Abstract
Ocular or eye pain is a frequent complaint encountered not only by eye care providers but neurologists. Isolated eye pain is non-specific and non-localizing; therefore, it poses significant differential diagnostic problems. A wide range of neurologic and ophthalmic disorders may cause pain in, around, or behind the eye. These include ocular and orbital diseases and primary and secondary headaches. In patients presenting with an isolated and chronic eye pain, neuroimaging is usually normal. However, at the beginning of a disease process or in low-grade disease, the eye may appear “quiet,” misleading a provider lacking familiarity with underlying disorders and high index of clinical suspicion. Delayed diagnosis of some neuro-ophthalmic causes of eye pain could result in significant neurologic and ophthalmic morbidity, conceivably even mortality. This article reviews some recent advances in imaging of the eye, the orbit, and the brain, as well as research in which neuroimaging has advanced the discovery of the underlying pathophysiology and the complex differential diagnosis of eye pain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ocular pain is defined as pain in the eyeball itself, but this localization is often uncertain; therefore, the term eye pain is frequently used to denote pain in, around, or behind the eye. In general, a patient referred to the neurologist for eye pain has an isolated and “quiet eye” with intra-, peri-, or retro-orbital pain that is often chronic and maybe associated with a headache [1••]. When the neuro-ophthalmic exam is normal, orbital and brain neuroimaging, including CT and MRI, is usually also normal, or it may show incidental findings, such as chronic, non-specific white matter signal changes, or atrophy, depending on the patient’s comorbidity and age [2, 3]. However, when the eye pain is non-isolated, but associated with abnormal external appearance, anisocoria, visual loss, or ophthalmoplegia, then neuroimaging is often the test of choice [4]. Neuroimaging may particularly be helpful at the early stage or in low-grade disease processes before other deficits develop [5]. The inflammatory and infectious, mechanical (abnormal intraocular or intracranial pressure), and vascular etiologies, and less likely tumors give rise to a painful eye. According to the site of the underlying pathology, the discomfort may arise from the eyeball, or the orbit, or be referred from the face or head and neck to the eye. This article focuses on the most frequent disorders presenting with eye pain to the neurologist, especially where neuroimaging is either diagnostic or excludes a structural lesion. However, when the diagnosis is clinically secured and an underlying pathology is not suspected, then imaging usually is not required [6].
Neuroimaging-Based Neuroanatomy of Eye Pain
Because the eye (conjunctiva, cornea, sclera, upper eyelid), the forehead, the tip of the nose, the scalp, the dura (anterior cranial fossa, falx cerebri, tentorium cerebelli), and the intracranial arteries and veins are all innervated by the first division of the trigeminal nerve (V1, ophthalmic), any painful process of these structures can cause eye pain. The ophthalmic branch is purely sensory. Its afferent fibers travel to the complex trigeminal nuclei in the dorsal brainstem, which expand as the trigeminal tract between the midbrain and upper cervical spinal cord at the level of C2 [7]. Familiarity with the anatomy of the pain pathway may allow the diagnosis of isolated eye pain by discovering subtle pathological changes. For example, Siritho reported a challenging case of herpes zoster ophthalmicus presenting without rash [8]. In another reported case, diffusion tensor MRI-derived fiber tractography and functional MRI were successfully used to detect brainstem dysfunction in patients with chronic temporomandibular pain syndromes [9, 10]. In a third example, a pontine descending tractotomy was done using imaging-based, neuronavigation-assisted microsurgery to help patients with medically and surgically intractable trigeminal neuralgia [11].
Neuroimaging in Neuro-Ophthalmology: Technical Considerations
MRI is the modality of choice when either neurologic or ophthalmic cause of eye pain is suspected. It provides non-invasiveness, full field of view imaging, high resolution, soft tissue contrast, and radiation-free benefits. Although MRI is a sensitive technique, its specificity is relatively low. For example, diffusion-weighted imaging (DWI), originally thought to be specific for vascular etiology, has been reported to show reduced diffusivity (suggesting axonal degeneration) in several diseases affecting the optic nerve and optic nerve head [12, 13]. Neuroimaging of the orbit poses technical challenges related to several factors; these include the artifacts arising at the different tissue interfaces and the techniques utilized for better visualization (fat suppression). A promising technique for orbital imaging is 3D radial-volumetric-interpolated breath-hold examination (VIBE). This is a motion robust fat-suppressed (FS), gradient echo, high-resolution (0.5–1 mm) isotropic sequence [14]. Another likely technique is 3D high-resolution MR angiography (MRA) with binomial off-resonant rectangular (BORR) pulse for imaging the orbital vasculature [15]. CT is helpful in the evaluation of calcified lesions such as aneurysm, optic nerve head drusen, optic nerve sheath meningioma, and retinoblastoma. However, it is limited by radiation exposure. Other techniques utilized in neuro-ophthalmology, often in conjunction with MRI, include optical coherence tomography (OCT) of the optic disc and the macula [16, 17], and chairside dynamic ophthalmic ultrasonography, which provides non-invasive evaluation of a variety of ocular and orbital pathologies [18].
Ophthalmic Causes of Eye Pain
Ophthalmic causes of eye pain may be divided into superficial and deep ocular, orbital (intra-and extraconal, muscle cone), and referred pain [19]. Features suggesting superficial eye pain include foreign body sensation, itching, and pain provoked by palpation. Photophobia can be associated with both superficial and deep eye pain and neurological disorders (most frequently migraine) [20, 21].
The most frequent ophthalmic cause of eye pain is chronic dry eye disease, a type of neuropathic ocular pain [22, 23]. In the neuropathic dry eye, orbital and brain MRI shows no relevant abnormality. However, familiarity with the condition is important for differential diagnostic purposes. From the imaging standpoint, the test of choice is corneal confocal microscopy, which allows direct observation of the corneal nerve endings, which show decreased density in patients with dry eye disease and with chronic migraine [24]. Neuropathic ocular pain has also been described following retina surgery and enucleation (phantom eye syndrome). Ophthalmic causes of referred eye pain or strain include refractive errors, accommodation spasm, anisometropia, astigmatism, and uncorrected presbyopia, especially in association with dry eye disease.
Infectious-Inflammatory Pain
Ocular pain provoked by eye movement suggests an orbital inflammatory, demyelinating, or infectious process, such as optic neuritis or perineuritis, scleritis, trochleitis, or orbital myositis. Posterior scleritis may be associated with anterior optic perineuritis, choroidal folds or effusion, serous retinal detachment, or macular edema. Orbital ultrasonography is very sensitive in detecting the associated posterior sub-tenon’s fluid accumulation [18], but MRI or CT with and without contrast is recommended as the initial diagnostic study [25]. Thin-section axial contrast-enhanced (CE) T1W FS sequence typically demonstrates thickening and enhancement of the posterior sclera. Patients with trochleitis present with severe episodic uni- or bilateral ocular or peri-ocular ache that worsens on adduction in downgaze, with greatest discomfort at the superomedial orbital rim. It may herald an autoimmune disorder [26]. MRI and CT high-resolution images may show thickening and enhancement of the trochlea or the tendon of the superior oblique muscle. It should be differentiated from trochlear headaches [27].
Optic perineuritis (OPN) is a localized form of idiopathic orbital inflammatory disease, in which the specific target tissue is the optic nerve sheath. It can be confirmed by demonstrating peripheral thickening and enhancement of the involved optic nerve sheath without central (nerve) involvement on CE MRI FS sequence [28••]. Perineuritis may be isolated or associated with myositis, posterior scleritis, or inflammatory changes in the adjacent intraconal adipose tissue, which is suggested by enlargement and enhancement of these structures. OPN may be idiopathic or associated with a systemic disorder, such as Bechet’s disease, Lyme disease, and sarcoidosis [29]. In demyelinating optic neuritis (ON), pain on eye movement was reported by 95 % of patients in the Optic Neuritis Treatment Trial, typically presenting few days prior to the ipsilateral visual loss and subsiding after the onset of blurred vision. In anterior optic neuritis, optic disc protrusion into the posterior globe may be observed on MRI with enhancement depending on the degree of disc swelling, similarly to optic disc edema due to other causes, such as anterior ischemic optic neuropathy or papilledema. Typically, mild optic nerve enlargement with intrinsic T2 hyperintensity and enhancement of the affected optic nerve is seen [30]. The hyperintensity is best demonstrated on MRI STIR, T2 FLAIR-, or T2-weighted (T2W) FS sequences, while the enhancement is best evaluated on CE T1W FS sequence. Occasionally, optic nerve hyperintensity on DWI, with or without corresponding hypointensity on the apparent diffusion coefficient (ADC) map, has also been observed [12] and is consistent with water restriction, similar to ischemic optic neuropathy. MRI is not required for the diagnosis of optic neuritis, which is made clinically. However, MRI is the only tool that enables the physician to diagnose multiple sclerosis at the first clinical presentation by identifying spatial and temporal dissemination. In neuromyelitis optica (NMO), the optic neuritis may be uni- or bilateral, although the bilaterality or chiasmal involvement may only be appreciated on MRI [30]. NMO patients with myelopathy typically demonstrate an elongated segment (three or more vertebral body segments) of continuous cord T2 hyperintensity, the so-called longitudinal extensive transverse myelitis. This is contrary to MS lesions, which are short (less than one vertebral body segment) and scattered. The lesion shows enhancement when the disease is active, although this may persist for up to 4 months after a relapse. In neurosarcoidosis, the associated eye pain may be secondary to optic neuritis, an inflamed lacrimal gland, uveitis, or may be referred from the intracranial compartment [31]. On MRI, sarcoidosis-related ON may be undifferentiated from any other causes of ON, but additional lacrimal gland enlargement suggests the diagnosis. Frohman et al. reported the so-called stem-to-stern (from globe to chiasm) optic nerve enhancement or nodular non-contiguous involvement. In the presence of frontal basilar meningeal enhancement, this is very suggestive of sarcoidosis [32]. Idiopathic orbital inflammation (IOI) or orbital pseudotumor may be localized or diffuse. In suspected isolated orbital myositis, a localized form of IOI, MRI, or CT with and without contrast is the test of choice [33•], but the disease could be followed by orbital ultrasonography [18]. In isolated myositis, neuroimaging usually shows enlargement of the affected extraocular muscle with involvement of the tendinous insertion site. There is a broad imaging differential diagnosis, especially in diffuse IOI, including but not limited to lymphoma, IgG4-related orbital disease (IgG4-ROD) [34], orbital histiocytic disorders (e.g., Rosai-Dorfman and Erdheim-Chester disease, Langerhans’ cell histiocytosis) [35], orbital amyloidosis [36], and Tolosa-Hunt syndrome (THS) [37]. Any of these disorders may spread intracranially mimicking a meningioma or even a glioma [38, 39]. The MRI findings are non-specific and include homogeneous T1W isointensity and T2W hypo- or isointensity (suggesting high cellularity of the lesions) and homogeneous enhancement. Helpful signs that suggest IgG4-ROD include findings that it is typically painless [40], is often bilateral and involving the lacrimal and salivary glands, frequently causes infraorbital nerve enlargement (a pathognomic feature) [41], is associated with multimuscle extraocular myositis and compressive optic neuropathy, may be a sclerosing orbital inflammation [42], and presents with an intracranial manifestation such as hypophysitis and hypertrophic pachymeningitis. On the contrary, IOI is typically associated with severe eye pain, restriction of eye movement in the field of action of the affected muscles, and orbital myositis of one or two muscles (most frequently: medial > superior > lateral > inferior rectus).
Pain in and around the eye may be the presenting symptom of orbital granulomatosis with polyangiitis (Wegener’s granulomatosis); neuroimaging characteristically reveals paranasal sinus disease with bony erosion and orbital extension [43].
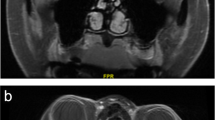
Fungal infections, especially in diabetic patients, may present with severe ocular or periorbital pain even without external infectious signs. Therefore, in the appropriate clinical settings, early sinonasal endoscopy with biopsy should be pursued to exclude rhino-orbital mucormycosis with the risk of intracranial spread or aspergillosis [44, 45]. Graves’ disease is the most frequent cause of thyroid eye disease (TED) and may present with ocular, orbital, or periorbital pain in a “quiet” eye. MRI has the advantage over CT by allow staging of the disease, as T2 hyperintensity of the most frequently involved muscles (inferior and medial rectus) suggests an active disease that is more amenable to treatment with corticosteroids [46] (Fig. 1). More recently, Kilicarslan et al. found DWI to be helpful in the detection of extraocular muscle involvement in the early stage of TED [47]. In TED, there is typically thickening and enhancement of the muscle itself, contrary to myositis where the muscle tendon is also involved.
Forty-two-year-old woman presented with binocular diplopia and “soreness” of both eyes. Coronal T2-weighted image demonstrates enlargement and heterogeneous signal of the medial more than lateral rectus muscles bilaterally at the mid-orbital level (a), and corresponding contrast-enhanced T1-weighted image (b) shows heterogeneous enhancement, more in the T2 hyperintense peripheral areas (arrows, marked in the right orbit only). The T2 hyperintensity suggests higher water content, consistent with active stage of thyroid eye disease
Mechanical Causes of Eye Pain (Glaucoma)
When the intraocular pressure is above 40 mmHg in an otherwise quiet eye, it may cause ocular or periocular pain or non-localizing episodic headache. This is especially true for patients with subacute angle-closure glaucoma, which may result in a significant delay in diagnosis [48, 49]. Therefore, glaucoma should be included in the differential diagnosis of an adult patient presenting with new onset headache. Because it is rare to provoke a glaucoma attach with the use of mydriatic drops, this fear should not discourage the neurologist to perform a dilated fundus examination [1••]. MRI is usually normal in new onset glaucoma patients; however, in association with chronic primary open-angle and more recently even early stages of glaucoma, several brain changes have been noted. These include atrophy and signal changes in the lateral geniculate body and decreased response on fMRI [50••, 51]. These imaging modalities are especially promising for securing the diagnosis in normal-tension glaucoma.
Vascular Pain
Following strabismus or surgery for scleral buckling, the development of achy pain may herald anterior segment ischemia. In any vascular lesion of the orbit, ocular or periocular pain may be the prodrome of a neuro-ophthalmic deficit, such as in ischemic optic neuropathy and microvascular ocular motor cranial nerve palsy. In ischemic optic neuropathy, MRI may demonstrate water restriction in the optic nerve on DWI [12], and in microvascular ocular motor cranial nerve palsy, it may show chronic microvascular changes of the brain or be normal.
Tumor-Related Pain
Ocular tumors usually present with painless visual disturbance but occasionally with pain in a “quiet eye.” Primary orbital tumors (eyelid, lacrimal gland) may also present with isolated eye pain (e.g., in lacrimal gland tumor, the eye pain may antecedes proptosis and gaze-evoked amaurosis) [52]. The differential diagnosis of lacrimal gland lesions includes pleomorphic adenoma (the most common benign tumor; however, malignant transformation may occur), adenoid cystic carcinoma (the most frequent malignant tumor), lymphoma (often a mucosa-associated lymphoid tissue type), and adenocarcinoma [53••]. CT may show calcification in pleomorphic adenoma and adenoid cystic carcinoma, but associated bone erosion and infiltration of adjacent structures only occurs in a malignancy. Adenoid cystic carcinoma may recur even after orbital exenteration and usually involves contiguous structures, such as the cavernous sinus secondary to perineural or perivascular spread. Other primary orbital tumors tend to present with painless visual symptoms and include cavernous hemangioma, lymphangioma, schwannoma, optic nerve glioma, and optic nerve sheath meningioma [52]. Tumorous processes of orbital bone may cause referred pain to the eye and include fibrous dysplasia, aneurysmal bone cyst, and chondrosarcoma.
Secondary orbital tumors that spread by direct extension from contiguous structures, especially in the immunocompromised, include cutaneous squamous cell carcinoma of the head and neck. Secondary metastatic orbital tumors have a predilection for highly vascular tissues (choroid, extraocular muscle, or marrow space of the greater wing of the sphenoid). Tumors that tend to metastasize to the orbital compartment include bladder, breast, lung, GI and prostate cancer, and lymphoma. On MRI, the lesion is usually hypointense on T1W and shows variable signal on T2W sequence (darker signal suggest higher cellularity) and enlargement and enhancement of the involved orbital structures. The most frequent skull base tumors that spread to the orbital compartment by direct extension from contiguous structures include nasopharyngeal carcinoma, angiofibroma, and esthesioneuroblastoma.
Neurologic Causes of Eye Pain
In general, any neurological disorder can cause referred eye pain, such as disorders of the cervical spine, carotid artery, sinuses [54], or temporomandibular joint. Therefore, neuroimaging should be obtained only when an underlying causative lesion is suspected, based on the patient’s history and neuro-ophthalmic examination [2]. This section will be limited to the retro-orbital pathologies with potential neuro-ophthalmic complications.
Primary Headache Disorders
The most frequent neurological cause of eye pain is migraine [1••, 3, 24], followed by primary stabbing headaches, trigeminal autonomic cephalalgias, and epicrania fugax [55]. A neuroimaging study is not indicated for the diagnosis of a primary headache disorder. However, when first evaluating a patient with new onset pain that localizes to the orbital region, especially when associated with autonomic features, then imaging is recommended to exclude cluster mimics, such as infection, tumor, and other pathologies [56]. Also, when a primary stabbing headache is persistently restricted to the same location, an underlying structural lesion in that location or within the distribution of the affected cranial nerve should be excluded by an imaging study.
Secondary Headache Disorders
The secondary causes of headaches and their neuroimaging features were recently reviewed in this journal by Chaudhry et al. Most of the reviewed disorders can be associated with referred ocular or periocular pain [57••].
Infectious-Inflammatory Pain
Cavernous sinus syndrome (CSS) can be caused by any pathology involving the cavernous sinus, but an infectious or inflammatory process is the most likely to present with intra- or periorbital pain before other more localizing signs appear. Most of the pathologies involving the cavernous sinus have the potential to involve adjacent structures, including the superior orbital fissure and orbital apex. Hence, the differential diagnosis of CSS includes disorders described under IOI, in addition to lymphoma, meningioma, sarcoidosis, THS, and less frequently Lemierre syndrome. Lemierre syndrome is a thrombophlebitis due to infection, typically untreated periodontal disease. This results in uni- or bilateral cavernous sinus thrombosis and may cause DWI hyperintensity in the superior ophthalmic vein(s) [58]. The diagnosis of THS is a diagnosis that is largely based on neuroimaging by excluding other disorders and demonstrating an inflammatory lesion [37]; however, recently reports of THS with normal MRI have been described [59]. THS typically presents with episodic but progressive eye pain, followed by diplopia. MRI with high-resolution images through the orbital apex and cavernous sinus demonstrates widening, T2 hyperintensity, and enhancement in the cavernous sinus, superior orbital fissure, and orbital apex; narrowing of the ipsilateral intracavernous internal carotid artery; and enlargement of the optic nerve and extraocular muscles. After initiation of high-dose corticosteroid treatment, symptoms should resolve within 24–72 h; however, improvement of cranial nerve deficits and regression of MRI abnormalities may take 2–8 weeks. CNS involvement is seen in about 25 % of sarcoidosis cases, but many of these are subclinical. Sarcoidosis is named the master mimicker of several disorders clinically and on imaging because it has a wide spectrum of intracranial imaging appearances. On brain MRI, lesions tend to be isointense relative to gray matter on T1W and hypointense on T2W sequences, with thickening and enhancement of the affected structures. Typical sites of involvement include the dura and leptomeninges at base of the brain, hypothalamus, pituitary gland, infundibulum, and anterior visual pathway [32]. Typical pattern of involvement suggesting sarcoidosis includes granulomatous infiltration causing intense plaque-like or nodular dural and leptomeningeal thickening and enhancement and solitary or multifocal CNS and meningioma-like dural masses. Leptomeningeal granulomas may go unnoticed on non-CE MRI.
Mechanical (Abnormal Intracranial Pressure)
Change in intracranial pressure in any direction can cause a headache but not typically ocular pain. Spontaneous intracranial hypotension is characteristically associated with orthostatic, while intracranial hypertension with hydrocephalus with occipital headache. Intracranial hypertension without hydrocephalus, i.e., pseudotumor cerebri syndrome (PTCS), frequently presents with retro-ocular pain, which may increase on eye movements [60], but the headache could be in any location [61]. PTCS is either primary, so-called idiopathic intracranial hypertension or secondary when an underlying cause is identified; therefore, it is a diagnosis of exclusion. In patients with symptoms suggesting raised intracranial pressure (ICP), a brain MRI with and without contrast should be obtained to exclude a space-occupying lesion, ventriculomegaly, and meningeal enhancement [62]. MRI in a patient with idiopathic intracranial hypertension should be normal, except it may show any of the following structural changes: empty or partial empty sella (usually with enlargement of the sellar floor suggesting chronicity); dilation of the optic nerve sheath; flattening of the posterior sclera with or without protrusion into the eyeball; tortuous course of the intraorbital optic nerve(s); and lateral transverse sinus stenosis (usually bilaterally) on MR or CT venography [63••]. The recognition of these imaging epiphenomena resulted in the newest revision of PTCS diagnostic criteria [62]. Additional neuroimaging findings that support raised ICP include meningoceles, hyperintensity of the optic disc on DWI [13], and spontaneous CSF leak (most frequently observed at the olfactory recess). In patients with asymmetric papilledema, larger optic canal size on the side of more disc swelling was measured [63••]. Causes of secondary PTCS include cerebral venous sinus occlusion, such as might result from a meningioma adjacent to the superior sagittal sinus, thrombosis (Fig. 2), or giant arachnoid granulation [64]. Spinal leptomeningeal lymphoma may also present as PTCS. Herespinal MRI typically shows extensive leptomeningeal enhancement of the lumbar and cauda equina nerve roots [65]. When a paraganglioma arises from the jugular bulb along the tympanic branch of the glossopharyngeal or the auricular branch of the vagus nerve, it may obstruct the intracranial venous outflow, resulting in secondary PTCS [66].
Twenty-three-year-old woman with history of migraine and 1 week duration of left ear ache presented with the worst headache of her life, described as left-sided with radiation into the neck for 5 days. a Head CT without contrast shows hyperdense vascular structures along the left tentorium cerebelli (white arrow) and transverse sinus (red arrow) that are most consistent with acute cortical vein and venous sinus thrombosis, respectively. b Axial gradient echo sequence shows hypointensity in the left transverse and sigmoid sinuses (white arrow) consistent with thrombosis and mild, likely reactive, opacification of the left mastoid air cells (yellow arrow)
Vascular Pain
An unruptured supra- or parasellar aneurysm of the internal carotid or ophthalmic arteries may be associated with peri-ocular pain and may cause a compressive optic neuropathy or chiasmopathy. Typically, a MRI T1W image shows an extra-axial, round, and mixed signal lesion, indenting the intracranial optic nerve or chiasm. This corresponds to hypointense flow void on coronal T2W image and peripheral enhancement due to central thrombosis. Only a minority (5 %) of trigeminal neuralgia patients present with periocular pain due to involvement of the ophthalmic division. Brain MRI is obtained to exclude neurovascular compression of the nerve at the trigeminal root entry zone by the superior cerebellar artery and less frequently a posterior fossa tumor or multiple sclerosis plaques.
Cervical carotid dissection may present with acute onset of ocular pain [67], retro-orbital headaches, and neck pain radiating to the jaw or eye and is frequently associated with ipsilateral Horner [68] syndrome. CT scan of the head without contrast in the acute setting is often interpreted as “normal,” but even early on, it may reveal enlargement with central area of hypo-and peripheral hyperdensity of the involved carotid artery at the skull base (Fig. 3a). The imaging evaluation of a suspected Horner syndrome can be lengthy and costly because of the long oculosympathetic pupillomotor pathway; therefore, the imaging study should be chosen according to the suspected site of injury [69]. However, recently, a single CE brain MRI with coverage extending to the T2 vertebral body level was found to be efficacious and cost effective [70]. MRI scan T1, T2, and DWI sequences (Fig. 3b) typically show peripheral hyper- and central hypointensity in the cervical segment of the involved artery with corresponding ADC map hypointensity consistent with water restriction in the vessel wall.
Forty-eight-year-old man with history of hypertension presented with new onset, persistent left retro-orbital headaches and an enlarged right pupil for 1 week. a Head CT without contrast demonstrates enlargement of the left internal carotid artery (ICA) with central area of hypodensity and peripheral hyperdensity (a, red arrow) and on corresponding axial diffusion-weighted image peripheral hyperintensity and central area of hypointensity in the left ICA (b, red arrow), consistent with an acute dissection of the left ICA, and normal right ICA (a, yellow arrow)
Carotid artery stenosis with resultant ocular hypoperfusion may cause a dull retro-orbital or frontal headache and various fundus abnormalities, consistent with ocular ischemic syndrome [71]. Typically, color Doppler flow imaging of the ophthalmic and central retinal artery demonstrates decreased velocity. Paradoxically, surgery for carotid artery stenosis may result in unilateral headache, with face and eye pain secondary to cerebral hyperperfusion syndrome [72]. Reversible cerebral vasoconstriction syndrome (RCVS) typically presents with an occipital onset bilateral thunderclap headache, followed by a more diffuse pain within a few seconds [73], though the headache may be retro-orbital initially, and subsequently visual symptoms, such as homonymous hemianopia or Bálint syndrome [74, 75], may develop. Recently, RCVS was found to be the most frequent cause of non-aneurysmal convexal subarachnoid hemorrhage with resultant superficial siderosis that is best observed on susceptibility-weighted or other gradient echo sequences [76].
A carotid-cavernous sinus dural arteriovenous fistula characteristically presents with retro-orbital headaches that may antedate other symptoms by 1–7 months [77]. MRI and MRA head source images frequently show the earliest findings: abnormal signal intensity and small flow voids in the cavernous sinus [78] (Fig. 4), asymmetry of the cavernous internal carotid arteries (smaller on the affected side), enlargement of the superior ophthalmic vein with flow void suggests reversed flow (arterialization), and tortuous intracranial ipsilateral hemispheric cortical veins with risk of venous infarction. In addition, the refluxed blood may flow to the contralateral cavernous sinus via the intercavernous sinus and further to the opposite superior ophthalmic vein. Carotid-cavernous fistula is treated with transvenous (with or without transarterial) coiling, depending on the route of refluxed (shunted) blood. Following coiling of the fistula, the periocular pain should resolve.
Seventy-five-year-old woman presented with 1-year duration of binocular diplopia and progressive redness of the eyes, right greater than left, for 6 months. a Brain MRA scan without contrast source image demonstrates abnormal signal in the right cavernous sinus (white arrow), dilated right Sylvian vein (yellow arrow), abnormal signal and dilation of the intercavernous sinus (a, red arrow), and dilation and arterialization of the right superior ophthalmic vein (b, arrow), consistent with right carotid-cavernous sinus fistula
A patient with giant cell arteritis (temporal arteritis) may present with retro-orbital pain; temporal, frontal, or occipital headache; jaw claudication; scalp tenderness; anorexia; and fever, with or without visual symptoms. It is a vasculitis of the middle and large size arteries. The gold standard for diagnosing giant cell arteritis is a positive temporal artery biopsy. Conventional neuroimaging techniques, including MRI and MRA, are usually unrevealing. Color Doppler ultrasound of the superficial temporal artery may demonstrate the “halo sign,” stenosis or complete occlusion of the small arterial branches, and the so-called ultrasound “compression sign,” suggesting giant cell arteritis [79, 80]. Primary angiitis of the central nervous system may present with periocular pain, especially when the angiitis involves the optic nerve or chiasm [81, 82]. MRI characteristically reveals T2 hyperintense signal and associated fine linear enhancement pattern in the brain parenchyma, frequently along the lenticulostriate vessels, and the optic nerve.
Conclusion
In patients with chronic isolated eye pain and normal neuro-ophthalmic examination, neuroimaging usually does not show an underlying causative lesion. However, early or in low-grade, progressive disease processes, neuroimaging may show a diagnostic, but often subtle, abnormality. Therefore, high index of clinical suspicion and familiarity with neuroimaging findings for secondary ocular pain are necessary. This may preserve vision or even be lifesaving.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Friedman DI. The eye and headache. Continuum (Minneap Minn). 2015;21(4 Headache):1109–17. This is a comprehensive review article with illustrative cases of ophthalmic and neurological causes of eye pain, emphasizing those with normal neurologic examination.
Harooni H, Golnik KC, Geddie B, Eggenberger ER, Lee AG. Diagnostic yield for neuroimaging in patients with unilateral eye or facial pain. Can J Ophthalmol. 2005;40(6):759–63.
Wolfe S, Van Stavern G. Characteristics of patients presenting with ocular pain. Can J Ophthalmol. 2008;43(4):432–4.
Prasad S. Diagnostic neuroimaging in neuro-ophthalmic disorders. Continuum (Minneap Minn). 2014;20(4 Neuro-ophthalmology):1023–62.
Mehta S1, Loevner LA, Mikityansky I, Langlotz C, Ying GS, Tamhankar MA, et al. The diagnostic and economic yield of neuroimaging in neuro-ophthalmology. J Neuroophthalmol. 2012;32(2):139–44.
Volpe Nicholas J, Lee Andrew G. Do patients with neurologically isolated ocular motor cranial nerve palsies require prompt neuroimaging? J Neuroophthalmol. 2014;34(3):301–5.
Voirol JR, Vilensky JA. The normal and variant clinical anatomy of the sensory supply of the orbit. Clin Anat. 2014;27(2):169–75.
Siritho S, Pumpradit W, Suriyajakryuththana W, Pongpirul K. Severe headache with eye involvement from herpes zoster ophthalmicus, trigeminal tract, and brainstem nuclei. Case Rep Radiol. 2015;2015:402015.
Wilcox SL, Gustin SM, Macey PM, Peck CC, Murray GM, Henderson LA. Anatomical changes at the level of the primary synapse in neuropathic pain: evidence from the spinal trigeminal nucleus. J Neurosci. 2015;35(6):2508–15.
Wilcox SL, Gustin SM, Macey PM, Peck CC, Murray GM, Henderson LA. Anatomical changes within the medullary dorsal horn in chronic temporomandibular disorder pain. Neuroimage. 2015;117:258–66.
Ibrahim TF, Garst JR, Burkett DJ, Toia GV, Braca JA 3rd, Hill JP, Anderson DE. Microsurgical pontine descending tractotomy in cases of intractable trigeminal neuralgia. Neurosurgery. 2015 Jul 31
Bender B, Heine C, Danz S, Bischof F, Reimann K, Bender M, et al. Diffusion restriction of the optic nerve in patients with acute visual deficit. J Magn Reson Imaging. 2014;40(2):334–40.
Salvay David M, Padhye Leena V, Huecker Julie B, Gordon Mae O, Viets R, Sharma A, et al. Correlation between papilledema grade and diffusion-weighted magnetic resonance imaging in idiopathic intracranial hypertension. J Neuroophthalmol. 2014;34(4):331–5.
Seeger A, Schulze M, Schuettauf F, Klose U, Ernemann U, Hauser TK. Feasibility and evaluation of dual-source transmit 3D imaging of the orbits: comparison to high-resolution conventional MRI at 3T. Eur J Radiol. 2015;84(6):1150–8.
Ye Y, Wu Z, Lewis NA, Fan Q, Haacke EM. Retrobulbar magnetic resonance angiography using binomial off-resonant rectangular (BORR) pulse. Magn Reson Med. 2015;74(4):1050–6.
Kupersmith MJ. Optical imaging of the optic nerve: beyond demonstration of retinal nerve fiber layer loss. J Neuroophthalmol. 2015;35(2):210–9.
Sibony P, Kupersmith MJ, Rohlf FJ. Shape analysis of the peripapillary RPE layer in papilledema and ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2011;52(11):7987–95.
Kendall CJ, Prager TC, Cheng H, Gombos D, Tang RA, Schiffman JS. Diagnostic ophthalmic ultrasound for radiologists. Neuroimaging Clin N Am. 2015;25(3):327–65.
Lee AG, Al-Zubidi N, Beaver HA, Brazis PW. An update on eye pain for the neurologist. Neurol Clin. 2014;32(2):489–505.
Katz BJ, Digre KB. Diagnosis, pathophysiology and treatment of photophobia. Surv Ophthalmol. 2016;61(4):466–77.
Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol. 2012;32(1):68–81.
Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What causes eye pain? Curr Ophthalmol Rep. 2015;3(2):111–21.
Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128–34.
Kinard KI, Smith AG, Singleton JR, Lessard MK, Katz BJ, Warner JE, et al. Chronic migraine is associated with reduced corneal nerve fiber density and symptoms of dry eye. Headache. 2015;55(4):543–9.
Lavric A, Gonzalez-Lopez JJ, Majumder PD, Bansal N, Biswas J, Pavesio C, et al. Posterior scleritis: analysis of epidemiology, clinical factors, and risk of recurrence in a cohort of 114 patients. Ocul Immunol Inflamm. 2015;2:1–10.
Fonseca P, Manno RL, Miller NR. Bilateral sequential trochleitis as the presenting feature of systemic lupus erythematosus. J Neuroophthalmol. 2013;33(1):74–6.
Smith JH, Garrity JA, Boes CJ. Clinical features and long-term prognosis of trochlear headaches. Eur J Neurol. 2014;21(4):577–85.
Hickman SJ. Optic perineuritis. Curr Neurol Neurosci Rep. 2016;16(2):16. This review article discusses the presentation, treatment, and prognosis of idiopathic and secondary optic perineuritis.
Lai C, Sun Y, Wang J, Purvin VA, He Y, Yang Q, et al. Optic perineuritis in Behçet disease. J Neuroophthalmol. 2015;35(4):342–7.
Costello F. Inflammatory optic neuropathies. Continuum (Minneap Minn). 2014;20(4 Neuro-ophthalmology):816–37.
Koczman JJ, Rouleau J, Gaunt M, Kardon RH, Wall M, Lee AG. Neuro-ophthalmic sarcoidosis: the University of Iowa experience. Semin Ophthalmol. 2008;23(3):157–68.
Frohman LP, Guirgis M, Turbin RE, Bielory L. Sarcoidosis of the anterior visual pathway: 24 new cases. J Neuroophthalmol. 2003;23(3):190–7.
Montagnese F, Wenninger S, Schoser B. "Orbiting around" the orbital myositis: clinical features, differential diagnosis and therapy. J Neurol. 2015;263(4):631–40. This is a comprehensive review of orbital myositis and its mimics, highlighting the value of orbital MRI in the differential diagnosis.
Ferry JA, Klepeis V, Sohani AR, Harris NL, Preffer FI, Stone JH, et al. IgG4-related orbital disease and its mimics in a Western population. Am J Surg Pathol. 2015;39(12):1688–700.
McClellan SF, Ainbinder DJ. Orbital Rosai-Dorfman disease: a literature review. Orbit. 2013;32(5):341–6.
Shah VS, Cavuoto KM, Capo H, Grace SF, Dubovy SR, Schatz NJ. Systemic amyloidosis and extraocular muscle deposition. J Neuroophthalmol. 2016;36(2):167–73.
Hao R, He Y, Zhang H, Zhang W, Li X, Ke Y. The evaluation of ICHD-3 beta diagnostic criteria for Tolosa-Hunt syndrome: a study of 22 cases of Tolosa-Hunt syndrome. Neurol Sci. 2015;36(6):899–905.
Tedeschi E, Ugga L, Caranci F, Califano F, Cocozza S, Lus G, et al. Intracranial extension of orbital inflammatory pseudotumor: a case report and literature review. BMC Neurol. 2016;16(1):29.
Lou X, Chen ZY, Wang FL, Ma L. MR findings of Rosai-Dorfman disease in sellar and suprasellar region. Eur J Radiol. 2012;81(6):1231–7.
Kashii S. IgG4-related disease: a neuro-ophthalmological perspective. J Neuroophthalmol. 2014;34(4):400–7.
Lee KH, Han SH, Yoon JS. Implications of enlarged infraorbital nerve in idiopathic orbital inflammatory disease. Br J Ophthalmol. 2015;30.
Sa HS, Lee JH, Woo KI, Kim YD. IgG4-related disease in idiopathic sclerosing orbital inflammation. Br J Ophthalmol. 2015;99(11):1493–7.
Isse N, Nagamatsu Y, Yoshimatsu N, Obata T, Takahara N. Granulomatosis with polyangiitis presenting as an orbital inflammatory pseudotumor: a case report. J Med Case Rep. 2013;7:110.
Anders UM, Taylor EJ, Martel JR, Martel JB. Acute orbital apex syndrome and rhino-orbito-cerebral mucormycosis. Int Med Case Rep J. 2015;8:93–6.
Arndt S, Aschendorff A, Echternach M, Daemmrich TD, Maier W. Rhino-orbital-cerebral mucormycosis and aspergillosis: differential diagnosis and treatment. Eur Arch Otorhinolaryngol. 2009;266(1):71–6.
Tortora F, Cirillo M, Ferrara M, Belfiore MP, Carella C, Caranci F, et al. Disease activity in Graves’ ophthalmopathy: diagnosis with orbital MR imaging and correlation with clinical score. Neuroradiol J. 2013;26(5):555–64.
Kilicarslan R, Alkan A, Ilhan MM, Yetis H, Aralasmak A, Tasan E. Graves’ ophthalmopathy: the role of diffusion-weighted imaging in detecting involvement of extraocular muscles in early period of disease. Br J Radiol. 2015;88(1047):20140677.
Nesher R, Mimouni MD, Khoury S, Nesher G, Segal O. Delayed diagnosis of subacute angle closure glaucoma in patients presenting with headaches. Acta Neurol Belg. 2014;114(4):269–72.
Nesher R, Epstein E, Stern Y, Assia E, Nesher G. Headaches as the main presenting symptom of subacute angle closure glaucoma. Headache. 2005;45(2):172–6.
Altobelli S, Toschi N, Mancino R, Nucci C, Schillaci O, Floris R, et al. Brain imaging in glaucoma from clinical studies to clinical practice. Prog Brain Res. 2015;221:159–75. This is a comprehensive review of the latest neuroimaing findings, including diffusion tensor imaging and functional MRI, in patients with glaucoma.
Zhang P, Wen W, Sun X, He S. Selective reduction of fMRI responses to transient achromatic stimuli in the magnocellular layers of the LGN and the superficial layer of the SC of early glaucoma patients. Hum Brain Mapp. 2016;37(2):558–69.
Szatmáry G. Imaging of the orbit. Neurol Clin. 2009;27(1):251–84. x.
Purohit BS, Vargas MI, Ailianou A, Merlini L, Poletti PA, Platon A, et al. Orbital tumours and tumour-like lesions: exploring the armamentarium of multiparametric imaging. Insights Imaging. 2015;7(1):43–68. A comprehensive review of orbital tumors and tumor mimics with emphasis on imaging findings.
Al-Hashel JY, Ahmed SF, Alroughani R, Goadsby PJ. Migraine misdiagnosis as a sinusitis, a delay that can last for many years. J Headache Pain. 2013;14:97.
Cuadrado ML, Guerrero AL, Pareja JA. Epicrania fugax. Curr Pain Headache Rep. 2016;20(4):21.
Carter DM. Cluster headache mimics. Curr Pain Headache Rep. 2004;8(2):133–9.
Chaudhry P, Friedman DI. Neuroimaging in secondary headache disorders. Curr Pain Headache Rep. 2015;19(7):30. This article reviews important causes of, and offers a neuroimaging-based diagnostic approach to, secondary headaches.
Miller B, Khalifa Y, Feldon SE, Friedman DI. Lemierre syndrome causing bilateral cavernous sinus thrombosis. J Neuroophthalmol. 2012;32(4):341–4.
Abdelghany M, Orozco D, Fink W, Begley C. Probable Tolosa-Hunt syndrome with a normal MRI. Cephalalgia. 2015;35(5):449–52.
Wall M. The headache profile of idiopathic intracranial hypertension. Cephalalgia. 1990;10(6):331–5.
Mallery RM, Friedman DI, Liu GT. Headache and the pseudotumor cerebri syndrome. Curr Pain Headache Rep. 2014;18(9):446.
Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159–65.
Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V. Brain imaging in idiopathic intracranial hypertension. J Neuroophthalmol. 2015;35(4):400–11. Excellent review article of the increasing number of recognized neuroimaging epiphenomenon in association with idiopathic intracranial hypertension.
Rosenberg KI, Banik R. Pseudotumor cerebri syndrome associated with giant arachnoid granulation. J Neuroophthalmol. 2013;33(4):417–9.
Ahmed RM, King J, Gibson J, Buckland ME, Gupta R, Gonzales M, et al. Spinal leptomeningeal lymphoma presenting as pseudotumor syndrome. J Neuroophthalmol. 2013;33(1):13–6.
Lertakyamanee P, Srinivasan A, De Lott LB, Trobe JD. Papilledema and vision loss caused by jugular paragangliomas. J Neuroophthalmol. 2015;35(4):364–70.
Richoz O, Scott Schutz J, Mégevand P. Pearls & oysters: unusual manifestations of bilateral carotid artery dissection: deep monocular pains. Neurology. 2012;78(3):e16–7.
Caplan LR, Biousse V. Cervicocranial arterial dissections. J Neuroophthalmol. 2004;24(4):299–305.
Almog Y, Gepstein R, Kesler A. Diagnostic value of imaging in horner syndrome in adults. J Neuroophthalmol. 2010;30(1):7–11.
Chen Y, Morgan ML, Barros Palau AE, Yalamanchili S, Lee AG. Evaluation and neuroimaging of the Horner syndrome. Can J Ophthalmol. 2015;50(2):107–11.
Newman N, Biousse V. Diagnostic approach to vision loss. Continuum (Minneap Minn). 2014;20(4 Neuro-ophthalmology):785–815.
Zhao WG, Luo Q, Jia JB, Yu JL. Cerebral hyperperfusion syndrome after revascularization surgery in patients with moyamoya disease. Br J Neurosurg. 2013;27(3):321–5.
Ducros A, Wolff V. The typical thunderclap headache of reversible cerebral vasoconstriction syndrome and its various triggers. Headache. 2016;56(4):657–73.
Raven ML, Ringeisen AL, McAllister AR, Knoch DW. Reversible cerebral vasoconstriction syndrome presenting with visual field defects. J Neuroophthalmol. 2016;36(2):187–90.
Walsh RD, Floyd JP, Eidelman BH, Barrett KM. Bálint syndrome and visual allochiria in a patient with reversible cerebral vasoconstriction syndrome. J Neuroophthalmol. 2012;32(4):302–6.
Graff-Radford J, Fugate JE, Klaas J, Flemming KD, Brown RD, Rabinstein AA. Distinguishing clinical and radiological features of non-traumatic convexal subarachnoid hemorrhage. Eur J Neurol. 2016;23(5):839–46.
Nomura M, Mori K, Tamase A, Kamide T, Seki S, Iida Y, et al. Cavernous sinus dural arteriovenous fistula patients presenting with headache as an initial symptom. J Clin Med Res. 2016;8(4):342–5.
Rucker JC, Biousse V, Newman NJ. Magnetic resonance angiography source images in carotid cavernous fistulas. Br J Ophthalmol. 2004;88(2):311.
Aschwanden M, Imfeld S, Staub D, Baldi T, Walker UA, Berger CT, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol. 2015;33(2 Suppl 89):S-113–5.
Landau K, Savino Peter J, Gruber P. Diagnosing giant cell arteritis: is ultrasound enough. J Neuroophthalmol. 2013;33(4):394–400.
Benson CE, Knezevic A, Lynch SC. Primary central nervous system vasculitis with optic nerve involvement. J Neuroophthalmol. 2015;16.
Rao NM, Prasad PS, Flippen 2nd CC, Wagner AS, Yim CM, Salamon N, et al. Primary angiitis of the central nervous system presenting as unilateral optic neuritis. J Neuroophthalmol. 2014;34(4):380–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gabriella Szatmáry declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Szatmáry, G. Neuroimaging in the Diagnostic Evaluation of Eye Pain. Curr Pain Headache Rep 20, 52 (2016). https://doi.org/10.1007/s11916-016-0582-8
Published:
DOI: https://doi.org/10.1007/s11916-016-0582-8