Abstract
Purpose of Review
Invasive aspergillosis is a frequent opportunistic infection in the intensive care unit. Diagnostic strategies within this setting are not well-defined.
Recent Findings
In the absence of histopathological proof, estimation of likelihood of disease is required, based on a combination of host factors with clinical, microbiological and radiologic findings. Importantly, the at-risk critically ill population has expanded beyond the classical immunosuppressed patients. Clinical features are non-specific, yet overt organ failure is associated with an increased risk and should raise the suspicion of invasive fungal disease. Validation of serological and molecular diagnostics is complicated by the lack of universal definitions in critically ill patients.
Summary
Improved awareness of novel risk groups can promote early diagnosis. Development of novel, host immune response driven biomarkers and universal definitions of invasive fungal disease in intensive care units is urgently needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive aspergillosis (IA) is a life-threatening infection caused by the ubiquitous fungus Aspergillus, which typically occurs in immunocompromised patients. After inhalation of spores (conidia), the fungus can disseminate hematogenously from the respiratory tract (as the entry point) towards different organs. Classic host factors defined by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) include stem cell and solid organ transplantation, prolonged neutropenia and use of corticosteroid or other immunosuppressive therapy [1, 2••]. In the last two decades, however, IA has been recognized as an emerging disease in intensive care unit (ICU) patients without these classic host factors [3,4,5,6]. Diagnosing IA in these critically ill patients remains challenging, for a multitude of reasons (Table 1). First, gold standard diagnosis of Aspergillus disease relies on culture from a normally sterile site or pathologic evidence of fungal hyphal growth within inflamed or necrotic tissue. To obtain this requires an invasive procedure that is rarely employed in critically ill patients due to coagulation abnormalities or respiratory instability (in case of lung biopsy) [1, 4]. Second and similar to other immunocompromised patients, clinical and radiological signs of fungal disease are non-specific in an ICU population. Third, data on the diagnostic performance and validity of laboratory tests within the ICU setting are limited by differences in study protocols for the evaluation of diagnostic tests and the reference standard employed in such studies. Although the EORTC/MSG consensus group has developed a reference standard for research purposes, many ICU patients fall outside the scope of these definitions due to the lack of a proper host factor [7,8,9,10,11]. Moreover, highly sensitive and specific diagnostic tests with rapid turnaround time are limited. Finally, the heterogeneity of the ICU population with respect to comorbidities and technical support measures required further increases the diagnostic challenge [1, 6, 9, 12]. A difficult/problematic diagnosis of IA impairs patient care by increasing the risk of missed diagnosis and late initiation of appropriate antifungal therapy, resulting in an associated high mortality rate. Indeed, IA still ranks as an important missed diagnosis in the ICU, based on a recent autopsy study [13•]. On the other hand, broad-spectrum empirical therapy without clear diagnosis leads to over-treatment with the associated risk of increased side-effects and costs, and the emergence of azole resistance.
In this review, we focus on recent work that has been published regarding clinical risk factors, laboratory and imaging modalities in the ICU setting and highlight gaps in the current literature that require urgent attention.
Clinical Disease Spectrum and Risk Factors
IA typically manifests as pulmonary disease or tracheobronchitis in critically ill patients. Fever, dyspnoea, cough, pleural rub and worsening respiratory function are part of the AspICU algorithm for diagnosing IA, yet these clinical signs/symptoms are neither sensitive nor specific [4, 9, 14, 15]. Disease severity, as expressed by APACHE II or Sequential Organ Failure Assessment (SOFA) score, and need for organ support (renal replacement therapy, mechanical ventilation or extra-corporeal membrane oxygenation (ECMO)), are associated with an increased risk of IA [4, 5, 9, 16••, 17••].
Well-established risk factors for IA include neutropenia, haematological malignancies, allogeneic stem cell transplantation, solid organ transplantation, solid organ cancer and AIDS [18]. However, epidemiological studies in ICU have identified additional risk categories for IA, such as chronic obstructive pulmonary disease (COPD GOLD III or IV), Child C liver cirrhosis, renal dysfunction, malnutrition and diabetes mellitus [6, 19,20,21,22,23]. Adjunct use of corticosteroid therapy forms a major risk factor, even at lower doses than stated in the EORTC definitions or when used for a more limited period of time [7]. In a recent autopsy series of ICU patients with IA, 56% received systemic steroid therapy prior to or during their ICU stay [13•]. Additionally, recent work argues for the inclusion of severe influenza infection in the ever-expanding list of risk factors [24, 25]. Though many critically ill influenza patients received steroid therapy, the viral infection itself has been identified as an independent risk factor for pulmonary IA in a retrospective cohort study [26•]. Whether this predisposition to IA is specific to influenza or can be found with other respiratory viral infections in ICU patients is currently not clear. Lastly, novel immunomodulators are increasingly used for treatment of cancer and autoimmune diseases. Many opportunistic infections have been associated with these therapies, both directly- or indirectly-linked by need for steroid treatment of side-effects [27,28,29]. In brief, a broad range of factors can predispose critically ill patients to the development of IA. Absence of well-known underlying conditions associated with IA should thus not rule-out IA. High diagnostic awareness for IA is thus warranted in critically ill patients, especially in tertiary care settings that manage a high number of immunocompromised patients.
Microbiological Diagnosis
A wide panel of direct and biomarker tests can be performed. Procurement of appropriate clinical samples is needed (e.g. bronchoalveolar lavage (BAL) fluid in case of suspicion of pulmonary aspergillosis, cerebrospinal fluid (CSF) if suspicion of cerebral aspergillosis). Although the risks of invasive procedures in a patient population characterized by coagulopathy, hypoxemia and high levels of organ support needs to be evaluated against the diagnostic merits on a case-by-case basis, performing bronchoscopy for BAL is generally safe in critically ill patients. In a study of 100 mechanically ventilated patients (APACHE II of 22, fiO2 up to 90%) only 2% of patients needed increased PEEP and fiO2 due to hypoxemia after bronchoscopy with an average amount of lavage fluid instilled of 188 ml [30]. Reducing the volume of fluid instilled to about 50 ml will probably reduce complication rate even more. All tests are corroborated by a bulk of evidence in high-risk, haematological patient groups, yet data on ICU patients are scarce. Providing a complete overview of the diagnostic performances of fungal assays in critically ill patients is thus complicated, a fact that is made worse by the heterogeneity of study populations and definitions of IA used as reference standard. Moreover, most studies focus on pulmonary IA with limited reports on other forms of IA. Given these limitations, a brief outline of different microbiological assays for diagnosis of IA in critically ill patients is given.
A positive fungal culture allows for species identification and antifungal susceptibility testing, yet its use as a diagnostic tool is hampered by low yield (sensitivity of 30–50%), required turnaround time and difficulty of differentiation between colonization and invasive fungal disease (IFD) [6, 8, 31,32,33]. Since COPD patients are frequently colonized with Aspergillus, diagnosing invasive disease is particularly challenging in this patient group [7, 34]. Use of appropriate (i.e. lower respiratory tract) sampling and interpretation within the individual patient context, as addressed by the AspICU clinical algorithm, can circumvent the latter limitation [9].
The most extensively used biomarker test for IA is the detection of galactomannan (GM), a polysaccharide component of the Aspergillus cell wall which is released during growth of the fungus. The test is validated for use in serum and BAL samples, and has been moderately evaluated for detection of cerebral aspergillosis based on CSF analysis [35]. Although GM detection with an optical index (OD) of 0.5 in serum is associated with very good sensitivity in neutropenic patients, diagnostic validity is markedly reduced in critically ill patients. This is explained by the ability of circulating neutrophils to clear the antigen and type of disease (airway-invasive versus angio-invasive in neutropenic setting). Based on a vast amount of additional evidence, the novel EORTC guidelines have increased the OD threshold of a positive GM detection in serum to 1.0 for IA diagnosis [2, 36]. Despite its limitations, GM evaluation in serum remains valuable, especially since it does not require invasive sampling and can be used to evaluate therapeutic responses in any positive case [37]. GM detection of BAL fluid is another valuable test for early diagnosis of IA in ICU patients, with higher sensitivity (up to 100%) compared to serum analysis. Cut-off values of 1.0 for a single measurement on BAL sample and 0.8 for BAL sample if in conjunction with a serum GM index of at least 0.7 have recently been proposed [11, 32, 33, 38,39,40,41]. False-positivity of GM detection in serum and BAL has been linked to different causes [38, 42, 43]. Recently, Candida in the respiratory tract was associated with GM positivity in 29% of tested BAL fluids, using a cut-off of 0.5. However, by using a cut-off of > 1.0, this association disappeared, providing further argument for the use of a cut-off of 1.0 [44]. Repeated analysis of the same sample (to detect false-positivity by technical issues) and simultaneous evaluation of serum and BAL can reduce the number of false-positive results. Very recently, the American Thoracic Society (ATS) has published their new guidelines on microbiological testing in the diagnosis of fungal infections in critical care, however, the majority of evidence regarding IA reviewed included immunosuppressed patients, limiting the applicability of the recommendations to this patient setting [45•].
1,3-β-D-glucan (BDG) is an abundant fungal cell wall component of most pathogenic fungi, except for Mucorales spp. and Cryptococcus spp. During invasive fungal disease, it can be detected in serum with one of several commercially available tests. Only limited data on the overall performance of the BDG assay in serum is available in a population of critically ill patients, but it shows a relatively good sensitivity and a broad-ranged specificity for the diagnosis of IA [41, 46,47,48,49]. BDG testing in BAL fluid is not recommended due to poor specificity, which can be explained by Candida colonization of the respiratory tract [31, 47•, 50, 51]. Moreover, in the ICU setting, many confounders leading to false positive BDG results can be found, such as haemodialysis with cellulose membranes, exposure to wound gauze, administration of intravenous albumin, immunoglobulins or antibiotic therapy and presence of bacteraemia [52, 53]. As a result, the usefulness of the BDG analysis as a diagnostic test for IA in the ICU mainly relies on its negative predictive value to exclude invasive fungal disease.
Aspergillus nucleic acid detection by PCR can be performed on serum, BAL samples and CSF samples [10, 33, 54, 55]. Advantages include high specificity and the capacity to detect mutations associated with antifungal resistance. Methods for optimization and standardization of Aspergillus PCRs have been published by the European Aspergillus PCR initiative (EAPCRI), which paved the way for inclusion of PCR in the updated EORTC/MSG diagnostic definitions [56, 57]. Current data in non-neutropenic, critically ill patients suggest a moderate benefit of PCR analysis, specifically in BAL samples, in combination with other biomarkers such as GM in the diagnostic evaluation of IA. Difficulties in differentiation between colonization and IFD remain a limitation [47•, 58,59,60].
Two lateral flow assays are currently CE-marked and commercialized for diagnosing IA on BAL samples. The technology is particularly attractive because of its potential of ease of use and short turnaround time, making it suitable as a point-of-care test within the ICU. The first assay, the lateral flow device (LFD) by OLM Diagnostics (Newcastle Upon Tyne, United Kingdom), detects an extracellular glycoprotein released by Aspergillus during active growth via a mouse monoclonal antibody (JF5) [61]. Both the prototype of this assay as well as the current, newly formatted, LFD were evaluated mainly in immunocompromised patients, though some data on ICU patients exist [31, 50, 62, 63]. The second CE-marked device, a lateral flow assay (LFA) commercialized by IMMY (Norman, OK, USA) is based on detection of GM. In a retrospective analysis of haematology patients, the two assays have shown comparable specificity for detection of proven and probable IA, yet the LFD showed lower sensitivity [64•]. Data on ICU patients are scarce, yet might indicate a similar problem of sensitivity [63, 65]. Another possible limitation for use as a point-of-care diagnostic test in ICU setting is the need for a pre-treatment in case of viscous or haemorrhagic sampling. Further evaluation of test performances and ease of use of lateral flow technology in critically ill patients is warranted.
In conclusion, diagnosis of IA in the ICU is currently not possible based on a single microbiological test. An optimal diagnostic strategy consists of simultaneous execution of several mycological tests and evaluation of test results in combination with clinical and radiological assessment [17••]. Indeed, combining multiple fungal readouts has been shown to improve diagnostic performance in critically ill patients [47•]. A multidisciplinary evaluation of microbiological test results along with host factors and imaging results can further improve diagnosis by providing a weighted estimate of likelihood of disease. Repeated positive testing can enhance diagnostic certainty as well, yet the benefit of this strategy should be evaluated against the risks associated with a time delay in initiation of antifungal therapy whereas repeated testing after therapy initiation may yield negative results [36]. Finally, there is a clear need for new, highly sensitive, diagnostic tests on non-invasively obtained clinical samples with rapid turnaround time for IA detection. Most importantly, differentiation of colonization and invasive disease remains a limitation for all currently available tests. Novel biomarkers, evaluating host immune response, might aid in this evaluation. In a cohort of lung transplant recipients, detection of the pro-inflammatory marker pentraxin 3 in conjunction with GM or fungal culture on BAL has been shown to increase the likelihood of IPA [66, 67]. Additionally cytokine studies, performed predominantly in high-risk cohorts of patients, have shown significant associations between IA and IL-6 and IL-8 elevations in serum and BAL fluid [68, 69]. Combination of cytokine analyses with fungal biomarker testing improved diagnostic performance of IA in a single-center cohort study of adult patients with haematological malignancy and suspected pulmonary infection [70]. Moreover, studies indicate diagnostic value of mould-reactive T cell evaluation from peripheral blood in high-risk populations [71, 72]. Further evaluation of these immunological test approaches in different patient populations is warranted, yet combined analysis of fungal biomarkers and host immunological signals is likely to help unravel the current diagnostic puzzle [73•].
Radiological Diagnosis
Imaging findings of IA on lung CT can vary between the most typical signs, such as halo sign and air crescent sign, which are rarely encountered in non-neutropenic critically ill patients [4, 19, 74, 75], to non-specific abnormalities such as ground-glass opacity, multiple nodules along broncho-vascular bundles or located subpleurally [76]. The overlap of non-specific radiological findings in ICU patients with ARDS is large, further complicating diagnosis. Moreover, in cases of tracheobronchial aspergillosis, radiological manifestations can be subtle or even absent, although bronchoscopy can show typical macroscopic images (ulceration, nodule, pseudomembrane, plaque or eschar) [77]. Recently, antibody-guided immunoPET/MR based on mAb mJF5 has shown promising results in small animal models and is fundamentally translatable to human IA diagnosis in the near future, since the same antibody has already been validated for diagnostic LFD tests on BAL fluids [78]. Importantly, performing these imaging procedures in unstable critically ill patients may not be feasible. Despite these limitations, imaging can aid in diagnosis and prognostic evaluation of non-neutropenic patients. In a retrospective evaluation of 61 critically ill COPD patients, a larger size of lung consolidation implicated poor prognosis of IA [76]. Airway-invasive disease manifestations were associated with higher mortality in non-neutropenic heart transplant patients with IA [79] and recently, the vessel occlusion sign (VOS) on contrast-enhanced pulmonary angiography (CTPA) has shown promising results in non-haematological immunocompromised patients with a sensitivity of 73% and a specificity of 93% for IA [80].
Diagnostic Approaches in Critically Ill Patients
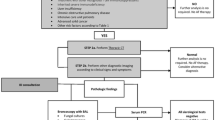
In the absence of histopathological proof, diagnosis of IA in critically ill patients requires a high-level of suspicion, based on judgement of clinical presentation and risk factors (both the classical host factors and recently identified risk factors) combined with work-up including imaging and procurement of relevant clinical samples for culture and biomarker testing. The need for combining clinical, host and mycological factors is generally accepted, yet standardization of diagnostic criteria in ICU is currently absent [1, 7, 9, 11, 31, 39, 58, 62]. This leads to difficulties in interpretation of test results and bedside diagnosis. We therefore recommend a multidisciplinary patient evaluation by ICU, infectious diseases, microbiology and radiology physicians to diagnose fungal infections in critically ill patients. However, logistical issues of availability of laboratory tests or inability to perform invasive procedures or to transport unstable patients to radiology departments should not delay empirical treatment in patients with high likelihood of IA. Blot et al. provided a nice overview of the different pillars of IA diagnosis and associated arbitrary likelihood ratio [17••]. Finally, as the validity of the 2008 EORTC/MSG as well as the recently published 2019 EORTC/MSG definitions for diagnosing IA in critically ill patients have been questioned, new collaborative initiatives have been taken to develop a standard set of definitions for IA in a general ICU population (FUNDICU study group) and in ICU patients with severe influenza (IAPA study group) [81••]. Publications can be expected soon.
Conclusions
The diagnosis of IA in critically ill patients remains a major challenge, with a diversity of clinical and radiological findings and an increasing spectrum of patients at risk. Besides gold standard histopathological proof of disease, no single test provides sufficient sensitivity and specificity. Estimation of likelihood of disease is required, based on a combination of clinical, mycological and imaging findings (sometimes requiring repetition). Novel diagnostic tests, evaluating host immune responses, and development of universal definitions of invasive fungal disease for intensive care setting will bring the field a major step forward [81••].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/invasive fungal infections cooperative group and the National Institute of Allergy and Infectious Diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46(12):1813–21.
•• Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz1008Updated definitions of invasive fungal disease, incorporating evidence onAspergillusPCR.
Meersseman W, Lagrou K, Maertens J, Van Wijngaerden E. Invasive aspergillosis in the intensive care unit. Clin Infect Dis. 2007;45(2):205–16.
Taccone FS, Van den Abeele AM, Bulpa P, Misset B, Meersseman W, Cardoso T, et al. Epidemiology of invasive aspergillosis in critically ill patients: Clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19(1):7.
Vandewoude K, Blot S, Benoit D, Depuydt P, Vogelaers D, Colardyn F. Invasive aspergillosis in critically ill patients: analysis of risk factors for acquisition and mortality. Acta Clin Belg. 2004;59(5):251–7.
Garnacho-Montero J, Amaya-Villar R, Ortiz-Leyba C, Leon C, Alvarez-Lerma F, Nolla-Salas J, et al. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: risk factors, clinical presentation and outcome. Crit Care. 2005;9(3):R191–9.
Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J. 2007;30(4):782–800.
Vandewoude KH, Blot SI, Depuydt P, Benoit D, Temmerman W, Colardyn F, et al. Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients. Crit Care. 2006;10(1):R31.
Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186(1):56–64.
Husain S, Sole A, Alexander BD, Aslam S, Avery R, Benden C, et al. The 2015 International Society for Heart and Lung Transplantation guidelines for the management of fungal infections in mechanical circulatory support and cardiothoracic organ transplant recipients: executive summary. J Heart Lung Transplant. 2016;35(3):261–82.
Schroeder M, Simon M, Katchanov J, Wijaya C, Rohde H, Christner M, et al. Does galactomannan testing increase diagnostic accuracy for IPA in the ICU? A prospective observational study. Crit Care. 2016;20(1):139.
Koulenti D, Garnacho-Montero J, Blot S. Approach to invasive pulmonary aspergillosis in critically ill patients. Curr Opin Infect Dis. 2014;27(2):174–83.
• Tejerina EE, Abril E, Padilla R, Rodriguez Ruiz C, Ballen A, Frutos-Vivar F, et al. Invasive aspergillosis in critically ill patients: an autopsy study. Mycoses. 2019;62(8):673–9 Recent autopsy study showing frequent misdiagnosis of IA in critically ill patients.
Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P, Belleguic C, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis. 2006;43(5):577–84.
Chakrabarti A, Kaur H, Savio J, Rudramurthy SM, Patel A, Shastri P, et al. Epidemiology and clinical outcomes of invasive mould infections in Indian intensive care units (FISF study). J Crit Care. 2019;51:64–70.
•• Cavayas YA, Yusuff H, Porter R. Fungal infections in adult patients on extracorporeal life support. Crit Care. 2018;22(1):98. https://doi.org/10.1186/s13054-018-2023-zRetrospective cohort study of adult patients on ECMO between 2006 and 2016, showing association betweenAspergillusinvolvement and use of ECMO as respiratory support as well as an association betweenAspergillusand influenza in this study cohort.
•• Blot S, Rello J, Koulenti D. Diagnosing invasive pulmonary aspergillosis in ICU patients: putting the puzzle together. Curr Opin Crit Care. 2019;25(5):430–7 Comprehensive review of IPA in intensive care setting, attributing degrees of likelihood for invasive pulmonary fungal disease to different pillars of diagnosis.
Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and Management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60.
Meersseman W, Vandecasteele SJ, Wilmer A, Verbeken E, Peetermans WE, Van Wijngaerden E. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170(6):621–5.
Prodanovic H, Cracco C, Massard J, Barrault C, Thabut D, Duguet A, et al. Invasive pulmonary aspergillosis in patients with decompensated cirrhosis: case series. BMC Gastroenterol. 2007;7:2. https://doi.org/10.1186/1471-230X-7-2.
Levesque E, Ait-Ammar N, Dudau D, Clavieras N, Feray C, Foulet F, et al. Invasive pulmonary aspergillosis in cirrhotic patients: analysis of a 10-year clinical experience. Ann Intensive Care. 2019;9(1):31.
Baddley JW, Stephens JM, Ji X, Gao X, Schlamm HT, Tarallo M. Aspergillosis in intensive care unit (ICU) patients: epidemiology and economic outcomes. BMC Infect Dis. 2013;13:29. https://doi.org/10.1186/1471-2334-13-29.
• Danion F, Rouzaud C, Dureault A, Poiree S, Bougnoux ME, Alanio A, et al. Why are so many cases of invasive aspergillosis missed? Med Mycol. 2019;57(Supplement_2):S94–s103 Elaborate review of causes of IA misdiagnosis in a general population, focussing on the different aspects of diagnosis (host factors, radiology and microbiological tests).
Wauters J, Baar I, Meersseman P, Meersseman W, Dams K, De Paep R, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. 2012;38(11):1761–8.
van de Veerdonk FL, Kolwijck E, Lestrade PP, Hodiamont CJ, Rijnders BJ, van Paassen J, et al. Influenza-associated Aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2017;196(4):524–7.
• Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–92 This retrospective cohort study of multiple ICUs in Belgium and the Netherlands showed for the first time that severe influenza in critically ill patients is an independent risk factor for IPA development.
Baron M, Zini JM, Challan Belval T, Vignon M, Denis B, Alanio A, et al. Fungal infections in patients treated with ibrutinib: two unusual cases of invasive aspergillosis and cryptococcal meningoencephalitis. Leuk Lymphoma. 2017;58(12):2981–2.
Fujita K, Kim YH, Kanai O, Yoshida H, Mio T, Hirai T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir Med. 2019;146:66–70.
Salmon-Ceron D, Tubach F, Lortholary O, Chosidow O, Bretagne S, Nicolas N, et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis. 2011;70(4):616–23.
Prebil SE, Andrews J, Cribbs SK, Martin GS, Esper A. Safety of research bronchoscopy in critically ill patients. J Crit Care. 2014;29(6):961–4.
Prattes J, Flick H, Pruller F, Koidl C, Raggam RB, Palfner M, et al. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am J Respir Crit Care Med. 2014;190(8):922–9.
He H, Ding L, Sun B, Li F, Zhan Q. Role of galactomannan determinations in bronchoalveolar lavage fluid samples from critically ill patients with chronic obstructive pulmonary disease for the diagnosis of invasive pulmonary aspergillosis: a prospective study. Crit Care. 2012;16(4):R138.
Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–e38.
Bulpa P, Bihin B, Dimopoulos G, Taccone FS, Van den Abeele AM, Misset B, et al. Which algorithm diagnoses invasive pulmonary aspergillosis best in ICU patients with COPD? Eur Respir J. 2017;50(3):1700532. https://doi.org/10.1183/13993003.00532-2017.
Chong GM, Maertens JA, Lagrou K, Driessen GJ, Cornelissen JJ, Rijnders BJ. Diagnostic performance of Galactomannan antigen testing in cerebrospinal fluid. J Clin Microbiol. 2016;54(2):428–31.
Leeflang MM, Debets-Ossenkopp YJ, Wang J, Visser CE, Scholten RJ, Hooft L, et al. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. 2015;(12):Cd007394. Doi: https://doi.org/10.1002/14651858.CD007394.pub2
Mercier T, Guldentops E, Lagrou K, Maertens J. Galactomannan, a surrogate marker for outcome in invasive Aspergillosis: finally coming of age. Front Microbiol. 2018;9:661. https://doi.org/10.3389/fmicb.2018.00661.
Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med. 2008;177(1):27–34.
Fortun J, Martin-Davila P, de la Gomez Garcia Pedrosa E, Silva JT, Garcia-Rodriguez J, Benito D, et al. Galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive aspergillosis in non-hematological patients. J Inf Secur. 2016;72(6):738–44.
Khodavaisy S, Hedayati MT, Alialy M, Habibi MR, Badali H. Detection of galactomannan in bronchoalveolar lavage of the intensive care unit patients at risk for invasive aspergillosis. Curr Med Mycol. 2015;1(1):12–7.
Acosta J, Catalan M, del Palacio-Perez-Medel A, Lora D, Montejo JC, Cuetara MS, et al. A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: comparison with the diagnostic performance of galactomannan and of (1--> 3)-beta-d-glucan chromogenic assay in serum samples. Clin Microbiol Infect. 2011;17(7):1053–60.
Petraitiene R, Petraitis V, Witt JR 3rd, Durkin MM, Bacher JD, Wheat LJ, et al. Galactomannan antigenemia after infusion of gluconate-containing plasma-Lyte. J Clin Microbiol. 2011;49(12):4330–2.
Spriet I, Lagrou K, Maertens J, Willems L, Wilmer A, Wauters J. Plasmalyte: no longer a culprit in causing false-positive Galactomannan test results. J Clin Microbiol. 2016;54(3):795–7.
Aigner M, Wanner M, Kreidl P, Lass-Florl C, Lackner M. Candida in the Respiratory Tract Potentially Triggers Galactomannan Positivity in Nonhematological Patients. Antimicrob Agents Chemother. 2019;63(6):e00138–19.
• Hage CA, Carmona EM, Epelbaum O, Evans SE, Gabe LM, Haydour Q, et al. Microbiological Laboratory Testing in the Diagnosis of Fungal Infections in Pulmonary and Critical Care Practice. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2019;200(5):535–50 Novel guideline of the American Thoracic Society focussing on microbiological aspects of invasive fungal disease in pulmonary and critical care setting. This paper entails specific recommendations on use of galactomannan andAspergillusPCR for IA diagnosis.
Acosta J, Catalan M, del Palacio-Perez-Medel A, Montejo JC, De-La-Cruz-Bertolo J, Moragues MD, et al. Prospective study in critically ill non-neutropenic patients: diagnostic potential of (1,3)-beta-D-glucan assay and circulating galactomannan for the diagnosis of invasive fungal disease. Eur J Clin Microbiol Infect Dis. 2012;31(5):721–31.
• Boch T, Reinwald M, Spiess B, Liebregts T, Schellongowski P, Meybohm P, et al. Detection of invasive pulmonary aspergillosis in critically ill patients by combined use of conventional culture, galactomannan, 1–3-beta-D-glucan and Aspergillus specific nested polymerase chain reaction in a prospective pilot study. J Crit Care. 2018;47:198–203 Prospective pilot study of a combination biomarker diagnostic strategy in 44 critically ill patients, both hematological as non-hematological, showing a benefit of integrating BAL and blood sample biomarkers for diagnosis of IPA.
De Vlieger G, Lagrou K, Maertens J, Verbeken E, Meersseman W, Van Wijngaerden E. Beta-D-glucan detection as a diagnostic test for invasive aspergillosis in immunocompromised critically ill patients with symptoms of respiratory infection: an autopsy-based study. J Clin Microbiol. 2011;49(11):3783–7.
Lahmer T, Neuenhahn M, Held J, Rasch S, Schmid RM, Huber W. Comparison of 1,3-beta-d-glucan with galactomannan in serum and bronchoalveolar fluid for the detection of Aspergillus species in immunosuppressed mechanical ventilated critically ill patients. J Crit Care. 2016;36:259–64.
Hoenigl M, Prattes J, Spiess B, Wagner J, Prueller F, Raggam RB, et al. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol. 2014;52(6):2039–45.
Meersseman W, Lagrou K, Spriet I, Maertens J, Verbeken E, Peetermans WE, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med. 2009;35(9):1526–31.
Digby J, Kalbfleisch J, Glenn A, Larsen A, Browder W, Williams D. Serum glucan levels are not specific for presence of fungal infections in intensive care unit patients. Clin Diagn Lab Immunol. 2003;10(5):882–5.
Alexander BD, Smith PB, Davis RD, Perfect JR, Reller LB. The (1,3){beta}-D-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J Clin Microbiol. 2010;48(11):4083–8.
Hummel M, Spiess B, Kentouche K, Niggemann S, Bohm C, Reuter S, et al. Detection of Aspergillus DNA in cerebrospinal fluid from patients with cerebral aspergillosis by a nested PCR assay. J Clin Microbiol. 2006;44(11):3989–93.
Imbert S, Brossas JY, Palous M, Joly I, Meyer I, Fekkar A. Performance of Aspergillus PCR in cerebrospinal fluid for the diagnosis of cerebral aspergillosis. Clin Microbiol Infect. 2017;23(11):889.e1–4.
White PL, Wingard JR, Bretagne S, Loffler J, Patterson TF, Slavin MA, et al. Aspergillus polymerase chain reaction: systematic review of evidence for clinical use in comparison with antigen testing. Clin Infect Dis. 2015;61(8):1293–303.
Mengoli C, Cruciani M, Barnes RA, Loeffler J, Donnelly JP. Use of PCR for diagnosis of invasive aspergillosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9(2):89–96.
Imbert S, Gauthier L, Joly I, Brossas JY, Uzunov M, Touafek F, et al. Aspergillus PCR in serum for the diagnosis, follow-up and prognosis of invasive aspergillosis in neutropenic and nonneutropenic patients. Clin Microbiol Infect. 2016;22(6):562.e1–8.
Orsi CF, Gennari W, Venturelli C, La Regina A, Pecorari M, Righi E, et al. Performance of 2 commercial real-time polymerase chain reaction assays for the detection of Aspergillus and Pneumocystis DNA in bronchoalveolar lavage fluid samples from critical care patients. Diagn Microbiol Infect Dis. 2012;73(2):138–43.
Torelli R, Sanguinetti M, Moody A, Pagano L, Caira M, De Carolis E, et al. Diagnosis of invasive aspergillosis by a commercial real-time PCR assay for Aspergillus DNA in bronchoalveolar lavage fluid samples from high-risk patients compared to a galactomannan enzyme immunoassay. J Clin Microbiol. 2011;49(12):4273–8.
Thornton CR. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol. 2008;15(7):1095–105.
Eigl S, Prattes J, Lackner M, Willinger B, Spiess B, Reinwald M, et al. Multicenter evaluation of a lateral-flow device test for diagnosing invasive pulmonary aspergillosis in ICU patients. Crit Care. 2015;19:178.
Hoenigl M, Eigl S, Heldt S, Duettmann W, Thornton C, Prattes J. Clinical evaluation of the newly formatted lateral-flow device for invasive pulmonary aspergillosis. Mycoses. 2018;61(1):40–3.
• Mercier T, Dunbar A, de Kort E, Schauwvlieghe A, Reynders M, Guldentops E, et al. Lateral flow assays for diagnosing invasive pulmonary aspergillosis in adult hematology patients: A comparative multicenter study. Med Mycol. 2019. https://doi.org/10.1093/mmy/myz079Comparative study of current CE-marked lateral flow assays for IPA diagnosis, evaluated in a multicenter study of hematological patients.
Jenks JD, Mehta SR, Taplitz R, Aslam S, Reed SL, Hoenigl M. Point-of-care diagnosis of invasive aspergillosis in non-neutropenic patients: Aspergillus Galactomannan lateral flow assay versus Aspergillus-specific lateral flow device test in bronchoalveolar lavage. Mycoses. 2019;62(3):230–6.
Kabbani D, Bhaskaran A, Singer LG, Bhimji A, Rotstein C, Keshavjee S, et al. Pentraxin 3 levels in bronchoalveolar lavage fluid of lung transplant recipients with invasive aspergillosis. J Heart Lung Transplant. 2017;36(9):973–9.
Li H, Liu L, Zhou W, Rui Y, He B, Shi Y, et al. Pentraxin 3 in bronchoalveolar lavage fluid and plasma in non-neutropenic patients with pulmonary aspergillosis. Clin Microbiol Infect. 2019;25(4):504–10.
Heldt S, Eigl S, Prattes J, Flick H, Rabensteiner J, Pruller F, et al. Levels of interleukin (IL)-6 and IL-8 are elevated in serum and bronchoalveolar lavage fluid of haematological patients with invasive pulmonary aspergillosis. Mycoses. 2017;60(12):818–25.
Rawlings SA, Heldt S, Prattes J, Eigl S, Jenks JD, Flick H, et al. Using interleukin 6 and 8 in blood and Bronchoalveolar lavage fluid to predict survival in hematological malignancy patients with suspected pulmonary Mold infection. Front Immunol. 2019;10:1798.
Heldt S, Prattes J, Eigl S, Spiess B, Flick H, Rabensteiner J, et al. Diagnosis of invasive aspergillosis in hematological malignancy patients: performance of cytokines, asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J Inf Secur. 2018;77(3):235–41.
Bacher P, Steinbach A, Kniemeyer O, Hamprecht A, Assenmacher M, Vehreschild MJ, et al. Fungus-specific CD4(+) T cells for rapid identification of invasive pulmonary mold infection. Am J Respir Crit Care Med. 2015;191(3):348–52.
Steinbach A, Cornely OA, Wisplinghoff H, Schauss AC, Vehreschild JJ, Rybniker J, et al. Mould-reactive T cells for the diagnosis of invasive mould infection-a prospective study. Mycoses. 2019;62(7):562–9.
• Jenks JD, Rawlings SA, Garcia-Vidal C, Koehler P, Mercier T, Prattes J, et al. Immune parameters for diagnosis and treatment monitoring in invasive Mold infection. J Fungi (Basel). 2019;5(4):116. https://doi.org/10.3390/jof5040116Elaborate review on recent advances in immunological diagnostic tools for invasive fungal disease.
Caillot D, Couaillier JF, Bernard A, Casasnovas O, Denning DW, Mannone L, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19(1):253–9.
Park SY, Lim C, Lee SO, Choi SH, Kim YS, Woo JH, et al. Computed tomography findings in invasive pulmonary aspergillosis in non-neutropenic transplant recipients and neutropenic patients, and their prognostic value. J Inf Secur. 2011;63(6):447–56.
Huang L, He H, Ding Y, Jin J, Zhan Q. Values of radiological examinations for the diagnosis and prognosis of invasive bronchial-pulmonary aspergillosis in critically ill patients with chronic obstructive pulmonary diseases. Clin Respir J. 2018;12(2):499–509.
Krenke R, Grabczak EM. Tracheobronchial manifestations of Aspergillus infections. Sci World J. 2011;11:2310–29.
Thornton CR. Molecular imaging of invasive pulmonary Aspergillosis using ImmunoPET/MRI: the future looks bright. Front Microbiol. 2018;9:691.
Munoz P, Vena A, Ceron I, Valerio M, Palomo J, Guinea J, et al. Invasive pulmonary aspergillosis in heart transplant recipients: two radiologic patterns with a different prognosis. J Heart Lung Transplant. 2014;33(10):1034–40.
Henzler C, Henzler T, Buchheidt D, Nance JW, Weis CA, Vogelmann R, et al. Diagnostic performance of contrast enhanced pulmonary computed tomography angiography for the detection of Angioinvasive pulmonary Aspergillosis in Immunocompromised patients. Sci Rep. 2017;7(1):4483. https://doi.org/10.1038/s41598-017-04470-6.
•• Bassetti M, Scudeller L, Giacobbe DR, Lamoth F, Righi E, Zuccaro V, et al. Developing definitions for invasive fungal diseases in critically ill adult patients in intensive care units. Protocol of the FUNgal infections definitions in ICU patients (FUNDICU) project. Mycoses. 2019;62(4):310–9 Protocol of project aimed to develop definitions for invasive fungal diseases in intensive care setting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lore Vanderbeke is a research fellow of the Research Foundation - Flanders (FWO Vlaanderen). Johan Maertens reports grants, personal fees and non-financial support from MSD; grants, personal fees and non-financial support from Gilead Sciences; grants, personal fees and non-financial support from Pfizer, Inc.; personal fees and non-financial support from Basilea; personal fees and non-financial support from Cidara; personal fees and non-financial support from F2G; grants from Immy; and grants from OLM outside the submitted work. Joost Wauters reports grants from MSD, other from Gillead, and grants from Pfizer outside the submitted work. Katrien Lagrou reports non-financial support from Pfizer, personal fees and non-financial support from MSD, personal fees from SMB Laboratoires, personal fees from Gilead, and personal fees from FUJIFILM Wako outside the submitted work. Eric Van Wijngaerden declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Advances in Diagnosis of Invasive Fungal Infections
Rights and permissions
About this article
Cite this article
Vanderbeke, L., Van Wijngaerden, E., Maertens, J. et al. Diagnosis of Invasive Aspergillosis in Intensive Care Unit Patients. Curr Fungal Infect Rep 14, 166–173 (2020). https://doi.org/10.1007/s12281-020-00383-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-020-00383-6