2000). The thoracic spine is best surveyed on sagittal and coronal images.
Solitary bone lesions are common IFs and may demonstrate either focal increased (sclerotic) or decreased (lytic) density. The differential diagnosis for a solitary bone lesion is broad, ranging from clearly benign findings to malignancy. A commonly encountered sclerotic lesion is the bone island, representing a focus of compact (cortical) bone within the medullary cavity. Bone islands are homogenously sclerotic and featureless, and often demonstrate hazy margins along their periphery (Fig. 2). Bone metastases may also appear sclerotic, particularly those from prostate and breast malignancies. However, their prevalence is low (0.1 %) on cardiac CT [10]. Features favoring sclerotic metastases include multiplicity, bone destruction, and history of prostate or breast cancer.
Solitary sclerotic lesion. (a) Axial and (b) sagittal images in bone windows demonstrate a solitary, homogenous sclerotic lesion (arrow) within an upper thoracic vertebral body, statistically likely a benign bone island. An osseous metastasis could have a similar appearance in a patient with prostate or breast cancer
Healing rib fractures are frequent benign findings encountered on cardiac CT and demonstrate smooth, irregular contours with callus formation. Sharp margins and discontinuity suggest acute fracture. Vertebral compression fractures are most common in elderly women with osteoporosis. They are demonstrated by wedge-shaped loss of anterior vertebral body height, typically in the middle or lower thoracic spine (Fig. 3). Compression fractures are most conspicuous in the sagittal plane (image). If the compression fracture is a new finding, follow-up is warranted as the patient may be at risk for additional fractures [12].
Chest Wall
Survey of the chest wall should include a review of the breasts and subcutaneous soft tissues using soft tissue window settings (center 40 HU, width 400 HU). Axial and sagittal imaging planes are most helpful for depicting chest wall pathology. In a review of 503 cardiac CT exams by Onuma et al [13], incidental breast masses were detected in 0.8 % of patients. Patchy, symmetric fibroglandular tissue is a normal finding in premenopausal female breasts. CT features most predictive of breast malignancy include focal masses with irregular margins and peripheral (rim) enhancement (Fig. 4) [14]. When calcifications are identified in the breast on CT, they are nearly all benign. The patient’s past surgical history and prior imaging studies should be reviewed when a focal breast lesion is encountered, as postoperative changes may mimic a malignancy.
Pectus deformity of the chest wall may be seen on cardiac CT. The most common variant is pectus excavatum, represented by a depressed sternum with the anterior ribs protruding anterior to the sternum. Commonly the heart is rotated and displaced to the left. This entity occurs more often in males (4:1) and may be associated with mitral valve prolapse and scoliosis [15]. Reporting pectus excavatum deformities is important as it may explain an abnormal appearance of the right ventricle on echocardiography.
Diaphragm and Pleura
Sagittal and coronal planes can be helpful for identifying IFs of the pleural and diaphragm. Images should be reviewed in soft tissue CT window settings (center 40 HU, width 400 HU). Lung settings (center -500, width 1800) are best to exclude pneumothorax, an abnormal collection of air within the pleural space. Pneumothorax is most often a complication of blunt or penetrating traumatic injury or iatrogenic and is infrequently encountered on cardiac CT. However, pneumothorax is an emergent finding that usually warrants immediate attention.
Pleural effusions are common IFs on cardiac CT. In a review of 1764 exams by Koonce et al [10], pleural effusions were identified in 2.8 % of patients. Most effusions are simple fluid collections measuring between 0–20 HU within the dependent thorax. Often a meniscus is formed along the posterolateral chest wall. While usually benign, follow-up imaging may be warranted to ensure resolution over time. Complicated, or exudative, pleural effusions are more concerning and warrant closer attention. Exudative effusions are most often the sequelae of pulmonary infection, and are less frequently associated with malignancy. Specific features suggesting exudative effusion include loculation, subjacent pleural thickening, unilateral effusion, higher density fluid, and small bubbles of air within the fluid collection [16].
Congenital defects in the diaphragm may be seen at cardiac CT and are usually of no clinical concern. They are optimally depicted on sagittal or coronal planes by demonstrating transdiaphragmatic herniation of abdominal fat into the thorax. Posterior (Bochdalek-type) hernias are more common than anterior (Morgagni-type) hernias, and both variants are more frequently right sided (Fig. 5) [17]. If the hernia is large, solid organs may protrude through the defect.
Esophagus and Mediastinum
Survey of the esophagus and mediastinum should be performed using soft tissue CT window settings (center 40 HU, width 400 HU). Hiatal hernia is one of the most common noncardiac IFs detected on cardiac CT. In a review of 395 cardiac CT exams, Lehman et al [18] reported a 3.5 % incidence of hiatal hernia. The prevalence of hiatal hernia increases with age and obesity. Hiatal hernias are best depicted on sagittal and coronal CT images by upward migration of the gastroesophageal junction and proximal stomach above the level of the esophageal hiatus. While hiatal hernias are most often asymptomatic, they may account for a patient’s chest pain and should be reported.
Mediastinal lymph node enlargement is another common noncardiac IF on cardiac CT, with a reported prevalence ranging from 0.7 %–2.3 % [10, 18, 19]. There are many causes of lymph node enlargement, including pulmonary infection, collagen vascular disease, sarcoidosis, and neoplastic processes (metastatic disease, lymphoma). When attempting to elucidate the significance of enlarged lymph nodes, many factors should be considered, including the distribution and number of enlarged lymph nodes and the patient’s clinical history. In general, lymph nodes within the superior mediastinum should measure less than 7 mm in the short-axis dimension, and lower paratracheal and subcarinal lymph nodes should measure up less than 11 mm (Fig. 6) [20]. A common approach is to consider all mediastinal lymph nodes enlarged if they measure greater than or equal to 10 mm in short-axis. While there are no specific follow-up guidelines for incidentally encountered enlarged lymph nodes, follow-up should be recommended for patients with known or suspected primary malignancy.
Lung Fields
The term lung ‘fields’ has fallen out of favor and is not part of the official nomenclature of chest imagers. ‘Field’ is used here solely to allow use of the systematic review using the “ABCDEFG” search pattern mnemonic. The lung parenchyma and airways should be surveyed first using lung window CT settings (center -500, width 1800). Next, the pulmonary arteries should be evaluated using soft tissue windows (center 40 HU, width 400 HU) with manual adjustment to avoid complete whiteout of the pulmonary arterial lumen.
Pulmonary embolism (PE) is an uncommon but emergent IF on cardiac CT that should be excluded, particularly in patients presenting with acute chest pain. Koonce et al [10] reported a 0.4 % prevalence at cardiac CT. On contrast-enhanced exams, PE are identified as partial or complete filling defects within an opacified pulmonary artery (Fig. 7 ). Sensitivity for detecting PE is influenced by the degree of pulmonary artery contrast opacification. As most cardiac CT exams are acquired in the arterial phase, there is paucity of contrast within the pulmonary circulation and evaluation for PE is limited. Nevertheless, large and proximal emboli are still commonly distinguishable on arterial phase acquisitions. Secondary signs of PE include a dilated main pulmonary artery (greater than 3 cm in diameter), right ventricular enlargement, and bowing of the interventricular septum toward the left ventricle. These signs portend a poor prognosis and should prompt emergent management [21].
Lung nodules are the most common pulmonary IF detected on cardiac CT, with a reported prevalence of 5 %–20 % depending upon the patient population being studied [2]. However, based on a meta-analysis of 15 studies, clinically significant or indeterminate nodules requiring intervention or follow-up imaging present in only 3 % of patients [3]. Detection of lung nodules is enhanced by utilization of maximum intensity projections (MIP) [22]. While lung nodules may be benign or malignant in etiology, several CT features can assist the reviewer in their classification to guide appropriate follow-up and management. For example, several patterns of calcification are predictive of benign and malignant nodules. Benign patterns of calcification include central, concentric, popcorn, and diffuse (homogenous) calcification. Malignant patterns include eccentric (asymmetric) and amorphous calcification. The presence of macroscopic fat within a well circumscribed nodule is essentially pathognomonic for a benign pulmonary hamartoma, especially if the lesion has been stable in size over time (Fig. 8).
Nodule size is a very important criterion for predicting the likelihood of malignancy. In a meta-analysis of 8 large screening trials [22], the prevalence of malignancy correlated with nodule size, ranging from 0–1 % for nodules 5 mm or smaller, 6 %–28 % for nodules between 5 and 10 mm, and 64 %–82 % for nodules 20 mm or larger. Nodules larger than 30 mm are termed masses, and virtually all are malignant. The Fleischner Society has issued guidelines for CT nodule follow-up based on size at first detection, and all cardiac CT reviewers should be familiar with these evidence-based criteria [23]. Prior chest CTs must be reviewed before follow-up recommendations are provided in order to prevent unnecessary follow-up imaging of known stable nodules. Generally, lung nodules are considered benign if stability can be documented over a 2 year period. Longer follow-up periods may be necessary for part-solid or ground-glass nodules to exclude slow-growing adenocarcinoma [23]. Nodules with spiculated margins are more concerning for malignancy (Fig. 9).
Consolidation is another IF occasionally seen at cardiac CT, identified by focal, patchy, or diffuse homogenous attenuation that obscures the underlying pulmonary architecture and may exhibit air bronchograms (visualization of air-filled bronchi within the opacity) (Fig. 10). The underlying abnormality is the presence of blood, pus, or fluid replacing alveolar air spaces. While the CT appearance usually implies infection (bacterial, viral, or fungal), consolidation can also be seen in pulmonary edema, pulmonary hemorrhage, eosinophilic lung disease, pulmonary infarction, and neoplastic conditions such as lymphoma and bronchoalveolar carcinoma [24]. Reviewing the patient’s clinical information is imperative for elucidating the etiology of consolidation. In most cases follow-up imaging is warranted to document resolution or improvement with therapy.
Emphysema is found in approximately 9 % of patients undergoing chest CT and 2.1 % of patients undergoing cardiac CT [9, 10]. Emphysema appears as regions of lung destruction with low attenuation and the lack of visible walls (Fig. 11). As emphysema becomes more severe, regions of lung destruction become more confluent. Centrilobular emphysema is the most common subtype and has a strong association with cigarette smoking. Lung destruction is most predominant in upper lung fields. Bullae and blebs are focal air-containing spaces surrounded by thin walls less than 1 mm thick. Both entities are subpleural in location and are encountered most often in the lung apices. Bullae rupture is a known cause of spontaneous (nontraumatic) pneumothorax.
Great Vessels and Aorta
Because the clinical syndrome of acute aortic disease can overlap that of acute coronary syndrome, the visualized thoracic aorta and origins of the great vessels if included (brachiocephalic artery, left common carotid artery, and left subclavian artery) should be reviewed on all cardiac CT exams using soft tissue window settings (center 40 HU, width 400 HU). Often several important aortic diagnoses can be made or suggested on cardiac CT, since the visualized anatomy includes both the origin of the aorta (ie, the aortic annulus) as well as the descending thoracic aorta at these same levels [25].
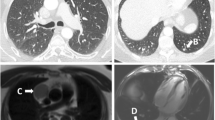
Three pathologies comprising the acute aortic syndrome warrant evaluation, particularly for the evaluation of acute chest pain: penetrating atherosclerotic ulcer (PAU), intramural hematoma (IMH), and aortic dissection. In the case of PAU, atheroma of the aortic wall disrupts the vasa vasorum causing focal ischemia of the aortic wall blood supply. PAU itself can cause pain, and it is treated as a potential aortic dissection since the process can lead to frank dissection if left unmanaged. IMH is considered a dissection equivalent, representing an unseen disruption of the intimal layer of the aorta with subtle hemorrhage into the medial layer of the aortic wall. It is best detected as intramural hyperdensity on noncontrast images, or more subtly, as irregular thickening of the aortic wall on contrast-enhanced images. Aortic dissection is identified by a distinct intimal flap with dissection of blood below the intimal layer (Fig. 12). In most cases, ECG-gated CT allows for straightforward identification of the intimal flap, which can be highly mobile and cause dynamic obstruction, including obstruction of the coronary ostia. In all 3 conditions, if the full extent of aortic pathology is not imaged, additional CT, or MRI imaging should be recommended [26]. Each should be considered potentially life-threatening and requires direct communication with the referring physician.
Finally, the thoracic aorta should be evaluated for dilatation and aneurysm. Generally, a thoracic aortic diameter greater than 3.5 cm is considered enlarged, and a diameter greater than 4.5 cm is considered aneurysmal. Although the specific threshold for abnormality varies with genetic syndromes, age, and body surface area, ECG-gated cardiac CT offers an ideal means to measure aortic diameter, allowing for robust visualization of the aortic wall compared to other modalities [25]. When available, end-systolic images should be reviewed at specific locations along the aortic wall, including the aortic annulus, sinuses of Valsalva, and the sinotubular junction [27]. Diameter measurements should be made on double-oblique, short axis planes to prevent over-sizing of the vessel due to obliquity.
Step 2: Review Clinical History and Prior Imaging Studies
To ensure cost-effective management of noncardiac IFs, the patient’s clinical history and prior imaging studies should be reviewed by a qualified specialist before providing follow-up recommendations. Clinical history can play an important role in the analysis and interpretation of noncardiac IFs, especially for incidental pulmonary consolidations and solitary bone lesions. For example, a lower lobe pulmonary consolidation in a febrile patient with elevated white blood cell count and productive cough is most compatible with pneumonia. A 1 cm sclerotic nodule in the rib of an otherwise healthy young patient with no history of malignancy is almost certainly a benign lesion (ie, bone island). The same lesion in an elderly male with history of prostate cancer would raise greater suspicion for an osseous metastasis, prompting closer follow-up. Reviewing relevant prior imaging studies is equally important for documenting the stability of IFs and to prevent unintentional duplication of follow-up CT exams. This is particularly important for patients in whom pulmonary nodules are incidentally discovered at cardiac CT. Consider, for example, a 65 year old male smoker in whom a 4 mm pulmonary nodule is incidentally discovered. If the patient had no prior CT imaging studies, Fleischner Society guidelines [23] would recommend interval CT follow-up at 12 months. However, if the patient had a chest CT performed 3 years prior, which demonstrated the same 4 mm nodule, no additional follow-up would be necessary. To facilitate the review of clinical history and prior imaging studies, cardiac CT exams should be reviewed on workstations with access to PACS and integrated electronic health records.
Step 3: Classify IFs Based on Clinical Significance and Provide Follow-Up Recommendations
Once the patient’s clinical history and relevant prior imaging studies have been reviewed, all noncardiac IFs should be classified into 1 of 4 categories based on their clinical significance: emergent, requiring immediate therapy; potentially clinically significant or indeterminate, requiring additional work-up; likely benign, outpatient follow-up recommended; or definitely benign/not important, usually no follow-up required (Table 1). As mentioned above, Fleischner Society guidelines [23] provide specific follow-up recommendations for incidentally discovered pulmonary nodules based on nodule size and the pre-test probability for lung cancer. It should be noted, however, that Fleischner Society guidelines do not apply to patients with known history of malignancy. Any newly discovered pulmonary nodule in a patient with history of malignancy should be classified as indeterminate, and follow-up management should be determined by protocol or the referring physician or oncologist.
Follow-up recommendations should also be provided for IFs described within the CT report. Emergent and potentially clinically significant/indeterminate findings should be described within the Impression section. Existing evidence-based guidelines, such as those put forth by the Fleischner Society, should guide the reviewer to recommend follow-up imaging studies at intervals outlined within consensus documents [28]. Incorporating real-time decision support into cardiac CT reporting systems may improve conformance to evidence-based guidelines.
Conclusions
Reviewing cardiac CT exams for noncardiac IFs requires careful evaluation and reporting. A particularly thorough search for IFs should be made in older patients and in cases evaluating CABG graft patency, as these exams are likely to yield a greater number of clinically significant noncardiac Ifs [10]. While currently there are no professional consensus statements on the best approach to reading cardiac CT exams for noncardiac IFs, our 3-step process outlined above offers a straightforward and cost-effective strategy. Noncardiac structures should be reviewed by appropriately trained readers, and follow-up recommendations should adhere to established evidence-based consensus documents where available.
References
Mark DB, Berman DS, Budoff MJ, et al. Accf/acr/aha/nasci/saip/scai/scct. 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on expert consensus documents. Circulation. 2010;121:2509–43.
Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. Am J Roentgenol. 2013;201:555–64.
Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261:342–6.
Kim JW, Kang EY, Yong HS, et al. Incidental extracardiac findings at cardiac CT angiography: comparison of prevalence and clinical significance between precontrast low-dose whole thoracic scan and postcontrast retrospective ECG-gated cardiac scan. Int J Cardiovasc Imaging. 2009;25 Suppl 1:75–81.
Kim TJ, Han DH, Jin KN, et al. Lung cancer detected at cardiac CT: prevalence, clinicoradiologic features, and importance of full-field-of-view images. Radiology. 2010;255:369–76.
Budoff MJ, Fischer H, Gopal A. Incidental findings with cardiac CT evaluation: should we read beyond the heart? Catheter Cardiovasc Interv. 2006;68:965–73.
Lee CI, Tsai EB, Sigal BM, et al. Incidental extracardiac findings at coronary CT: clinical and economic impact. Am J Roentgenol. 2010;194:1531–8.
Mayo-Smith WW, Gupta H, Ridlen MS, et al. Detecting hepatic lesions: the added utility of CT liver window settings. Radiology. 1999;210:601–4.
Killeen RP, Cury RC, McErlean A, et al. Noncardiac findings on cardiac CT. Part II: spectrum of imaging findings. J Cardiovasc Comput Tomogr. 2009;3:361–71.
Koonce J, Schoepf JU, Nguyen SA, et al. Extra-cardiac findings at cardiac CT: experience with 1764 patients. Eur Radiol. 2009;19:570–6.
Boyce CJ, Pickhardt PJ, Kim DH, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. Am J Roentgenol. 2010;194:623–8.
Von Feldt JM. Managing osteoporotic fractures minimizing pain and disability. J Clin Rheumatol. 1997;3:65–8.
Onuma Y, Tanabe K, Nakazawa G, et al. Noncardiac findings in cardiac imaging with multidetector computed tomography. J Am Coll Cardiol. 2006;48:402–6.
Harish MG, Konda SD, MacMahon H, et al. Breast lesions incidentally detected with CT: what the general radiologist needs to know. Radiographics. 2007;27 Suppl 1:S37–51.
Restrepo CS, Martinez S, Lemos DF, et al. Imaging appearances of the sternum and sternoclavicular joints. Radiographics. 2009;29:839–59.
Aquino SL, Webb WR, Gushiken BJ. Pleural exudates and transudates: diagnosis with contrast-enhanced CT. Radiology. 1994;192:803–8.
Sandstrom CK, Stern EJ. Diaphragmatic hernias: a spectrum of radiographic appearances. Curr Prob Diagn Radiol. 2011;40:95–115.
Lehman SJ, Abbara S, Cury RC, et al. Significance of cardiac computed tomography incidental findings in acute chest pain. Am J Med. 2009;122:543–9.
Kawano Y, Tamura A, Goto Y, et al. Incidental detection of cancers and other noncardiac abnormalities on coronary multislice computed tomography. Am J Cardiol. 2007;99:1608–9.
Genereux GP, Howie JL. Normal mediastinal lymph node size and number: CT and anatomic study. Am J Roentgenol. 1984;142:1095–100.
Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the Pioped II investigators. Radiology. 2007;242:15–21.
Brandman S, Ko JP. Pulmonary nodule detection, characterization, and management with multidetector computed tomography. J Thor Imaging. 2011;26:90–105.
MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237:395–400.
Kazerooni EA. High-resolution CT, of the lungs. Am J Roentgenol. 2001;177:501–19.
Chung JH, Ghoshhajra BB, Rojas CA, et al. CT angiography of the thoracic aorta. Radiol Clin NA. 2010;48:249–64. VII.
Gomes AS, Bettmann MA, Boxt LM, et al. Acute chest pain—suspected aortic dissection. Am Coll Radiol. ACR appropriateness criteria. Radiology. 2000;215(Suppl):1–5.
Lin FY, Devereux RB, Roman MJ, et al. Assessment of the thoracic aorta by multidetector computed tomography: age- and sex-specific reference values in adults without evident cardiovascular disease. J Cardiovasc Comp Tomogr. 2008;2:298–308.
Kassing P, Duszak R. Neiman report brief #02: repeat medical imaging: a classification system for meaningful policy analysis and research. Harvey L. Neiman Health Policy Institute. 2013.
Compliance with Ethics Guidelines
Conflict of Interest
Christopher D. Maroules, Brian B. Ghoshhajra, Nagina Malguria, Michael Landay, Jed Hummel, Maros Ferencik, and Suhny Abbara declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Cardiac Computed Tomography
Rights and permissions
About this article
Cite this article
Maroules, C.D., Ghoshhajra, B.B., Malguria, N. et al. Noncardiac Incidental Findings on Cardiac CT: A Step-by-Step Approach. Curr Cardiovasc Imaging Rep 7, 9283 (2014). https://doi.org/10.1007/s12410-014-9283-z
Published:
DOI: https://doi.org/10.1007/s12410-014-9283-z