Abstract
The outcomes of total shoulder arthroplasty (TSA) for painful arthritis of the glenohumeral joint are excellent with significant improvement in pain and function. Increased use of total shoulder arthroplasty over the past decade has led to identification of common complications. Although the complication rate is low, accurate and timely diagnosis, appropriate management, and implementation of methods for prevention are critical to a successful long-term outcome. The most common complications include infection, glenoid and humeral component loosening, rotator cuff tear, periprosthetic fracture, and neurologic injury. The purpose of this review is to outline the best practices for diagnosing, managing, and preventing these complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glenohumeral arthritis is a disabling condition that affects up to 20 % of the older population. When conservative management fails to improve pain, total shoulder arthroplasty has shown to reliably relieve pain and improve function in 95 % of patients [1]. The incidence of shoulder arthroplasty has increased 2.5-fold over the past decade, which may be due in large part to the aging population and the introduction of the reverse total shoulder arthroplasty [2]. Therefore, as the volume of shoulder arthroplasty increases, surgeons are encountering more intraoperative and postoperative complications. The purpose of this review is to discuss the complications of total shoulder arthroplasty, including diagnosis, radiographic or laboratory workup, appropriate management, and methods for prevention.
History, presentation, and physical examination
Patients with glenohumeral arthritis frequently describe an insidious onset of pain often present for many years and associated with progressive worsening. Pain is worse with activity and relieved with rest and is commonly present at night and impairs sleep. The clinician should discern from the history whether the pain is secondary to primary osteoarthritis or posttraumatic based on any history of trauma or recurrent instability. Additionally, any associated neck pain, numbness, tingling, or weakness in the upper extremity should indicate that the symptoms are secondary to a cervical disk herniation with resultant radiculopathy. Furthermore, medical comorbidities, prior shoulder surgery, tobacco usage, and social support system should all be addressed prior to indicating patients for surgery. Optimizing preoperative medical comorbidities and addressing postoperative expectations will improve outcome and patient satisfaction.
Physical examination commonly reveals posterior joint line pain, restricted symmetric passive and active range of motion, rotator cuff and periscapular muscle atrophy secondary to disuse, and intact neurologic function. The clinician must determine the integrity of the rotator cuff muscles. The empty can and drop arm tests (supraspinatus), external rotation lag sign (supraspinatus, infraspinatus), Hornblower’s test (teres minor), and lift off and belly press tests (subscapularis) are effective physical exam tests useful for determining the functional integrity of the rotator cuff [3, 4]. Park et al. evaluated the diagnostic accuracy of clinical tests for full-thickness rotator cuff tears and found that the combination of a painful arc, drop arm sign, and infraspinatus muscle test produced the best probability for a full-thickness rotator cuff tear [5]. In a subsequent study, Bak et al. supported the above finding and found that a positive lag sign (external rotation lag or drop arm test) is indicative of a full-thickness rotator cuff tear and specificity is further improved with a subacromial lidocaine injection [6]. However, in patients with severe shoulder arthritis and limitation of range of motion, using the above tests may be difficult to diagnose rotator cuff tears. Thus, advanced imaging (MRI or CT) is essential to evaluate for rotator cuff status.
Diagnostic imaging
Findings of shoulder osteoarthritis are diagnosed on orthogonal radiographic views of the shoulder (true AP [Grashey] view and axillary view). Joint space narrowing, subchondral sclerosis and cysts, and inferior humeral osteophytes are the hallmark radiographic findings of primary shoulder osteoarthritis. Retained implants in the glenoid rim or implants in the proximal humerus are indicators of prior instability surgery. Advanced imaging including MRI and CT arthrogram gives additional information regarding rotator cuff muscle quality (fatty infiltration—Goutallier) and integrity, humeral head subluxation, and glenoid morphology including version and wear pattern as described by Walch (Table 1) [7].
Glenoid deformity identified on CT scan is classified according to Walch [7].
Indications for total shoulder arthroplasty
The preferred indications for total shoulder arthroplasty include the following:
-
1.
Age >50 years old with activity-limiting shoulder pain and dysfunction which has failed nonsurgical conservative measures
-
2.
Absence of medical comorbidities which would preclude joint replacement surgery
-
3.
Physical exam findings that correlate with a history of pain and dysfunction, and an intact or reparable rotator cuff
-
4.
Radiographic findings of osteoarthritis and adequate glenoid bone stock
-
5.
Willing and able to comply with postoperative activity restrictions and rehabilitation protocols and accept the risks of surgery
The contraindications are as follows:
-
1.
Age <50 years old
-
2.
Medical comorbidities which would preclude joint replacement surgery
-
3.
Active infection
-
4.
Irreparable rotator cuff tear
-
5.
Inadequate glenoid bone stock
-
6.
Brachial plexus palsy (axillary nerve palsy)
Outcomes
The outcomes of total shoulder arthroplasty are excellent with significant improvement in pain and function in 95 % of patients [1]. Implant survivorship is reported by one study to be 85 % at 15-year follow-up and 82 % at 20-year follow-up [8]. The rate of revision total shoulder arthroplasty is approximately 7 %, with an overall mean complication rate ranging from 10 to 16 % [9, 10].
Complications after total shoulder arthroplasty
Infection
The incidence of infection after total shoulder arthroplasty is reported to be 0–4 % [11, 12]. Risk factors for infection include diabetes mellitus, chronic diseases, and history of acute infection at another joint. The diagnosis of infection after primary total shoulder arthroplasty can be difficult. Pain, prosthetic loosening, or a combination of these findings may be the only indicators of an acute or indolent periprosthetic infection. The average time following surgery to the development of deep periprosthetic infection is 3.5 years (range 0–14.8 years) [12]. Rarely do patients have elevated erythrocyte sedimentation rate (ESR), white blood cell (WBC) count, or C-reactive protein (CRP). One study demonstrated that CRP was elevated in 25 % of infected total shoulder arthroplasties at the time of revision [13]. Propionibacterium acnes (P. acnes) is the most common organism responsible for periprosthetic infection, and it is important to know that these bacteria require incubation for culture of an average of 5.1 days [12].
Infection should always be considered in the differential when considering patients who develop pain after a total shoulder arthroplasty (TSA). The following studies and parameters should always be investigated in the setting of shoulder pain after TSA given the relative indolent nature of P. acnes:
-
1.
Infection labs: CRP, ESR, WBC count with differential.
-
2.
Aspiration and holding of cultures for a minimum of 2 weeks [14].
-
3.
Orthogonal X-rays and intraarticular gadolinium CT scan to evaluate for glenoid lucency, component loosening, or osteomyelitis. Extravasation of dye around pegs or keel is highly suggestive of glenoid component loosening.
Normal infection labs do not preclude the diagnosis of infection. Furthermore, the absence of positive cultures does not rule out infection either. If radiographic imaging does not suggest component loosening and other sources of pain are ruled out, then serial monitoring of shoulder pain and function is reasonable. If loosening is suggested but not confirmed by the appearance of the imaging studies, then a diagnostic arthroscopy with tissue cultures, repeat aspiration, and evaluation of glenoid component stability can be considered.
Ultimately, if infection is determined to be present, an open debridement is indicated with component removal. Matsen and associates outlined intraoperative findings which were predictive of a P. acnes-positive culture. Male sex, humeral osteolysis, and cloudy fluid were associated with a 600 % increased likelihood of obtaining a positive P. acnes culture. Identification of these predictive factors assists surgical decision-making and antibiotic therapy before the results of intraoperative cultures [14]. The elusiveness of a definitive diagnosis of infection even with negative intraoperative gram stains and frozen sections highlights the need for detailed preoperative planning and shared decision-making with the patient.
In the setting of an infected TSA, component removal and placement of an antibiotic spacer are indicated. Not infrequently, as Warner and colleagues found, patients can have remarkable function and pain relief with an antibiotic spacer alone. Their function and pain relief is often durable enough to preclude further surgery [15]. Furthermore, Walch et al. have shown a high failure rate of revision anatomic shoulder arthroplasty in the setting of loose glenoids [16•]. Given the potential for adequate pain relief and function with an antibiotic spacer, the role of a single-stage revision in the setting of infection is uncertain and probably should be discouraged.
Other considerations for the management of periprosthetic TSA infections are based on the timing for the development of infection relative to the timing of the development of symptoms. Sperling et al. provided a classification to guide treatment for periprosthetic TSA infection based on level 4 evidence and is largely similar to management of periprosthetic infection in knee and hip arthroplasty (Table 2) [17].
For patients with a history of an infected TSA who are not satisfied with their function and pain with an antibiotic spacer or in whom a reimplantation is already planned, a revision TSA can be considered. As mentioned previously, the failure rate of revision TSAs is high in general as noted in Walch and colleagues’ experience which were performed for a variety of reasons including infection [16•]. Zhang and colleagues, however, further clarified their experience with 18 patients with infected TSA managed with staged reimplantation of components after a scheduled open biopsy. They found that 22 % of open biopsy specimens obtained prior to the scheduled second-stage reimplantation had evidence of persistent infection. Seventy-five percent of the persistent infections were P. acnes positive on culture. These patients then underwent another course of debridement and intravenous antibiotics for 6 weeks prior to reimplantation. At the final follow-up, all patients in this study were infection free after staged revision and open biopsy [18•]. This recent study offers an effective treatment algorithm for approaching two-stage reimplantation and also highlights the challenge of eradicating a P. acnes periprosthetic infection.
Given the poor outcomes and complications associated with revision TSA, an alternative approach for revision in the setting of an eradicated infection is conversion to reverse total shoulder arthroplasty (RTSA). Several case series indicate that two-stage revision with RTSA can be effective at eradicating infection and provides improved outcomes over revision to a TSA [19, 20]. Patient age, activity level, and implant longevity, however, must be considered when deciding to use a RTSA in the revision setting.
Instability following TSA
Instability following TSA is uncommon and can be associated with component-related factors and further classified based on the primary direction of instability [21]. These component factors include malpositioning and incorrect sizing of the humeral or glenoid side, as well as anatomic factors such as glenoid version, humeral subluxation, rotator cuff insufficiency, or a combination of factors resulting in imbalanced or improperly tensioned soft tissues. Recognizing which elements are causing the instability is critical before embarking on revision surgery. Despite recognition of the cause of instability, in general, revision surgery frequently results in a high rate of failure [22].
Superior instability is essentially a result of an incompetent superior-posterior rotator cuff, namely the infraspinatus. This problem is usually the result of an attritional rupture of the rotator cuff and treatment options are limited. Consideration for rotator cuff repair can be made but the outcome is unpredictable. Hattrup et al. reported the results of 20 patients with rotator cuff tear and repair after TSA and found success in only 4 patients (20 %) [23]. Majority of patients still had pain with limitation of range of motion. Thus, the most reliable option in the setting of persistent pain and weakness/loss of motion would be revision to a reverse total shoulder arthroplasty.
Anterior instability is usually associated with subscapularis insufficiency. Again, subscapular insufficiency is addressed elsewhere in this chapter, but choices include subscapularis repair if possible (depending on the chronicity from the time of injury and condition of the tendon or muscle), pectoralis major tendon transfer (not effective when static anterior subluxation is present), or revision to a reverse total shoulder arthroplasty. Revision to a reverse total shoulder is an option but not an optimal option in younger individuals (age <60). A modified Latarjet procedure is an option in the setting of a young patient with anterior static subluxation and can restore function [24].
Likewise, posterior shoulder instability can result from a variety of reasons but most commonly from preexisting posterior humeral subluxation and glenoid retroversion (Fig. 1). Fortunately, recurrent posterior instability is uncommon after TSA.
Glenoid component loosening
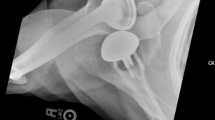
Glenoid component loosening is the most common complication of total shoulder arthroplasty and is reported to have a prevalence of 6 % [1]. Pain may be the presenting sign of a loose glenoid component. Orthogonal radiographs of the shoulder and CT scan assist in the diagnosis of a loose glenoid component (Fig. 2).
It is not known if the presence of radiolucent lines predicts or indicates glenoid component loosening and failure. Radiolucent lines at the cement-bone interface are present in 15–84 % of TSAs but do not necessarily correlate to symptomatic loosening [17]. Lack of long-term outcome studies correlating the presence and severity of radiographic radiolucent lines with glenoid loosening limits our ability to truly discern their prognostic applicability.
Factors associated with glenoid component loosening include rotator cuff deficiency, glenoid morphology, and osteolysis. Mechanically, these factors contribute to eccentric loading of the humeral head on the glenoid component, with the end result being the “rocking horse phenomenon” and eventual failure. The rocking horse phenomenon can occur anterior to posterior as a function of increased glenoid retroversion. Walch et al. demonstrated that biconcave B2 glenoids with increased retroversion and associated posterior humeral subluxation experienced worse outcomes after TSA [25]. Additionally, the rocking phenomenon can occur superior to inferior in the setting of rotator cuff tear and disruption of the dynamic force couple. Furthermore, osteolysis of the glenoid has been shown to be secondary to asymmetric wear and development of minute polyethylene (PE) particles which are consumed by macrophages. This initiates a cascade of osteoclastic activation and bone resorption, mediated by receptor activator of nuclear factor-kappa beta (RANK) ligand and interleukin-1 (IL-1) [26].
Another category of glenoid component failure and loosening that deserves mention are metal-backed glenoids. Introduced as an alternative to cemented polyethylene glenoids, metal-backed glenoids have consistently resulted in higher rates of failure and requirements for revision surgery [27–29]. The main mode of failure in an all-PE glenoid component is aseptic loosening, whereas in a metal-backed glenoid design, failure is related to PE liner wear and dissociation [16•, 27, 30]. The reasons metal-backed glenoid components fail at a higher rate than that of cemented all-PE glenoids are not clear but may be related to the increased stresses given they are fixed rigidly. Finite element analysis has shown that a PE component fixed in a metal-backed glenoid experiences greater amounts of stress in comparison to a cemented all-PE component [31]. Despite the historically poor outcomes for metal-backed glenoids, newer designs with alternative materials including those with porous coating and trabecular metal designs are being introduced. Unfortunately, similarly high failure rates are being found even with the newer trabecular metal designs [32].
Prevention strategies at the time of implantation include preservation of glenoid bone stock and the subchondral bone plate with minimal reaming and using cementation techniques that decrease porosity and maximize cement-bone integration [17].
Surgical management of glenoid component loosening is indicated in the symptomatic patient. Revision TSA is the preferred surgical management in the majority of patients. Another option for management of a symptomatic loose glenoid component is arthroscopic removal of the component and the cement mantle, as described by O’Driscoll and associates [33]. Their report of five cases demonstrated the technical feasibility, and their small sample was satisfied with the surgery. Potential indications for this procedure are those individuals with multiple medical comorbidities and the morbidly obese. Infection should be the diagnosis until proven otherwise. Additionally, rotator cuff insufficiency should be suspected, which can be evaluated with a good physical exam and advanced imaging.
The outcomes after revision arthroplasty for glenoid component loosening are guided by case series. Cheung et al. retrospectively reviewed 68 shoulders in 66 patients who underwent revision TSA for glenoid component loosening [34]. Thirty-three shoulders underwent placement of a new glenoid component, and the remaining 35 shoulders underwent removal of the glenoid component and bone grafting without glenoid reimplantation. At 5 years, 91 % of primary glenoid reimplantation patients had satisfactory outcome without a reoperation, and 78 % of patients with glenoid bone grafting had survival free of operation. In contrast, Boileau et al. recently reported the results of a retrospective multicenter review of 42 revision TSA for glenoid component loosening. The rate of recurrent glenoid loosening was 67 % with 17 % of patients requiring rerevision. The overall complication rate was 45 % [16•]. Due to recent poor outcomes with revision TSA for glenoid component loosening, RTSA is being investigated as a surgical alternative with initial results reported as favorable. Walch and Boileau and colleagues recently reported on their results with 37 patients who underwent RTSA for aseptic glenoid loosening of an anatomic TSA associated with the presence of another complication including rotator cuff tears, subscapularis insufficiency, prosthetic instability, or glenoid bone deficiency. Overall, 86 % of patients were satisfied or very satisfied with the outcome. There was a 21 % reoperation rate secondary to complications [35••]. Therefore, it is essential to take into consideration the age, activity level, and implant longevity when pursuing RTSA in the setting of a failed TSA secondary to glenoid loosening.
Humeral component loosening
The overall prevalence of humeral component loosening is approximately 1 % [36]. As with the glenoid component, radiolucent lines may be a harbinger for future component loosening requiring revision [37]. However, various studies have shown radiolucent lines to be present without any clinically evident loose humeral prostheses [38]. Sperling et al. identified humeral components “at risk” of loosening as those with subsidence, implant tilt, and at least 2-mm lucent lines around the stem [37]. If patients present with pain and “at risk” signs based on radiographs, humeral component revision may be indicated. Infection should also always be suspected as the etiologic source of loosening, and the patient should be managed accordingly.
Removal of a well-fixed humeral component can be difficult. If the component is not easily extracted from above, a longitudinal humeral split osteotomy or development of a cortical window is an effective technique [39]. Sahota et al. reported their results comparing these two techniques in 26 patients who required humeral component removal. The authors found no difference in intraoperative or postoperative complications, and there were no cases of malunion or nonunion. Osteotomies are effectively fixated with cerclage wires or heavy nonabsorbable sutures.
Rotator cuff tear and subscapularis failure after total shoulder arthroplasty
The reported incidence of postoperative TSA rotator cuff tears is 1.3 % (32 of 2540 shoulders) [40]. Subscapularis muscles tears were the most common rotator cuff tear, comprising approximately 50 % of the rotator cuff tears in the cohort and are primarily due to overstuffing the joint, tendon insufficiency after multiple open surgeries or secondary to fatty infiltration or aggressive passive external rotation rehabilitation. Primary repair of the subscapularis tendon tear is indicated if the tissue is of sufficient quality and amenable to repair. Pectoralis major tendon transfer can be considered in the setting of an irreparable subscapularis tear. This is a salvage procedure and is contraindicated in the setting of a humeral head that is no longer centered or statically subluxated anteriorly. Furthermore, the success of a pectoralis major tendon transfer appears also to be dependent on the quality of the existing subscapularis muscle. Patients with chronic fatty infiltrated subscapularis tears should be addressed with caution as pectoralis major tendon transfer does not provide an acceptable outcome in these patients [40]. Disruption of the subscapularis and supraspinatus results in a so-called rotator interval lesion and is exemplified by anterior and superior escape on radiographs (Fig. 3a, b). The integrity of the rotator cuff during the lifespan of a total shoulder arthroplasty is critical to continued function. Walch and colleagues recently demonstrated that the rate of secondary rotator cuff dysfunction with moderate or severe superior subluxation of the humeral prosthesis increased over time and significantly influenced the clinical and radiographic outcome of total shoulder arthroplasty. The authors also found that preoperative fatty infiltration of the infraspinatus muscle and implantation of the glenoid component with superior tilt were negative prognostic factors [41].
Periprosthetic fractures after TSA
The prevalence of periprosthetic humerus fractures is 0.6 to 2.3 % [42]. Identified risk factors include osteopenia, cortical thinning secondary to osteolysis, and eccentric reaming at the time of arthroplasty. Periprosthetic humerus fractures can be classified according to location in relation to the humeral component and whether the component is loose or well fixed. Orthogonal radiographs of the humerus are necessary, and many times, a CT scan aids determining the stability of the humeral stem.
The classification by Wright and Cofield described a classification system for periprosthetic humerus fractures primarily based on the location of the fracture [43]. This classification is useful as it guides treatment. A recent analysis by Andersen et al. showed low interobserver reliability for this classification system. Their experience showed a high union rate (97 %), but a high incidence of loose stems (50 %) and reoperation rate (19 %) indicated that periprosthetic fractures represent a difficult clinical problem [44].
-
Type A:
fracture at the tip of the component with proximal extension
Treatment (well-fixed component)—revision arthroplasty
Treatment (loose component)—revision arthroplasty, with allograft strut and internal fixation
-
Type B:
fracture at the tip of the component with no extension
Treatment (well-fixed component)—trial of nonoperative treatment with fracture functional bracing is reasonable, and if no evidence of healing after 3 months, then internal fixation with cerclage supplementation and allograft versus autograft strut graft
Treatment (loose component)—revision arthroplasty with long cemented stem bypassing the fracture, plus or minus allograft or autograft strut with internal fixation
-
Type C:
fracture at the tip of the component with distal extension
Treatment (well-fixed component)—nonoperative treatment with functional bracing if acceptable closed reduction can be maintained
Treatment (loose component)—revision arthroplasty with long stem component bypassing the fracture, plus or minus cortical strut graft with internal fixation
Neurologic injuries
The prevalence of neurologic injury after anatomic total shoulder arthroplasty is approximately 1 to 4.3 %. The axillary nerve is the most commonly injured nerve. Causes of injury include direct damage during the surgical approach, stretch injury from retractors, or compression from a postoperative hematoma, with the majority of cases resolving without surgical intervention needed [45, 46]. The musculocutaneous nerve is also at risk of injury with retractor placement deep to the conjoined tendon. Complete and partial brachial plexopathy after TSA is a rare complication thought to be due to traction but may also occur as a result of nerve block [47]. Most patients with brachial plexopathy experience improvement and frequently good functional recovery but can take over 12 months [46].
Interestingly, a recent study by Walch and associates demonstrated that RTSA had a 10.9 times higher incidence of transient peripheral nerve lesion than anatomic total shoulder arthroplasty, with the axillary nerve most commonly affected. The authors concluded that lengthening of the arm with RTSA may be the reason for the increased neurologic lesions in this group [45]. Evaluation of neurologic injury after TSA includes careful neurologic examination, EMG/NCS, and brachial plexus MRI (Fig. 4) with consideration for referral to neurology to identify the location of lesions. Electromyography is typically obtained 6 weeks after the initial onset of symptoms to obtain baseline results and may be repeated in 6 months to 1 year. In the absence of a clear nerve laceration or space-occupying lesion such as a hematoma or cement extrusion, watchful waiting is the method of management of traction-related palsies.
Conclusions
Total shoulder arthroplasty, originally used by Péan in 1893, has evolved over the past century and especially the last two decades to be a reliable surgical intervention to relieve the pain and dysfunction of glenohumeral osteoarthritis [48]. The most common complications are glenoid and humeral component loosening, periprosthetic fracture, rotator cuff tears, and infection. Orthopedic surgeons performing TSA must have a high index of suspicion of these complications when the TSA postoperative patient presents with pain and dysfunction. Many complications can present with similar clinical complaints of pain and decreasing function; therefore, differentiating between etiologies can be challenging. An algorithm to consider the steps for evaluating the painful total shoulder is listed in Fig. 5.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Should Elbow Surg. 2002;11(2):130–5.
Kim SH, Wise BL, Zhang Y, et al. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249–54.
Walch G, Boulahia A, Calderone S, et al. The ‘dropping’ and ‘hornblower’s’ signs in evaluation of rotator-cuff tears. J Bone Joint Surg (Br). 1998;80(4):624–8.
Gerber C, Hersche O, Farron A. Isolated rupture of the subscapularis tendon. J Bone Joint Surg Am. 1996;78(7):1015–23.
Park HB, Yokota A, Gill HS. Diagnostic accuracy of clinical tests for the different degrees of subacromial impingement syndrome. J Bone Joint Surg Am. 2005;87(7):1446–55.
Bak K, Sørensen AK, Jørgensen U. The value of clinical tests in acute full-thickness tears of the supraspinatus tendon: does a subacromial lidocaine injection help in the clinical diagnosis? A prospective study. Arthroscopy. 2010;26(6):734–42.
Walch G, Badet R, Boulahia A, et al. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756–60.
Cil A, Veillette CJ, Sanchez-Sotelo J, et al. Survivorship of the humeral component in shoulder arthroplasty. J Should Elbow Surg. 2010;19(1):143–50.
Bohsali KI, Wirth MA, Rockwood Jr CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279–92.
Wirth MA, Rockwood Jr CA. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603–16.
Coste JS, Reig S, Trojani C, et al. The management of infection in arthroplasty of the shoulder. J Bone Joint Surg (Br). 2004;86(1):65–9.
Sperling JW, Kozak TK, Hanssen AD, et al. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;382:206–16.
Topolski MS, Chin PY, Sperling JW, et al. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Should Elbow Surg. 2006;15(4):402–6.
Pottinger P, Butler-Wu S, Neradilek MB, et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012;94(22):2075–83.
Jawa A, Shi L, O’Brien T, et al. Prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) use for the treatment of infection after shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(21):2001–9.
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Should Elbow Surg. 2013;22(6):745–51. This is a retrospective review of 42 symptomatic failed TSAs secondary to glenoid component loosening and it demonstrated that revision TSA with reimplantation of an all-PE cemented glenoid component leads to high rate of recurrent glenoid loosening and failure.
Sperling JW, Hawkins RJ, Walch G, et al. Complications in total shoulder arthroplasty. J Bone Joint Surg Am. 2013;95(6):563–9.
Zhang AL, Feeley BT, Schwartz BS, et al.: Management of deep postoperative shoulder infections: is there a role for open biopsy during staged treatment? J Shoulder Elbow Surg. 2014. S1058-2746. This was a prospective study in which an open biopsy was performed as a separate procedure prior to second stage reimplantation for an infected TSA. Specimens obtained at time of open biopsy detected a high culture positive rate of 22% and in 38% of P. Acnes infections, which suggests a role for this procedure in the management of infected TSA undergoing two stage reimplantation.
Sabesan VJ, Ho JC, Kovacevic D, et al. Two-stage reimplantation for treating prosthetic shoulder infections. Clin Orthop Relat Res. 2011;469(9):2538–43.
Li X, Eichinger JK, Higgins LD. Management of failed metal-backed glenoid component in patients with bilateral total shoulder arthroplasty. Int J Shoulder Surg. 2013;7(4):143–8.
Moeckel BH, Altchek DW, Warren RF, et al. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492–7.
Sanchez-Sotelo J, Sperling JW, Rowland CM, et al. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85-A(4):622–31.
Hattrup SJ, Cofield RH, Cha SS. Rotator cuff repair after shoulder replacement. J Should Elbow Surg. 2006;15(1):78–83.
Endres NK, Warner JJ. Anterior instability after total shoulder replacement: salvage with modified Latarjet procedure. A report of 2 cases. J Should Elbow Surg. 2010;19(2):e1–5.
Walch G, Moraga C, Young A, et al. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Should Elbow Surg. 2012;21(11):1526–33.
Vermes C, Chandrasekaran R, Jacobs JJ, et al. The effects of particulate wear debris, cytokines, and growth factors on the functions of MG-63 osteoblasts. J Bone Joint Surg Am. 2001;83-A(2):201–11.
Fox TJ, Cil A, Sperling JW, et al. Survival of the glenoid component in shoulder arthroplasty. J Should Elbow Surg. 2009;18:859–63.
Taunton MJ, McIntosh AL, Sperling JW, et al. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90:2180–8.
Boileau P, Avidor C, Krishnan SG, et al. Cemented polyethylene versus uncemented metalbacked glenoid components in total shoulder arthroplasty: a prospective, double-blind, randomized study. J Should Elbow Surg. 2002;11:351–9.
Kasten P, Pape G, Raiss P, et al. Mid-term survivorship analysis of a shoulder replacement with a keeled glenoid and a modern cementing technique. J Bone Joint Surg (Br). 2010;92:387–92.
Stone KD, Grabowski JJ, Cofield RH, et al. Stress analyses of glenoid components in total shoulder arthroplasty. J Should Elbow Surg. 1999;8:151–8.
Budge MD, Nolan EM, Heisey MH, et al. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Should Elbow Surg. 2013;22:535–41.
O’Driscoll SW, Petrie RS, Torchia ME. Arthroscopic removal of the glenoid component for failed total shoulder arthroplasty. A report of five cases. J Bone Joint Surg Am. 2005;87(4):858–63.
Cheung EV, Sperling JW, Cofield RH. Revision shoulder arthroplasty for glenoid component loosening. J Should Elbow Surg. 2008;17(3):371–5.
Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Should Elbow Surg. 2012;21(3):342–9. This was a retrospective multicenter cohort study evaluating the outcomes of 37 consecutive anatomical TSA revised to RTSA for aseptic glenoid loosening or failure. Eighty six percent of patients were satisfied or very satisfied with their outcome; however, there was a 21% reoperation rate for either glenoid loosening, prosthetic instability or humeral subsidence. RTSA in the revision setting for glenoid loosening is a reliable option in the appropriate patient.
Deshmukh AV, Koris M, Zurakowski D, et al. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Should Elbow Surg. 2005;14(5):471–9.
Sperling JW, Cofield RH, O’Driscoll SW, et al. Radiographic assessment of ingrowth total shoulder arthroplasty. J Should Elbow Surg. 2002;9:507–13.
Matsen 3rd FA, Iannotti JP, Rockwood Jr CA. Humeral fixation by press-fitting of a tapered metaphyseal stem: a prospective radiographic study. J Bone Joint Surg Am. 2003;85:304–8.
Sahota S, Sperling JW, Cofield RH.: Humeral windows and longitudinal splits for component removal in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2014;S1058-2746.
Elhassan B, Ozbaydar M, Massimini D, et al. Transfer of pectoralis major for the treatment of irreparable tears of subscapularis: does it work? J Bone Joint Surg (Br). 2008;90(8):1059–65.
Young AA, Walch G, Pape G, et al. Secondary rotator cuff dysfunction following total shoulder arthroplasty for primary glenohumeral osteoarthritis: results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(8):685–93.
Kumar S, Sperling JW, Haidukewych GH, et al. Periprosthetic humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 2004;86-A(4):680–9.
Wright TW, Cofield RH. Humeral fractures after shoulder arthroplasty. J Bone Joint Surg Am. 1995;77:1340–6.
Andersen JR, Williams CD, Cain R, et al. Surgically treated humeral shaft fractures following shoulder arthroplasty. J Bone Joint Surg Am. 2013;95(1):9–18.
Lädermann A, Lübbeke A, Mélis B, et al. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(14):1288–93.
Lynch NM, Cofield RH, Silbert PL, et al. Neurologic complications after total shoulder arthroplasty. J Should Elbow Surg. 1996;5:53–61.
Walton JS, Folk JW, Friedman RJ, et al. Complete brachial plexus palsy after total shoulder arthroplasty done with interscalene block anesthesia. Reg Anesth Pain Med. 2000;25(3):318–21.
Lugli T. Artificial shoulder joint by Péan (1893): the facts of an exceptional intervention and the prosthetic method. Clin Orthop Relat Res. 1978;133:215–8.
Compliance with ethics guidelines
ᅟ
Conflict of interest
Josef K. Eichinger and Joseph W. Galvin declare that they have no conflict of interest. The authors are employees of the US Army. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army, the Department of Defense, or the US Government.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Shoulder Surgery: Complications
Rights and permissions
About this article
Cite this article
Eichinger, J.K., Galvin, J.W. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med 8, 83–91 (2015). https://doi.org/10.1007/s12178-014-9251-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12178-014-9251-x