Abstract
Purpose of Review
Septic coagulopathy is a complex disorder linked with multiple organ dysfunction and increased mortality, and definitive treatments are still lacking. This review summarizes the current understanding of septic coagulopathy, covering its pathophysiology, diagnosis, and debatable treatment approaches. Additionally, it provides a thorough overview of recent research and emerging trends in this area.
Recent Findings
Recent studies have highlighted the interplay between coagulation mechanisms in sepsis and inflammatory response. Diagnostic tools include the newly published scoring system for sepsis-induced coagulopathy and the existing scoring systems for disseminated intravascular coagulation, enhancing early detection and treatment. Several drugs targeting abnormal clotting have been investigated in septic coagulopathy or wider septic groups, including heparin, antithrombin, activated protein C, and human-soluble thrombomodulin. However, they have not yielded clear survival benefits. Nonetheless, recent studies indicate that some of those therapies may benefit specific groups with septic coagulopathy, emphasizing the growing interest in emerging biomarkers and precision medicine to enhance patient outcomes.
Summary
Despite recent advancements, no pharmaceutical intervention is currently endorsed for septic coagulopathy. However, a noted association exists between disseminated intravascular coagulation and unfavorable prognosis. Future research is imperative, especially in devising individualized treatment strategies tailored to each patient’s condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Septic coagulopathy is a prominent complication in patients with sepsis. This condition is marked by an abnormality in the coagulation system, leading to serious clotting disorders and bleeding complications [1]. Disordered coagulation is one of the characteristic features of sepsis, and almost every patient suffering from sepsis experiences increased coagulation activation, reduced anticoagulation, and impaired fibrinolysis [2]. The incidence of coagulopathy in septic patients ranges from 50 to 70%, depending on the specific definition used [3]. Furthermore, its correlation with increased mortality rates underscores the significance of this condition. In a post hoc analysis of two large randomized controlled trials (RCTs) on sepsis, it was associated with significantly higher 90-day mortality (13.9% vs. 26.8%) and morbidity in patients diagnosed with septic coagulopathy based on the latest diagnostic criteria [4].
The pathophysiology of septic coagulopathy is highly complex, involving the intricate interplay of coagulation mechanisms, anticoagulant pathways, and fibrinolysis, all intertwined with the systemic inflammatory response [5, 6]. These changes serve as both detrimental effects and defensive reactions to the invasion of pathogens [7]. Such intricacy poses significant challenges to effective management, as therapeutic strategies must adapt to their variable course and strive to strike a balance between controlling coagulation and minimizing the risk of bleeding [8]. Notably, there is no pharmaceutical treatment for septic coagulopathy with clinically solid evidence backing its recommendation. These aspects stress the necessity for a comprehensive understanding of septic coagulopathy, from its initial triggers to its ultimate manifestations, to optimize the management of sepsis.
Given these considerations, this review aims to consolidate the current knowledge on septic coagulopathy, detailing its pathophysiology, diagnostic tools, and debatable treatment options. Additionally, we seek to spotlight ongoing research initiatives, offering a strategic roadmap for future explorations in septic coagulopathy.
Methods
We conducted a comprehensive search on PubMed for relevant articles addressing septic coagulopathy published by June 22, 2023. The search strategy is shown in the Supplementary Information. To ensure consistent language throughout our review, we only included literature described in English. Additionally, we incorporated relevant articles obtained from a review of the references in the identified manuscripts.
Systemic Inflammatory Response and Coagulation in Sepsis: Immunothrombosis and Septic Coagulopathy
The essential mechanism of sepsis is an exaggerated systemic inflammatory response, leading to hypercoagulability. This response involves a complex interplay of immune cells, cytokines, and other mediators, collectively contributing to a pro-coagulant state. This state is characterized by the activation of coagulation pathways, inhibition of anticoagulant pathways, and impairment of fibrinolysis, culminating in the widespread formation of microvascular thrombi [9, 10].
In sepsis, the onset of the inflammatory response is driven by the detection of pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors (PRRs) on immune cells [11]. This recognition triggers the release of pro-inflammatory cytokines such as tumor necrosis factor (TNF), interleukin-1β (IL-1β), and interleukin-6 (IL-6), which subsequently activate the coagulation system. The activated coagulation system can further amplify the inflammatory response, creating a vicious cycle of inflammation and coagulation [11, 12].
A significant player in sepsis is immunothrombosis, a process that intertwines the immune response and coagulation (Fig. 1). Neutrophil extracellular traps (NETs), web-like structures released by neutrophils, are vital contributors to this process. NETs can trap and kill pathogens and provide a scaffold for platelets and coagulation factors, promoting thrombus formation [13]. This interaction between the immune system and coagulation can contribute to the pathogenesis of sepsis. NETs are formed by a unique NETosis process, a cell death distinct from apoptosis and necrosis [14, 15]. During NETosis, the nuclear membrane of the neutrophil ruptures, and the chromatin, mixed with granular proteins, is expelled into the extracellular space [16]. These NETs can trap and neutralize pathogens, preventing their dissemination. However, the exposed chromatin and granular proteins can activate platelets and coagulation, forming thrombi [7].
Pathogenesis of septic coagulopathy: immune activation and thrombotic response. In the initial process of septic coagulopathy, immune cells recognize pathogen-associated molecular patterns (PAMPs) through pattern recognition receptors (PRRs). This recognition triggers the release of pro-inflammatory cytokines such as tumor necrosis factor (TNF), interleukin-1β (IL-1β), and interleukin-6 (IL-6). The ensuing inflammation destroys the glycocalyx that covers the surface of vascular endothelial cells. Subsequently, the inflammatory cytokines promote the induction of neutrophils, which then adhere to the exposed vascular endothelial cells. Once activated, the neutrophils release neutrophil extracellular traps (NETs), networks of fibers primarily composed of deoxyribonucleic acid (DNA) from neutrophils, which serve to trap, localize, neutralize, and kill bacteria. These NETs also have the effect of activating platelets and promoting thrombosis. Excessively formed clots cause organ ischemia and gangrene. Platelets and coagulation factors are consumed, and fibrinolysis is activated, resulting in an increased risk of bleeding. Finally, the inflammation-injured vascular endothelium becomes hyperpermeable, leading to fluid loss
While essential for the body’s defense mechanism, the coagulation cascade can also harm septic patients. The widespread formation of microvascular thrombi can lead to organ dysfunction due to impaired perfusion [12]. Moreover, clot formation causes consumptive coagulopathy, resulting from the consumption of coagulation factors and platelets, and an increased fibrinolytic system, which increases the risk of bleeding. [17,18,19]. Therefore, patients with septic coagulopathy are at risk for both thrombotic and hemorrhagic complications due to immunothrombosis. The adverse effects of the coagulation cascade in sepsis extend beyond thrombosis and bleeding. The activation of coagulation can also lead to the generation of thrombin, which can have pro-inflammatory effects and cause endothelial dysfunction [20, 21, 22•]. Thrombin can induce the expression of adhesion molecules on endothelial cells, promoting the recruitment and adhesion of leukocytes. It can also cause the release of pro-inflammatory cytokines, further amplifying the inflammatory response and endothelial damage [23].
The systemic inflammatory response and coagulation in sepsis are closely intertwined, amplifying the other. This interplay can lead to a dysregulated coagulation, resulting in widespread thrombosis, consumptive coagulopathy, and organ dysfunction. The intricate balance between inflammation and coagulation and the role of NETs in sepsis remains to be explored and understood.
Definition and Diagnostic Criteria of Septic Coagulopathy: An Exploration of Terminology and Conceptual Framework
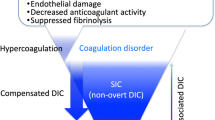
“Septic coagulopathy” is a term used to describe a multifactorial composite state that reflects the complex pathophysiology of sepsis, involving altered coagulation, immune response, and inflammatory pathways. Within this broad spectrum, two specific entities disseminated intravascular coagulation (DIC) and sepsis-induced coagulopathy (SIC), have been defined and distinguished but have commonalities between the two (Fig. 2). These scores are designed to identify the most severely ill population in terms of coagulation, and in this respect, they serve more as prognostic criteria to identify patients at high risk of death, rather than as diagnostic criteria. Due to their validated prognostic performance, these scores have been used in many clinical trials to select populations for therapeutic intervention.
Conceptual Venn diagram of septic coagulopathy, disseminated intravascular coagulation, and sepsis-induced coagulopathy. Septic coagulopathy is a conceptual term that refers to various degrees of coagulopathy occurring in patients with sepsis. Disseminated intravascular coagulation (DIC) is a condition of severe coagulopathy resulting from a variety of conditions, including sepsis, and is defined by criteria. Sepsis-induced coagulopathy (SIC) is defined by criteria specifically established for sepsis to identify severely ill patients, which broadly encompasses DIC due to sepsis
Disseminated Intravascular Coagulation
DIC is a complex and severe pathological condition encountered in critical illnesses, characterized by a simultaneous occurrence of hypercoagulability and fibrinolysis, resulting in widespread microvascular thrombosis and paradoxical bleeding [10, 24]. These manifestations impact multiple organ systems, leading to significant morbidity and mortality. Among conditions leading to DIC, sepsis is notable for its immune response to infection. This response causes a complex interplay between the inflammatory and coagulation systems, ultimately leading to DIC. The study of sepsis-associated DIC has been extensive, with research focusing on understanding the underlying mechanisms, identifying diagnostic markers, and developing targeted therapeutic strategies [25,26,27].
Two predominant scoring systems have been developed to facilitate the diagnosis of DIC: the International Society on Thrombosis and Hemostasis (ISTH) DIC diagnostic scoring system and the Japanese Association for Acute Medicine (JAAM) DIC diagnostic scoring system [28, 29].
The DIC Diagnostic Criteria by ISTH
The ISTH has formulated a concept of DIC and a scoring system to enhance patient outcomes [28]. The ISTH DIC scoring system is structured to be applied in the presence of an underlying disorder associated with DIC. This scoring system incorporates the results of routine laboratory tests, which are generally available daily in most hospitals. Depending on the type of test used, cutoff values for a “strongly” or “moderately” elevated result of the test for fibrin-related markers, including soluble fibrin monomers, D-dimer, or fibrin degradation products (FDP), are established. A score equal to or more than five is compatible with overt-DIC, whereas a score of less than five may be indicative (but not affirmative) for non-overt DIC (Table 1). A systematic review reported a consistent association between the presence of DIC, as determined by the ISTH DIC scoring system, and mortality [30]. Furthermore, the act of conducting ISTH overt-DIC screening itself on the day of intensive care unit admission was associated with a lower mortality rate, and this association became even stronger when the screening was repeated 2 days later [31]. These findings suggest that early and repeated screening using the ISTH DIC scoring system may decrease the mortality of patients afflicted with DIC.
The primary objective of developing ISTH DIC criteria was identifying patients who would benefit from specific treatments [32]. The ISTH DIC criteria have been used to select participants in clinical trials, underlining the practical utility of the criteria in both clinical practice and research settings [33].
The DIC Diagnostic Criteria by JAAM
The JAAM issued a distinct set of scoring systems for diagnosing DIC in 2005 [29]. This diagnostic criteria set was developed and validated based on data from sepsis and trauma populations [29, 34]. The criteria are designed with an emphasis on early detection and treatment. The distinguishing feature of the JAAM DIC criteria is its focus on systemic inflammatory response and organ dysfunction. They incorporate both clinical and laboratory factors that mirror inflammation and organ failure, including platelet count, fibrin/FDP, prothrombin time (PT) ratio, and the systemic inflammatory response syndrome (SIRS) score (Table 1).
The JAAM DIC diagnostic criteria have been used for patient selection and as endpoints in numerous clinical trials on DIC [35,36,37,38,39,40]. However, with the introduction of the updated Sepsis-3 definition [41] that eliminated the SIRS criterion for sepsis diagnosis, the JAAM DIC criteria became obsolete [42•]. In a study by Iba et al., analyzing data from 819 septic patients treated with recombinant human-soluble thrombomodulin (rhTM), they found a correlation between 28-day mortality and baseline coagulation parameters. They suggested that replacing the SIRS criteria with antithrombin activity in the JAAM DIC diagnostic criteria might be a feasible alternative [43]. However, the pertinent authorities have not formally approved the proposal.
Sepsis-Induced Coagulopathy
The SIC Diagnostic Criteria by ISTH
Following the introduction of the Sepsis-3 definition, the ISTH presented the SIC criteria, a scoring system specifically tailored for coagulation disturbances in sepsis [41, 44]. The SIC scoring system comprises three parameters: the platelet count, the PT international normalized ratio, and the Sequential Organ Failure Assessment (SOFA) score [45]. A SIC score of 4 points or higher is considered positive, indicating an elevated risk of mortality [44, 46]. This system seeks to pinpoint a consistent group of patients with analogous pathophysiology and clinical attributes, enhancing the prospects of improved outcomes through targeted treatment interventions [44].
Distinctively, the SIC score introduces features that differentiate it from both the JAAM and other ISTH diagnostic criteria. The SIC criteria utilize the SOFA score, a pivotal component of the Sepsis-3 diagnostic criteria, contrasting the JAAM DIC criteria [41, 47] that employ the SIRS score (Table 1) [41, 47]. Significantly, the SIC criteria deviate from the traditional ISTH DIC diagnostic approach by omitting the FDP criterion. Studies show a strong correlation between the SIC score and overt-DIC as delineated by the ISTH criteria, and DIC as described by JAAM [48, 49]. Additionally, the SIC score showcases predictive efficacy for mortality and is instrumental in discerning ideal candidates for anticoagulant treatments [49,50,51].
Furthermore, the ISTH’s Scientific Standardization Committee has proposed a two-stage sequential scoring strategy. This initiates with the SIC score for preliminary screening, succeeded by the calculation of the overt DIC score when the SIC criteria thresholds are met [52]. Such a bifurcated approach stands to bolster early detection and identification, thus optimizing the treatment trajectory for septic coagulopathy, paving the way for innovative therapeutic interventions.
Management of Septic Coagulopathy
Treating Septic Coagulopathy: A Therapeutic Target or a Risky Endeavor?
The decision to treat septic coagulopathy remains contentious, primarily due to the delicate equilibrium between potential therapeutic advantages and inherent intervention risks [42•, 53,54,55,56]. The interplay between coagulation and inflammation in sepsis provides a compelling argument for treatment [57,58,59]. Coagulation activation paired with fibrinolysis suppression in sepsis can amplify the inflammatory response, perpetuating a detrimental cycle that exacerbates disease progression [60]. Consequently, targeting coagulopathy might temper the sepsis inflammatory response, presenting a hopeful strategy for enhancing patient outcomes.
However, the decision to treat is not straightforward. The complexity of the coagulation system, coupled with the delicate equilibrium of coagulation, anticoagulation, and fibrinolysis, introduces a significant risk of bleeding complications due to intervention [60, 61]. These challenges emphasize the inherent dangers involved in treating septic coagulopathy and raise questions about the feasibility and safety of such an approach. In clinical practice, prevailing evidence does not advocate specific therapies targeting coagulation to enhance sepsis prognosis. Clinicians are encouraged to follow established research and guidelines for holistic sepsis management rather than isolating coagulopathy as a singular therapeutic focus.
In the following sections, we will examine various treatments for septic coagulopathy, including anticoagulant or anti-thrombotic therapies in general sepsis populations, providing a detailed insight into the current research landscape in this field.
Role of Antithrombotic Agents
Heparin
Heparin, known to amplify antithrombin activity and thereby inhibit thrombin formation, has been rigorously researched for its potential in treating septic coagulopathy. Both unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) have been evaluated for their efficacy in sepsis management.
The HETRASE RCT evaluated heparin’s efficacy in septic patients. Involving a cohort of 319 participants, the study compared mortality outcomes between those treated with heparin and a placebo group [62]. Nonetheless, no significant mortality difference was observed: 14% in the heparin group versus 16% in the placebo (odds ratio, 0.87; 95% confidence interval [CI], 0.44 to 1.69) [62]. A subsequent meta-analysis of nine studies hinted at a potential advantage of administering UFH or LMWH, indicating a decrease in mortality rates (risk ratio [RR], 0.88; 95% CI, 0.77 to 1.00) [63]. A recent systematic review focused solely on UFH’s impact on sepsis reported reduced 28-day mortality in the UFH group (RR, 0.82; 95% CI, 0.72 to 0.94) [64]. While these results indirectly suggest a promising role for heparin in treating coagulopathy, it is crucial to recognize that these reviews encompass general sepsis populations and not specifically those with septic coagulopathy.
These analyses were limited by underreported safety outcomes in the included studies, masking the potential risk of significant bleeding—a notable adverse effect of heparin. Addressing these ambiguities, subsequent clinical investigation, including a large-scale RCT evaluating UFH’s effectiveness in septic shock (ClinicalTrials.gov number, NCT03378466), was initiated. However, the study’s recruitment was prematurely halted due to the coronavirus disease 2019 pandemic.
Antithrombin
Antithrombin (AT), a serine protease inhibitor family (serpins) member, has been the focus of extensive research to explore its potential efficacy in managing septic coagulopathy. AT, recognized for its ability to inhibit thrombin and factor Xa, and to a lesser extent, factors IXa and XIa, initially emerged as a promising candidate for sepsis treatment due to its inherent anticoagulative properties.
The KyberSept trial, the most extensive study evaluating AT in sepsis, involved 1157 patients with severe sepsis. It aimed to determine whether AT therapy offered a survival advantage over placebo. However, the results showed no significant survival benefit with AT therapy (RR, 1.01; 95% CI, 0.91 to 1.11) [65]. Further, a meta-analysis of 30 RCTs, encompassing the KyberSept trial, also found no survival advantage for patients with severe sepsis complicated by DIC (RR, 0.95; 95% CI, 0.88 to 1.03) [66]. Importantly, this meta-analysis highlighted a significantly elevated risk of bleeding associated with AT use (RR, 1.58; 95% CI, 1.35 to 1.84), underscoring critical safety concerns.
Activated Protein C
The journey of recombinant human-activated protein C (rhAPC) in sepsis treatment is a significant chapter in clinical therapeutics. Defined by its antithrombotic and anti-inflammatory qualities, achieved through the inhibition of thrombin formation, rhAPC was once heralded as a promising remedy for sepsis. This enthusiasm was primarily fueled by the PROWESS trial, an RCT of 1690 severe sepsis patients, which reported a marked 28-day mortality reduction with rhAPC treatment to 24.7%, compared to 30.8% in the placebo group (RR, 0.80; 95% CI, 0.69 to 0.94) [67].
However, a pre-specified subgroup analysis suggested that rhAPC’s effectiveness was mainly in patients with an APACHE II score ≥ 25. Consequently, the Food and Drug Administration mandated a further RCT for septic patients with an APACHE II score < 25. The ensuing ADRESS trial of 2613 patients was prematurely halted due to its inconclusive nature, revealing no significant 28-day mortality difference between rhAPC and placebo groups (RR, 1.08; 95% CI, 0.92 to 1.28) [68]. Moreover, the PROWESS trial faced criticism for potential methodological lapses, particularly a mid-study protocol shift that questioned its initial findings’ credibility. In 2007, the European Medicines Agency sought another definitive RCT to validate rhAPC’s risk-benefit in sepsis. However, the subsequent PROWESS-SHOCK trial with 1697 participants did not indicate a survival advantage for rhAPC (RR, 1.09; 95% CI, 0.92 to 1.28) [61].
Given these trial setbacks and methodological concerns, rhAPC was withdrawn from the market. This sequence of events underscores the importance of consistent and transparent research when appraising potential treatments for intricate ailments like septic coagulopathy.
Thrombomodulin
Recombinant human-soluble thrombomodulin (rhTM), a co-factor for thrombin-activated protein C that inhibits thrombin generation, has garnered interest as a potential intervention in septic coagulopathy. An initial Japanese RCT that focused on patients with DIC from hematological malignancies or sepsis presented encouraging findings, demonstrating improved DIC scores with rhTM [69]. However, subsequent studies brought this optimism into question. The SCARLET trial, a comprehensive international RCT of 816 septic coagulopathy patients, found no discernible survival benefit with rhTM (28-day mortality, 26.8% in the rhTM group vs. 29.4% in the placebo group, with an absolute risk reduction of 2.55%; 95% CI, −3.68 to 8.77%) [70]. A meta-analysis covering five trials with 1762 patients echoed these findings [71].
Although the broad applicability of rhTM effectiveness in sepsis or septic coagulopathy has been largely discredited, recent analyses suggest its effectiveness might be tailored to specific patient characteristics. A post hoc review of the SCARLET trial indicated reduced mortality in patients exhibiting elevated baseline coagulation markers, specifically prothrombin fragment 1 + 2 and thrombin-antithrombin complex, when treated with rhTM [72]. Adding complexity, a review by Valeriani et al. posited that rhTM lowered 28-day mortality in sepsis patients with coagulopathy but not in those without [73]. These diverse outcomes suggest that the efficacy of rhTM may be contingent upon the severity of the coagulopathy. Therefore, future studies should explore whether rhTM’s effectiveness correlates with specific patient indicators, including distinct coagulopathy states denoted by select biomarkers.
Tissue Factor Pathway Inhibitor
Recombinant tissue factor pathway inhibitor (TFPI) has been investigated as a potential therapeutic agent for septic coagulopathy. Naturally synthesized by endothelial cells, TFPI is a crucial serine protease inhibitor that neutralizes factor Xa and the factor VIIa/tissue factor complex [74, 75]. The recombinant variant of TFPI may mitigate excessive coagulation, particularly in cases of sepsis, possibly protecting the microvascular endothelium from coagulation-related damage [76, 77].
Clinical trials focused on TFPI, including the OPTIMIST trial, took place in the early 2000s [76, 78, 79]. The OPTIMIST trial, a major RCT on sepsis involving TFPI, evaluated the safety and effectiveness of tifacogin, a recombinant TFPI, in patients with severe sepsis [76]. Although tifacogin was well-tolerated and maintained consistent endogenous TFPI levels, it failed to reduce the 28-day mortality rate compared to the placebo significantly. Consequently, tifacogin has not gained acceptance for clinical application in the treatment of septic coagulopathy.
Antiplatelet Agents
Platelets play a pivotal role in the inflammatory response associated with sepsis, contributing to the initiation and propagation of the inflammatory cascade [80•]. Therefore, antiplatelet agents have been hypothesized to mitigate the severity of sepsis through their anti-inflammatory effects. This theoretical benefit is grounded in the fundamental understanding of the inflammatory response and the role of platelets in this process [81].
Aspirin, a nonsteroidal anti-inflammatory drug, has been investigated in sepsis management due to its ability to inhibit cyclooxygenase enzymes, thereby exerting anti-inflammatory and anti-platelet effects [82]. The ANTISEPSIS trial, involving over 16,000 participants aged 70 years and older, examined the effect of aspirin on sepsis-associated deaths [83]. The participants were followed up for a median of 4.6 years, with approximately half receiving aspirin and the other half a placebo. The study found 203 deaths associated with sepsis, but univariate analysis showed similar death rates in both the aspirin and placebo groups (hazard ratio for aspirin vs. placebo 1.08; 95% CI, 0.82 to 1.43) [83]. These findings suggest that aspirin may not translate into a survival benefit in sepsis.
Other antiplatelet agents, such as P2Y12 inhibitors (clopidogrel, prasugrel, and ticagrelor) and glycoprotein (GP) IIb/IIIa inhibitors (abciximab, eptifibatide, and tirofiban), have also been evaluated in the context of sepsis. A study on the effect of antiplatelet therapy on the systemic inflammatory response in human endotoxemia found that aspirin did not significantly affect circulating cytokines, except for a slight attenuation of the aspirin-induced increase in TNFα by ticagrelor [84]. However, the literature on using P2Y12 inhibitors in sepsis is sparse, and the role of the GP IIb/IIIa inhibitors has not yet been well-studied [85, 86]. Thus, further studies are needed to investigate these antiplatelet agents’ potential benefits and risks in managing sepsis.
Transfusion Strategy in the Management of Septic Coagulopathy
Platelets
Thrombocytopenia is frequently observed in septic coagulopathy. Thrombocytopenia in the ICU often signals a more unfavorable prognosis [87]. The underlying mechanisms for this phenomenon are multifaceted, encompassing platelet consumption in the coagulation process, impaired platelet production, and platelet sequestration within the spleen [88]. “Septic thrombocytopenia” commonly refers to platelet count < 150,000/µL in septic patients but is not clearly defined; instead, it is included as an element in the definition of coagulation disorders such as DIC and SIC [89•]. Similarly, treatment strategies for septic thrombocytopenia have not been well-studied. The clinical guidelines advocate for platelet transfusion in sepsis patients who exhibit a platelet count of less than 50,000/µL and are either actively bleeding or at a high risk of bleeding, such as those scheduled for invasive procedures [90]. Without significant bleeding, platelet transfusions have not demonstrated notable benefits, and maintaining a platelet count of 20,000–30,000/µL is typically considered adequate [91].
Fresh-Frozen Plasma and Coagulation Factor Concentrates
When considering the transfusion of fresh frozen plasma (FFP) and coagulation factor concentrates, including prothrombin complex and fibrinogen products, the guidelines advise that decisions should not be made solely based on laboratory results. Instead, these interventions should be contemplated for patients who are actively bleeding or those who are due to undergo an invasive procedure [90]. In situations where FFP transfusion is not an option due to the risk of fluid overload, using factor concentrates may be an alternative. However, they may only partially rectify the defect as they contain a limited selection of factors. In contrast, DIC presents with a comprehensive deficiency of coagulation factors. In cases of severe hypofibrinogenemia that persist despite FFP replacement, fibrinogen concentrate or cryoprecipitate may be administered [90].
Therapeutic Plasma Exchange
Therapeutic plasma exchange (TPE) is a procedure wherein plasma is separated from blood cells using a specialized device. Once separated, the patient’s plasma—which may contain harmful or excess substances—is discarded and replaced with fresh plasma or a plasma substitute. This process allows for the removal of pathological factors from the blood. Some research indicates that the properties of human plasma and albumin may help stabilize or even restore glycocalyx components [92]. Additionally, TPE might play a role in modulating levels of heparanases, enzymes vital for maintaining the glycocalyx, thereby supporting endothelial integrity. Weng et al. recently suggested that TPE might be a promising treatment for sepsis [93]. Their findings reveal TPE’s superiority over heparin precipitation in enhancing platelet counts, bettering coagulation, increasing 28-day survival rates, and positively impacting biomarkers of endothelial injury [93]. Echoing this, a review by Lee et al. associated TPE with reduced mortality in severe sepsis and shock patients when using fresh frozen plasma (FFP) as the sole replacement fluid [94]. However, due to the small sample sizes in most RCTs and the predominance of patients in observational studies, considerable biases cannot be ignored in incorporating the results into clinical practice [94]. Further research is needed to understand these mechanisms and their implications for patient outcomes fully.
Current Research and Future Directions
Use of Viscoelastic Testing
Rotational thromboelastometry (ROTEM) and thromboelastography (TEG) are diagnostic techniques that serve as point-of-care tests that evaluate whole-blood coagulation. These tests can measure clots’ formation, stabilization, and breakdown, capturing the roles of both plasma and cellular elements [95]. These tests offer insightful data into sepsis-related coagulation disorders. The complexity and variability in coagulation disorders in septic patients have been reported, with hypocoagulable states observed more frequently in septic shock patients and those with overt-DIC than patients without these conditions [96, 97]. TEG parameters have shown good diagnostic value for diagnosing SIC [98].
The TEG profile can detect signs of hypocoagulability, even when the usual measurements, such as PT and activated partial thromboplastin time, may appear normal, indicating a higher sensitivity in detecting septic coagulopathy [99]. The lysis index of ROTEM has also shown superior accuracy in identifying patients with severe sepsis compared to biomarkers such as procalcitonin, IL-6, and C-reactive protein [100].
The relationship between viscoelastic tests and mortality in sepsis has been the subject of intensive research. Several studies and a meta-analysis have demonstrated that specific alterations in TEG and other viscoelastic parameters, such as prolonged clotting times and reduced clot firmness, are associated with an increased mortality risk [101,102,103]. Whole-blood hypocoagulable profiles also correlate with a greater risk of death within 28 days in severe sepsis patients [104]. The observation of acute fibrinolysis shutdown early in septic shock is another finding associated with increased morbidity and mortality [103].
Viscoelastic tests have been examined in attempts to predict DIC in septic patients. TEG values, except lysis after 30 min, have demonstrated significant differences between the DIC and non-DIC groups [105]. Future insights into the viscoelasticity assay in predictive and diagnostic contexts open new perspectives in the management of septic coagulopathy, including the targeted identification and treatment of at-risk patients.
Emerging Biomarkers to Detect Coagulation Abnormalities
Recent advancements in the study of septic coagulopathy have unveiled a range of innovative biomarkers associated with inflammation and coagulation, providing deeper insights into the complex nature of this condition. These biomarkers are currently under investigation for their potential to improve patient outcomes in sepsis, recognizing the crucial role of early and accurate diagnosis for effective intervention. These biomarkers are associated with DIC and heightened mortality rates, particularly when evaluated alongside traditional assays [106, 107]. These biomarker categories encompass indicators related to thrombin generation, fibrinolysis, endothelial dysfunction, and cellular responses. In recent years, microRNAs and other measurable genetic markers, including non-coding RNAs, have gained significant prominence in the pathways governing inflammation, coagulation, and vascular endothelial impairment [108•, 109, 110]. For a comprehensive understanding, a detailed compilation of these emerging biomarkers, along with their distinctive attributes and implications, is provided in Table 2. Introducing and utilizing these markers can reshape the landscape of septic coagulopathy diagnosis and management, ushering in a new era of personalized medical approaches and precision-targeted therapeutic strategies.
Role of Precision Medicine in Septic Coagulopathy
Precision medicine is increasingly recognized as a promising approach to managing sepsis. This approach, which tailors treatment to individual patient characteristics, can improve outcomes by addressing the heterogeneity of sepsis. Recent research has identified distinct clinical phenotypes of sepsis that correlate with host-response patterns and clinical outcomes [139]. These phenotypes may help understand the variability of treatment effects in sepsis, paving the way for more personalized therapeutic strategies.
Precision medicine has been applied to identify specific phenotypes with varying coagulation features in septic coagulopathy. For instance, one phenotype was notably impacted using rhTM. Specifically, patients exhibiting severe coagulopathy, characterized by low platelet counts, extremely high levels of FDP and D-dimer, and severe organ dysfunction, were found to have decreased in-hospital mortality when treated with rhTM [140]. Further developments in machine learning have resulted in a model capable of predicting the rhTM-responsive phenotype in septic patients. This model demonstrated high-performance metrics, boasting a C statistic of 0.996, sensitivity of 0.991, and specificity of 0.967, and was effective in predicting patients likely to have less severe coagulopathy [141].
Additionally, recent studies have identified three distinct subphenotypes of SIC, each demonstrating unique clinical and laboratory features. Interestingly, these subphenotypes responded differently to various anticoagulant treatments [142].
These findings highlight the potential of precision medicine in customizing treatments to individual patient phenotypes, leading to more favorable outcomes for those suffering from septic coagulopathy.
Conclusion
Septic coagulopathy presents a formidable challenge, distinguished by its intricate and multifaceted pathophysiology, linked to an elevated fatality risk. Despite dedicated investigations, the underlying mechanisms remain not fully elucidated. As of now, approved pharmacological interventions for septic coagulopathy remain limited. The intrinsic diversity and complexity of this condition defy one-size-fits-all solutions. Instead, the pursuit of tailored therapeutic approaches guided by biomarkers and scoring systems emerges as an immediate research priority, potentially revolutionizing the current landscape of septic coagulopathy management.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. https://doi.org/10.1016/j.thromres.2016.11.007.
Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569]. Crit Care (Lond Engl). 2004;8:R82–90. https://doi.org/10.1186/cc2459.
Winer LK, Salyer C, Beckmann N, Caldwell CC, Nomellini V. Enigmatic role of coagulopathy among sepsis survivors: a review of coagulation abnormalities and their possible link to chronic critical illness. Trauma Surg Acute Care Open. 2020;5:e000462. https://doi.org/10.1136/tsaco-2020-000462.
Schmoch T, Möhnle P, Weigand MA, et al. The prevalence of sepsis-induced coagulopathy in patients with sepsis - a secondary analysis of two German multicenter randomized controlled trials. Ann Intensive Care. 2023;13:3. https://doi.org/10.1186/s13613-022-01093-7.
Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16:231–41. https://doi.org/10.1111/jth.13911.
Simmons J, Pittet JF. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. 2015;28:227–36. https://doi.org/10.1097/ACO.0000000000000163.
Delabranche X, Helms J, Meziani F. Immunohaemostasis: a new view on haemostasis during sepsis. Ann Intensive Care. 2017;7:117. https://doi.org/10.1186/s13613-017-0339-5.
Czempik PF, Wiórek A. Management strategies in septic coagulopathy: a review of the current literature. Healthcare (Basel Switz). https://doi.org/10.3390/healthcare11020227.
Levi M, Schultz M, van der Poll T. Sepsis and thrombosis. Semin Thromb Hemost. 2013;39:559–66. https://doi.org/10.1055/s-0033-1343894.
Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129:290–5. https://doi.org/10.1016/j.thromres.2011.10.013.
Bleiblo F, Michael P, Brabant D, Ramana CV, Tai T, Saleh M, Parrillo JE, Kumar A, Kumar A. The role of immunostimulatory nucleic acids in septic shock. Int J Clin Exp Med. 2012;5:1–23.
Lupu F. “Crossroads in sepsis research” Review series overview of the pathophysiology of sepsis. J Cell Mol Med. 2008;12:1072–3. https://doi.org/10.1111/j.1582-4934.2008.00366.x.
Abrams ST, Morton B, Alhamdi Y, Alsabani M, Lane S, Welters ID, et al. A novel assay for neutrophil extracellular trap formation independently predicts disseminated intravascular coagulation and mortality in critically ill patients. Am J Respir Crit Care Med. 2019;200:869–80. https://doi.org/10.1164/rccm.201811-2111OC.
Yu X, Tan J, Diamond SL. Hemodynamic force triggers rapid NETosis within sterile thrombotic occlusions. J Thromb Haemost. 2018;16:316–29. https://doi.org/10.1111/jth.13907.
Delabranche X, Stiel L, Severac F, et al. Evidence of netosis in septic shock-induced disseminated intravascular coagulation. Shock (Augusta Ga). 2017;47:313–7. https://doi.org/10.1097/SHK.0000000000000719.
Kambas K, Mitroulis I, Apostolidou E, et al. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS ONE. 2012;7: e45427. https://doi.org/10.1371/journal.pone.0045427.
van Vught LA, Uhel F, Ding C, et al. Consumptive coagulopathy is associated with a disturbed host response in patients with sepsis. J Thromb Haemost. 2021;19:1049–63. https://doi.org/10.1111/jth.15246.
Muronoi T, Koyama K, Nunomiya S, Lefor AK, Wada M, Koinuma T, et al. Immature platelet fraction predicts coagulopathy-related platelet consumption and mortality in patients with sepsis. Thromb Res. 2016;144:169–75. https://doi.org/10.1016/j.thromres.2016.06.002.
Scarlatescu E, Tomescu D, Arama SS. Sepsis-associated coagulopathy. J. Crit Care Med. 2016;2:156–63. https://doi.org/10.1515/jccm-2016-0024.
Levi M, Keller TT, van Gorp E, ten Cate H. Infection and inflammation and the coagulation system. Cardiovasc Res. 2003;60:26–39. https://doi.org/10.1016/s0008-6363(02)00857-x.
Levy JH, Sniecinski RM, Welsby IJ, Levi M. Antithrombin: anti-inflammatory properties and clinical applications. Thromb Haemost. 2016;115:712–28. https://doi.org/10.1160/TH15-08-0687.
• Lupu F, Kinasewitz G, Dormer K. The role of endothelial shear stress on haemodynamics, inflammation, coagulation and glycocalyx during sepsis. J Cell Mol Med. 2020;24:12258–71. https://doi.org/10.1111/jcmm.15895. This review offers insights into the influence of endothelial shear stress on various aspects such as hemodynamics, inflammation, and coagulation during sepsis.
Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808–14. https://doi.org/10.1074/jbc.M500747200.
Carey MJ, Rodgers GM. Disseminated intravascular coagulation: clinical and laboratory aspects. Am J Hematol. 1998;59:65–73. https://doi.org/10.1002/(sici)1096-8652(199809)59:1<65::aid-ajh13>3.0.co;2-0.
Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010;2:e2010024. https://doi.org/10.4084/MJHID.2010.024.
Okamoto K, Tamura T, Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J Intensive Care. 2016;4:23. https://doi.org/10.1186/s40560-016-0149-0.
Iba T, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology. 2020;132:1238–45. https://doi.org/10.1097/ALN.0000000000003122.
Taylor FB, Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30.
Gando S, Wada H, Asakura H, et al. Evaluation of new Japanese diagnostic criteria for disseminated intravascular coagulation in critically ill patients. Clin Appl Thromb. 2005;11:71–6. https://doi.org/10.1177/107602960501100108.
Frank CS, Larsen JB. Prognostic impact of the international society on thrombosis and hemostasis disseminated intravascular coagulation score in sepsis: a systematic review. Semin Thromb Hemost. 2023;49:471–87. https://doi.org/10.1055/s-0043-1761216.
Umemura Y, Yamakawa K, Hayakawa M, Hamasaki T, Fujimi S, Japan Septic Disseminated Intravascular Coagulation (J-Septic DIC) study group. Screening itself for disseminated intravascular coagulation may reduce mortality in sepsis: a nationwide multicenter registry in Japan. Thromb Res. 2018;161:60–6. https://doi.org/10.1016/j.thromres.2017.11.023.
Iba T, Umemura Y, Watanabe E, Wada T, Hayashida K, Kushimoto S. Diagnosis of sepsis-induced disseminated intravascular coagulation and coagulopathy. Acute Med Surg. 2019;6:223–32. https://doi.org/10.1002/ams2.411.
Dhainaut JF, Yan SB, Joyce DE, Pettilä V, Basson B, Brandt JT, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2:1924–33. https://doi.org/10.1111/j.1538-7836.2004.00955.x.
Gando S, Iba T, Eguchi Y, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria*. Crit Care Med. 2006;34:625. https://doi.org/10.1097/01.CCM.0000202209.42491.38.
Gando S, Saitoh D, Ishikura H, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care (Lond Engl). 2013;17:R297. https://doi.org/10.1186/cc13163.
Tanaka K, Takeba J, Matsumoto H, Ohshita M, Annen S, Moriyama N, et al. Anticoagulation therapy using rh-thrombomodulin and/or antithrombin III agent is associated with reduction in in-hospital mortality in septic disseminated intravascular coagulation: a nationwide registry study. Shock (Augusta Ga). 2019;51:713–7. https://doi.org/10.1097/SHK.0000000000001230.
Endo S, Shimazaki R, Antithrombin Gamma Study Group. An open-label, randomized, phase 3 study of the efficacy and safety of antithrombin gamma in patients with sepsis-induced disseminated intravascular coagulation syndrome. J Intensive Care. 2018;6:75. https://doi.org/10.1186/s40560-018-0339-z.
Jiang S, Ma J, Ye S, Meaney C, Moore TE, Pan S, et al. Associations among disseminated intravascular coagulation, thrombocytopenia cytokines/chemokines and genetic polymorphisms of toll-like receptor 2/4 in Chinese patients with sepsis. J Inflamm Res. 2022;15:1–15. https://doi.org/10.2147/JIR.S337559.
Gando S, Saitoh D, Ogura H, et al. Disseminated intravascular coagulation (DIC) diagnosed based on the Japanese Association for Acute Medicine criteria is a dependent continuum to overt DIC in patients with sepsis. Thromb Res. 2009;123:715–8. https://doi.org/10.1016/j.thromres.2008.07.006.
Kato T, Sakai T, Kato M, Hagihara M, Hasegawa T, Matsuura K, et al. Recombinant human soluble thrombomodulin administration improves sepsis-induced disseminated intravascular coagulation and mortality: a retrospective cohort study. Thromb J. 2013;11:3. https://doi.org/10.1186/1477-9560-11-3.
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10. https://doi.org/10.1001/jama.2016.0287.
• Iba T, Helms J, Connors JM, Levy JH. The pathophysiology, diagnosis, and management of sepsis-associated disseminated intravascular coagulation. J Intensive Care. 2023;11:24. https://doi.org/10.1186/s40560-023-00672-5. This article provides a comprehensive guide on the underlying mechanisms, diagnosis, and management of sepsis-associated disseminated intravascular coagulation.
Iba T, Di Nisio M, Thachil J, Wada H, Asakura H, Sato K, et al. Revision of the Japanese Association for Acute Medicine (JAAM) disseminated intravascular coagulation (DIC) diagnostic criteria using antithrombin activity. Crit Care Lond Engl. 2016;20:287. https://doi.org/10.1186/s13054-016-1468-1.
Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7:e017046. https://doi.org/10.1136/bmjopen-2017-017046.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996:22:707–10. https://doi.org/10.1007/BF01709751.
Tanaka C, Tagami T, Kudo S, et al. Validation of sepsis-induced coagulopathy score in critically ill patients with septic shock: post hoc analysis of a nationwide multicenter observational study in Japan. Int J Hematol. 2021;114:164–71. https://doi.org/10.1007/s12185-021-03152-4.
Vincent JL. Dear SIRS, I’m sorry to say that I don’t like you. Crit Care Med 1997:25:372–4.
Iba T, Arakawa M, Levy JH, Yamakawa K, Koami H, Hifumi T, et al. Sepsis-induced coagulopathy and Japanese Association for Acute Medicine DIC in coagulopathic patients with decreased antithrombin and treated by antithrombin. Clin Appl Thromb. 2018;24:1020–6. https://doi.org/10.1177/1076029618770273.
Yamakawa K, Yoshimura J, Ito T, Hayakawa M, Hamasaki T, Fujimi S. External validation of the two newly proposed criteria for assessing coagulopathy in sepsis. Thromb Haemost. 2019;119:203–12. https://doi.org/10.1055/s-0038-1676610.
Iba T, Arakawa M, Di Nisio M, Gando S, Anan H, Sato K, et al. Newly proposed sepsis-induced coagulopathy precedes international society on thrombosis and haemostasis overt-disseminated intravascular coagulation and predicts high mortality. J Intensive Care Med. 2020;35:643–9. https://doi.org/10.1177/0885066618773679.
Ding R, Wang Z, Lin Y, Liu B, Zhang Z, Ma X. Comparison of a new criteria for sepsis-induced coagulopathy and International Society on Thrombosis and Haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: a retrospective study. Blood Coagul Fibrinolysis. 2018;29:551–8. https://doi.org/10.1097/MBC.0000000000000755.
Iba T, Levy JH, Yamakawa K, Thachil J, Warkentin TE, Levi M. Proposal of a two-step process for the diagnosis of sepsis-induced disseminated intravascular coagulation. J Thromb Haemost. 2019;17:1265–8. https://doi.org/10.1111/jth.14482.
Ushio N, Wada T, Ono Y, Yamakawa K. Sepsis-induced disseminated intravascular coagulation: an international estrangement of disease concept. Acute Med Surg. 2023;10:e00843. https://doi.org/10.1002/ams2.843.
van der Poll T, Opal SM. Should all septic patients be given systemic anticoagulation? No. Intensive Care Med. 2017;43:455–7. https://doi.org/10.1007/s00134-016-4607-x.
Meziani F, Gando S, Vincent JL. Should all patients with sepsis receive anticoagulation? Yes. Intensive Care Med. 2017;43:452–4. https://doi.org/10.1007/s00134-016-4621-z.
Hotchkiss RS, Levy JH, Levi M. Sepsis-induced disseminated intravascular coagulation, symmetrical peripheral gangrene, and amputations. Crit Care Med. 2013;41:e290–1. https://doi.org/10.1097/CCM.0b013e31828cef48.
Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17:283–94. https://doi.org/10.1111/jth.14371.
Lopes-Pires ME, Frade-Guanaes JO, Quinlan GJ. Clotting dysfunction in sepsis: a role for ROS and potential for therapeutic intervention. Antioxidants (Basel Switz). https://doi.org/10.3390/antiox11010088
Lipinska-Gediga M. Coagulopathy in sepsis - a new look at an old problem. Anaesthesiol Intensive Ther. 2016;48:352–9. https://doi.org/10.5603/AIT.a2016.0051.
Scully M, Levi M. How we manage haemostasis during sepsis. Br J Haematol. 2019;185:209–18. https://doi.org/10.1111/bjh.15821.
Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. https://doi.org/10.1056/NEJMoa1202290.
Jaimes F, De La Rosa G, Morales C, Fortich F, Arango C, Aguirre D, et al. Unfractioned heparin for treatment of sepsis: a randomized clinical trial (the HETRASE study). Crit Care Med. 2009;37:1185–96. https://doi.org/10.1097/CCM.0b013e31819c06bc.
Zarychanski R, Abou-Setta AM, Kanji S, et al. The efficacy and safety of heparin in patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2015;43:511–8. https://doi.org/10.1097/CCM.0000000000000763.
Fu S, Yu S, Wang L, Ma X, Li X. Unfractionated heparin improves the clinical efficacy in adult sepsis patients: a systematic review and meta-analysis. BMC Anesthesiol. 2022;22:28. https://doi.org/10.1186/s12871-021-01545-w.
Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869–78. https://doi.org/10.1001/jama.286.15.1869.
Allingstrup M, Wetterslev J, Ravn FB, Møller AM, Afshari A. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2016;42:505–20. https://doi.org/10.1007/s00134-016-4225-7.
Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. https://doi.org/10.1056/NEJM200103083441001.
Abraham E, Laterre P-F, Garg R, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353:1332–41. https://doi.org/10.1056/NEJMoa050935.
Saito H, Maruyama I, Shimazaki S, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5:31–41. https://doi.org/10.1111/j.1538-7836.2006.02267.x.
Vincent J-L, François B, Zabolotskikh I, et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. J Am Med Assoc. 2019;321:1993–2002. https://doi.org/10.1001/jama.2019.5358.
Yamakawa K, Murao S, Aihara M. Recombinant human soluble thrombomodulin in sepsis-induced coagulopathy: an updated systematic review and meta-analysis. Thromb Haemost. 2019;119:56–65. https://doi.org/10.1055/s-0038-1676345.
Levi M, Vincent J-L, Tanaka K, Radford AH, Kayanoki T, Fineberg DA, et al. Effect of a recombinant human soluble thrombomodulin on baseline coagulation biomarker levels and mortality outcome in patients with sepsis-associated coagulopathy. Crit Care Med. 2020;48:1140–7. https://doi.org/10.1097/CCM.0000000000004426.
Valeriani E, Squizzato A, Gallo A, Porreca E, Vincent J-L, Iba T, et al. Efficacy and safety of recombinant human soluble thrombomodulin in patients with sepsis-associated coagulopathy: a systematic review and meta-analysis. J Thromb Haemost. 2020;18:1618–25. https://doi.org/10.1111/jth.14812.
Ameri A, Kuppuswamy MN, Basu S, Bajaj SP. Expression of tissue factor pathway inhibitor by cultured endothelial cells in response to inflammatory mediators. Blood. 1992;79:3219–26.
Bajaj MS, Kuppuswamy MN, Saito H, Spitzer SG, Bajaj SP. Cultured normal human hepatocytes do not synthesize lipoprotein-associated coagulation inhibitor: evidence that endothelium is the principal site of its synthesis. Proc Natl Acad Sci USA. 1990;87:8869–73. https://doi.org/10.1073/pnas.87.22.8869.
Abraham E, Reinhart K, Opal S, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–47. https://doi.org/10.1001/jama.290.2.238.
Walborn A, Rondina M, Mosier M, Fareed J, Hoppensteadt D. Endothelial dysfunction is associated with mortality and severity of coagulopathy in patients with sepsis and disseminated intravascular coagulation. Clin Appl Thromb. 2019;25:1076029619852163. https://doi.org/10.1177/1076029619852163.
de Jonge E, Dekkers PE, Creasey AA, Hack CE, Paulson SK, Karim A, et al. Tissue factor pathway inhibitor dose-dependently inhibits coagulation activation without influencing the fibrinolytic and cytokine response during human endotoxemia. Blood. 2000;95:1124–9.
Abraham E, Reinhart K, Svoboda P, et al. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: a multicenter, randomized, placebo-controlled, single-blind, dose escalation study. Crit Care Med. 2001;29:2081–9. https://doi.org/10.1097/00003246-200111000-00007.
• Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol. 2021;192:803–18. https://doi.org/10.1111/bjh.17172. This article offers a comprehensive overview of the epidemiology, biomarkers, and management strategies for disseminated intravascular coagulation.
Falcone M, Russo A, Farcomeni A, Pieralli F, Vannucchi V, Vullo V, et al. Septic shock from community-onset pneumonia: is there a role for aspirin plus macrolides combination? Intensive Care Med. 2016;42:301–2. https://doi.org/10.1007/s00134-015-4139-9.
Wang Y, Ouyang Y, Liu B, Ma X, Ding R. Platelet activation and antiplatelet therapy in sepsis: a narrative review. Thromb Res. 2018;166:28–36. https://doi.org/10.1016/j.thromres.2018.04.007.
Eisen DP, Leder K, Woods RL, et al. Effect of aspirin on deaths associated with sepsis in healthy older people (ANTISEPSIS): a randomised, double-blind, placebo-controlled primary prevention trial. Lancet Respir Med. 2021;9:186–95. https://doi.org/10.1016/S2213-2600(20)30411-2.
Kiers D, van der Heijden WA, van Ede L, et al. A randomised trial on the effect of anti-platelet therapy on the systemic inflammatory response in human endotoxaemia. Thromb Haemost. 2017;117:1798–807. https://doi.org/10.1160/TH16-10-0799.
Bestle MH, Clausen NE, Søe-Jensen P, Kristiansen KT, Lange T, Johansson PI, et al. Efficacy and safety of iloprost in patients with septic shock-induced endotheliopathy-Protocol for the multicenter randomized, placebo-controlled, blinded, investigator-initiated trial. Acta Anaesthesiol Scand. 2020;64:705–11. https://doi.org/10.1111/aas.13546.
Berthelsen RE, Ostrowski SR, Bestle MH, Johansson PI. Co-administration of iloprost and eptifibatide in septic shock (CO-ILEPSS)-a randomised, controlled, double-blind investigator-initiated trial investigating safety and efficacy. Crit Care (Lond Engl). 2019;23:301. https://doi.org/10.1186/s13054-019-2573-8.
Akca S, Haji-Michael P, de Mendonça A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med. 2002;30:753–6. https://doi.org/10.1097/00003246-200204000-00005.
Gonzalez DA, Kumar R, Asif S, Bali A, Dang AK. Sepsis and thrombocytopenia: a nowadays problem. Cureus. 2022;14:e25421. https://doi.org/10.7759/cureus.25421.
• Giustozzi M, Ehrlinder H, Bongiovanni D, Borovac JA, Guerreiro RA, Gąsecka A, et al. Coagulopathy and sepsis: pathophysiology, clinical manifestations and treatment. Blood Rev. 2021;50:100864. https://doi.org/10.1016/j.blre.2021.100864. This review delves deeply into a wide range of information related to the pathophysiology, diagnostic process, and treatment options for septic coagulopathy.
Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. https://doi.org/10.1111/j.1365-2141.2009.07600.x.
Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. British Committee for Standards in Haematology Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365–94. https://doi.org/10.1111/bjh.14423.
David S, Russell L, Castro P, et al. Research priorities for therapeutic plasma exchange in critically ill patients. Intensive Care Med Exp. 2023;11:26. https://doi.org/10.1186/s40635-023-00510-w.
Weng J, Chen M, Fang D, Liu D, Guo R, Yang S. Therapeutic plasma exchange protects patients with sepsis-associated disseminated intravascular coagulation by improving endothelial function. Clin Appl Thromb. 2021;27:10760296211053312. https://doi.org/10.1177/10760296211053313.
Lee OPE, Kanesan N, Leow EH, Sultana R, Chor YK, Gan CS, et al. Survival benefits of therapeutic plasma exchange in severe sepsis and septic shock: a systematic review and meta-analysis. J Intensive Care Med. 2023;38:598–611. https://doi.org/10.1177/08850666231170775.
Scarlatescu E, Juffermans NP, Thachil J. The current status of viscoelastic testing in septic coagulopathy. Thromb Res. 2019;183:146–52. https://doi.org/10.1016/j.thromres.2019.09.029.
Zhou W, Zhou W, Bai J, Ma S, Liu Q, Ma X. TEG in the monitoring of coagulation changes in patients with sepsis and the clinical significance. Exp Ther Med. 2019;17:3373–82. https://doi.org/10.3892/etm.2019.7342.
Kim S-M, Kim S-I, Yu G, Kim Y-J, Kim WY. Which septic shock patients with non-overt DIC progress to DIC after admission? Point-of-Care thromboelastography testing. Shock (Augusta Ga). 2022;57:168–74. https://doi.org/10.1097/SHK.0000000000001847.
Luo C, Hu H, Gong J, Zhou Y, Chen Z, Cai S. The value of thromboelastography in the diagnosis of sepsis-induced coagulopathy. Clin Appl Thromb. 2020;26:1076029620951847. https://doi.org/10.1177/1076029620951847.
Kim S-M, Kim SI, Yu G, Kim JS, Hong SI, Chae B, et al. Role of thromboelastography in the evaluation of septic shock patients with normal prothrombin time and activated partial thromboplastin time. Sci Rep. 2021;11:11833. https://doi.org/10.1038/s41598-021-91221-3.
Adamzik M, Eggmann M, Frey UH, Görlinger K, Bröcker-Preuss M, Marggraf G, et al. Comparison of thromboelastometry with procalcitonin, interleukin 6, and C-reactive protein as diagnostic tests for severe sepsis in critically ill adults. Crit Care (Lond Engl). 2010;14:R178. https://doi.org/10.1186/cc9284.
Boscolo A, Spiezia L, De Cassai A, et al. Are thromboelastometric and thromboelastographic parameters associated with mortality in septic patients? A systematic review and meta-analysis. J Crit Care. 2021;61:5–13. https://doi.org/10.1016/j.jcrc.2020.09.034.
Ninan KF, Iyadurai R, Varghese JK, et al. Thromboelastograph: a prognostic marker in sepsis with organ dysfunction without overt bleeding. J Crit Care. 2021;65:177–83. https://doi.org/10.1016/j.jcrc.2021.06.005.
Schmitt FCF, Manolov V, Morgenstern J, et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9:19. https://doi.org/10.1186/s13613-019-0499-6.
Boscolo A, Spiezia L, Campello E, Bertini D, Lucchetta V, Piasentini E, et al. Whole-blood hypocoagulable profile correlates with a greater risk of death within 28 days in patients with severe sepsis. Korean J Anesthesiol. 2020;73:224–31. https://doi.org/10.4097/kja.19396.
Kim SM, Kim SI, Yu G, Kim JS, Hong SI, Chae B, et al. Role of thromboelastography as an early predictor of disseminated intravascular coagulation in patients with septic shock. J Clin Med. 2020;9:3883. https://doi.org/10.3390/jcm9123883.
Koyama K, Madoiwa S, Nunomiya S, Koinuma T, Wada M, Sakata A, et al. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care (Lond Engl). 2014;18:R13. https://doi.org/10.1186/cc13190.
Jackson Chornenki NL, Dwivedi DJ, Kwong AC, Zamir N, Fox-Robichaud AE, Liaw PC, et al. Identification of hemostatic markers that define the pre-DIC state: a multi-center observational study. J Thromb Haemost. 2020;18:2524–31. https://doi.org/10.1111/jth.14973.
• Cánovas-Cervera I, Nacher-Sendra E, Osca-Verdegal R, Dolz-Andrés E, Beltrán-García J, Rodríguez-Gimillo M, et al. The intricate role of non-coding RNAs in sepsis-associated disseminated intravascular coagulation. Int J Mol Sci. https://doi.org/10.3390/ijms24032582. This article dives into the complex role of a wide variety of non-coding RNAs in disseminated intravascular coagulation associated with sepsis.
Meidert AS, Buschmann D, Brandes F, et al. Molecular RNA correlates of the SOFA Score in patients with sepsis. Diagnostics (Basel Switz). 2021;11:1649. https://doi.org/10.3390/diagnostics11091649.
Wang H, Zhang C, Zhang C, Wang Y, Zhai K, Tong Z. MicroRNA-122-5p regulates coagulation and inflammation through MASP1 and HO-1 genes. Infect Genet Evol. 2022;100:105268. https://doi.org/10.1016/j.meegid.2022.105268.
Hoshino K, Nakashio M, Maruyama J, Irie Y, Kawano Y, Ishikura H. Validating plasminogen activator inhibitor-1 as a poor prognostic factor in sepsis. Acute Med Surg. 2020;7:e581. https://doi.org/10.1002/ams2.581.
Wang D, Yang Y, Wang Y, Proulle V, Andreasen PA, Hong W, et al. Embelin ameliorated sepsis-induced disseminated intravascular coagulation intensities by simultaneously suppressing inflammation and thrombosis. Biomed Pharmacother. 2020;130:110528. https://doi.org/10.1016/j.biopha.2020.110528.
Catenacci V, Sheikh F, Patel K, Fox-Robichaud AE. The prognostic utility of protein C as a biomarker for adult sepsis: a systematic review and meta-analysis. Crit Care (Lond Engl). 2022;26:21. https://doi.org/10.1186/s13054-022-03889-2.
Niederwanger C, Hell T, Hofer S, Salvador C, Michel M, Schenk B, et al. Antithrombin deficiency is associated with mortality and impaired organ function in septic pediatric patients: a retrospective study. PeerJ. 2018;6:e5538. https://doi.org/10.7717/peerj.5538.
Al Otair HA, Abdel Gader AGM, Khurshid SM, Alzeer AH, Al Momen AK, Al Shaikh M, et al. The levels of tissue factor pathway inhibitor in sepsis patients receiving prophylactic enoxaparin. Turk J Haematol. 2016;33:112–8. https://doi.org/10.4274/tjh.2014.0312.
Ishikura H, Irie Y, Kawamura M, Hoshino K, Nakamura Y, Mizunuma M, et al. Early recognition of sepsis-induced coagulopathy using the C2PAC index: a ratio of soluble type C lectin-like receptor 2 (sCLEC-2) level and platelet count. Platelets. 2022;33:935–44. https://doi.org/10.1080/09537104.2021.2019694.
Azfar MF, Khan MF, Habib SS, Aseri ZA, Zubaidi AM, Aguila DO, et al. Prognostic value of ADAMTS13 in patients with severe sepsis and septic shock. Clin Investig Med. 2017;40:E49–58. https://doi.org/10.25011/cim.v40i2.28195.
Levi M, Scully M, Singer M. The role of ADAMTS-13 in the coagulopathy of sepsis. J Thromb Haemost. 2018;16:646–51. https://doi.org/10.1111/jth.13953.
Rodrigues AT, Rodrigues JT, Rodrigues CT, de Oliveira Volpe CM, Rocha-Silva F, Nogueira-Machado JA, et al. Association between thrombomodulin and high mobility group box 1 in sepsis patients. World J Crit Care Med. 2020;9:63–73. https://doi.org/10.5492/wjccm.v9.i4.63.
Hatanaka K, Ito T, Madokoro Y, Kamikokuryo C, Niiyama S, Yamada S, et al. Circulating Syndecan-1 as a predictor of persistent thrombocytopenia and lethal outcome: a population study of patients with suspected sepsis requiring intensive care. Front Cardiovasc Med. 2021;8:730553. https://doi.org/10.3389/fcvm.2021.730553.
Ikeda M, Matsumoto H, Ogura H, Hirose T, Shimizu K, Yamamoto K, et al. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J Crit Care. 2018;43:48–53. https://doi.org/10.1016/j.jcrc.2017.07.049.
Huang X, Hu H, Sun T, Zhu W, Tian H, Hao D, et al. Plasma endothelial glycocalyx components as a potential biomarker for predicting the development of disseminated intravascular coagulation in patients with sepsis. J Intensive Care Med. 2021;36:1286–95. https://doi.org/10.1177/0885066620949131.
Guitton C, Gérard N, Sébille V, Bretonnière C, Zambon O, Villers D, et al. Early rise in circulating endothelial protein C receptor correlates with poor outcome in severe sepsis. Intensive Care Med. 2011;37:950–6. https://doi.org/10.1007/s00134-011-2171-y.
Lafon T, Cazalis M-A, Vallejo C, Tazarourte K, Blein S, Pachot A, et al. Prognostic performance of endothelial biomarkers to early predict clinical deterioration of patients with suspected bacterial infection and sepsis admitted to the emergency department. Ann Intensive Care. 2020;10:113. https://doi.org/10.1186/s13613-020-00729-w.
Cheng TH, Puskarich M, Li X, Fang Z, Xu F, Chen Y, et al. Circulating complement C3-alpha chain levels predict survival of septic shock patients. Shock (Augusta Ga). 2020;54:190–7. https://doi.org/10.1097/SHK.0000000000001502.
Abe T, Kubo K, Izumoto S, Shimazu S, Goan A, Tanaka T, et al. Complement activation in human sepsis is related to sepsis-induced disseminated intravascular coagulation. Shock (Augusta Ga). 2020;54:198–204. https://doi.org/10.1097/SHK.0000000000001504.
Takahashi G, Shibata S, Ishikura H, Miura M, Fukui Y, Inoue Y, et al. Presepsin in the prognosis of infectious diseases and diagnosis of infectious disseminated intravascular coagulation: a prospective, multicentre, observational study. Eur J Anaesthesiol. 2015;32:199–206. https://doi.org/10.1097/EJA.0000000000000178.
Ishikura H, Nishida T, Murai A, Nakamura Y, Irie Y, Tanaka J, et al. New diagnostic strategy for sepsis-induced disseminated intravascular coagulation: a prospective single-center observational study. Crit Care (Lond Engl). 2014;18:R19. https://doi.org/10.1186/cc13700.
Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, et al. Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005;94:975–9. https://doi.org/10.1160/TH05-05-0316.
Patel P, Walborn A, Rondina M, Fareed J, Hoppensteadt D. Markers of inflammation and infection in sepsis and disseminated intravascular coagulation. Clin Appl Thromb. 2019;25:1076029619843338. https://doi.org/10.1177/1076029619843338.
Nakahara M, Ito T, Kawahara K, et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS ONE. 2013;8:e75961. https://doi.org/10.1371/journal.pone.0075961.
Abrams ST, Su D, Sahraoui Y, et al. Assembly of alternative prothrombinase by extracellular histones initiates and disseminates intravascular coagulation. Blood. 2021;137:103–14. https://doi.org/10.1182/blood.2019002973.
Rhodes A, Wort SJ, Thomas H, Collinson P, Bennett ED. Plasma DNA concentration as a predictor of mortality and sepsis in critically ill patients. Crit Care (Lond Engl). 2006;10:R60. https://doi.org/10.1186/cc4894.
Wang HJ, Deng J, Wang JY, Zhang PJ, Xin Z, Xiao K, et al. Serum miR-122 levels are related to coagulation disorders in sepsis patients. Clin Chem Lab Med. 2014;52:927–33. https://doi.org/10.1515/cclm-2013-0899.
Wang Y, Wang H, Zhang C, Zhang C, Yang H, Gao R, et al. Plasma Hsa-miR-92a-3p in correlation with lipocalin-2 is associated with sepsis-induced coagulopathy. BMC Infect Dis. 2020;20:155. https://doi.org/10.1186/s12879-020-4853-y.
Zhang R, Lu S, Yang X, et al. miR-19a-3p downregulates tissue factor and functions as a potential therapeutic target for sepsis-induced disseminated intravascular coagulation. Biochem Pharmacol. 2021;192:114671. https://doi.org/10.1016/j.bcp.2021.114671.
Zhu X. MiR-125b but not miR-125a is upregulated and exhibits a trend to correlate with enhanced disease severity, inflammation, and increased mortality in sepsis patients. J Clin Lab Anal. 2020;34:e23094. https://doi.org/10.1002/jcla.23094.
Ma H, Wang X, Ha T, et al. MicroRNA-125b prevents cardiac dysfunction in polymicrobial sepsis by targeting TRAF6-mediated nuclear factor κB activation and p53-mediated apoptotic signaling. J Infect Dis. 2016;214:1773–83. https://doi.org/10.1093/infdis/jiw449.
Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321:2003–15. https://doi.org/10.1001/jama.2019.5791.
Kudo D, Goto T, Uchimido R, Hayakawa M, Yamakawa K, Abe T, et al. Coagulation phenotypes in sepsis and effects of recombinant human thrombomodulin: an analysis of three multicentre observational studies. Crit Care (Lond Engl). 2021;25:114. https://doi.org/10.1186/s13054-021-03541-5.
Goto T, Kudo D, Uchimido R, Hayakawa M, Yamakawa K, Abe T, et al. Web-based application for predicting the potential target phenotype for recombinant human thrombomodulin therapy in patients with sepsis: analysis of three multicentre registries. Crit Care (Lond Engl). 2022;26:145. https://doi.org/10.1186/s13054-022-04020-1.
Cai D, Greco M, Wu Q, Cheng Y. Sepsis-induced coagulopathy subphenotype identification by latent class analysis. Balk Med J. 2023;40:244–51. https://doi.org/10.4274/balkanmedj.galenos.2023.2023-4-6.
Acknowledgements
All artworks in this article were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
All authors contributed to the drafting and review of this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karumai, T., Kotani, Y., Yamamoto, R. et al. Septic Coagulopathy: Pathophysiology, Diagnosis, and Therapeutic Strategies. Curr Infect Dis Rep 26, 91–106 (2024). https://doi.org/10.1007/s11908-024-00833-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-024-00833-z