Abstract
Elderly are at high risk for hospitalization for community-acquired pneumonia (CAP), especially due to Streptococcus pneumoniae, and seasonal influenza viruses. Data suggest PPV23’s influence on various CAP-related outcomes among the elderly may depend upon how many years have elapsed since they received this vaccine. PPV23’s protection against invasive pneumococcal disease and CAP hospitalizations are often limited to moderately ill elderly, who are less than 75 years old, or female. PCV13 demonstrates broad protection against a variety of CAPs, but ultimately, its influence on their outcomes among the elderly may be limited by herd immunity from PCV7 use. Influenza vaccine’s indirect protective effect against all-cause and non-invasive pneumococcal CAP in the elderly is difficult to ascertain. The use of both PPV23 and influenza vaccine shortens length of stay in hospitalized elderly with CAP, but whether that benefit would be realized in the presence of herd immunity is unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the USA, pneumonia and influenza are the leading infectious causes of death among those 65 years or older (hereafter “elderly”) [1]. Elderly are at high risk for pneumonia [2]. Although US hospitalizations for pneumonia in the elderly have been reduced through “herd” protection from childhood vaccination efforts since 2000; the pneumonia-related hospitalization rate in this population is still the highest among all age groups [2–5]. Community-acquired pneumonia (CAP) is the most common type of pneumonia, and the incidence of CAP requiring hospitalization is highest among the elderly [4]. Influenza-associated bacterial pneumonia (i.e., combined bacterial–viral, or secondary bacterial) is more prevalent during seasonal outbreaks and pandemics than primary viral pneumonia [6, 7]. The elderly are vulnerable to influenza-associated pneumonias and it is recognized that CAP may result from viral coinfections like influenza [5, 7–9].
Pneumococcal pneumonias including CAP begin with asymptomatic nasopharynx colonization [6, 8, 9]. Recently, an eloquent series of studies in a murine model of co-infection demonstrated how influenza promotes pneumococcal proliferation during colonization [9]. Viral and pneumococcal neuraminidase increase the local availability of sialic acid from airway epithelium and mucin from influenza-enhanced mucous secretion. This released sialic acid is catabolized by Streptococcus pneumoniae, which leads to its rapid growth that facilitates high-density pneumococcal colonization of the nasopharynx. The increased pneumococcal burden in the nasopharynx stimulated by influenza increases the likelihood of aspiration of pneumococci into the lungs [9].
Although the trends may be shifting, S. pneumoniae and influenza A or B virus are among the most common pathogens of CAP requiring hospitalization, particularly in elderly patients [4, 10]. The primary bacterial causes of influenza-associated pneumonias are S. pneumoniae and Staphylococcus aureus [6, 7]. The 30-day mortality among elderly with CAP has substantially declined in the past several decades, but estimates still suggest it is approximately 18 % [11]. Influenza-associated bacterial pneumonia morbidity and mortality are higher compared to influenza virus infection alone [6].
CAP and influenza disproportionately affect the elderly because physiologic changes associated with aging lower their pulmonary reserves [12]. CAP and influenza also aggravate underlying comorbidities (e.g., chronic heart disease, chronic liver disease, COPD, diabetes), producing complications that increase 30-day mortality rates [12–14]. Age-related declines in the immune system limit host response to colonization and infection [12–14].
In the USA, 13- and 23-valent pneumococcal vaccines are used in the elderly. The 23-valent pneumococcal vaccine, comprising capsular polysaccharide antigens from 23 (PPV23) of the more than 90 pneumococci serotypes, lacks immunogenicity across all ages [15]. In the elderly, PPV23 is effective in preventing invasive pneumococcal disease, but its efficacy declines in those over the age of 75 [16]. Historically, data on its effectiveness in preventing non-bacteremic CAP due to any cause including S. pneumonia are conflicting, but often suggest a lack of efficacy [15]. The 13-valent pneumococcal vaccine, a second-generation conjugated vaccine, is comprised of capsular polysaccharide antigens from 13 pneumococci serotypes (PCV13) conjugated to a protein that is immunogenic across all ages [14]. PCV13 is effective in preventing pneumococcal pneumonia, invasive pneumococcal disease, and otitis media in children [15]. Recently, the Advisory Committee on Immunization Practices (ACIP) added PCV13 to the vaccination schedule for the elderly because a high proportion of invasive pulmonary disease among the elderly in 2013 was caused by serotypes unique to PPV23, and it was believed that using both vaccines would provide broader protection to this vulnerable population [17]. In the USA, the live-attenuated influenza vaccine and the inactivated influenza vaccine are approved for use. However, for many reasons, it has been difficult to obtain quality evidence demonstrating influenza vaccination’s efficacy or effectiveness in preventing influenza-associated bacterial pneumonia, like CAP, in the elderly.
The elderly are highly vulnerable to influenza and CAP and may experience them either as coinfections or sequentially [5, 6]. In the elderly, until recently, evidence of a direct effect of pneumococcal vaccines to prevent non-invasive CAP due to any cause including S. pneumonia was uncertain for PPV23 and limited for PCV13. Furthermore, the indirect effect of the influenza vaccine to prevent non-invasive pneumococcal pneumonia or all-cause CAP has been difficult to ascertain. This manuscript reviewed recent studies published since 2014 that have examined pneumococcal and/or influenza vaccines’ influence on CAP-related outcomes among elderly patients.
The Influence of Pneumococcal Vaccines on CAP Outcomes in Elderly
Polysaccharide Pneumococcal Vaccines
There has been a longstanding debate regarding the effectiveness of polysaccharide pneumococcal vaccines in preventing non-invasive pneumococcal pneumonia or all-cause CAP among the elderly [16, 18]. Studies conducted nearly 40 years ago demonstrated the effectiveness of these vaccines in preventing pneumococcal bacteremia (i.e., invasive pneumococcal disease) and all-cause pneumonia in African gold miners [18]. However, inconsistent results from underpowered observational studies and conflicting meta-analyses, coupled with a lack of controlled studies, have created lingering uncertainty about the true effectiveness of PPV23 in preventing non-invasive pneumococcal pneumonia, which is the most common form of pneumococcal disease in the elderly [15–18].
The introduction of the pneumococcal conjugated vaccines like PCV7 into childhood vaccination efforts in the USA in 2000, and its replacement by PCV13 in 2010, has somewhat confounded the debate over the effectiveness of PPV23 in preventing non-invasive pneumococcal pneumonia in the elderly. The introduction of PCV7 has lowered the burden of PCV7-serotype pneumonia and has been associated with the decline in hospitalizations for pneumonia in the USA across all age groups including the elderly [2–4, 16–19]. Moreover, data suggest the switch from PCV7 to PCV13 use among children in 2010 is further reducing the burden of adult pneumococcal disease caused by PCV13 types [16–21]. Nonetheless, with its demonstrated effectiveness in preventing invasive pneumococcal disease, PPV23 has been the cornerstone of efforts to prevent pneumococcal infections in the elderly since 1983 [15, 17]. PPV23 elicits similar serological responses in the elderly and younger adults, but as a T cell-independent vaccine, it does not produce lasting immunologic memory, and the antibody response can rapidly wane. Thus, long-term clinical protection by PPV23 vaccination and revaccination has not been observed [18].
We identified three studies published since 2014 that have assessed the effectiveness of PPV23 on CAP in the elderly, all of which are observational studies. The first, known as the Community-Acquired Pneumonia, hospitalization for Acute Myocardial Infarction and Stroke (CAPAMIS) Study was a 3-year closed population-based cohort observational study that assessed clinical effectiveness of PPV23 in preventing CAP among 27,204 elderly in the general population in Tarragona, Spain [22]. The second was a retrospective nested case–control study of a population-based cohort from the largest Israeli healthcare provider assessing the effectiveness of PPV23 in preventing invasive pneumococcal disease and, subsequently, its effectiveness in preventing hospital-treated CAP in 23,952 participants [23]. The third known as the Community-Acquired Pneumonia Organization (CAPO) cohort study was a multicenter, international-nested case–control study of the CAPO database that assessed the effectiveness of PPV23 in preventing hospitalizations due to pneumococcal CAP among the elderly who were hospitalized for CAP and whether it differs by gender [24].
The CAPAMIS Study
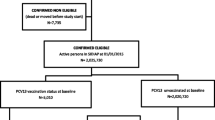
In the CAPAMIS study, a slightly different age threshold (defined as ≥60 years of age) was used. CAP cases were radiographically confirmed and validated by the clinical records [22]. CAP was defined as an acute respiratory illness, with evidence of a new infiltrate on a chest radiograph, excluding aspirative pneumonias and nosocomial pneumonias [22]. Cases where S. pneumoniae was isolated from blood or other sterile site specimens were considered bacteremic pneumococcal CAP [22]. Non-bacteremic pneumococcal CAP was defined as a case without bacteremia (blood culture negative or not performed), with a sputum culture positive for pneumococci (and no other likely bacterial pathogens), and/or a positive S. pneumoniae urinary antigen test [22]. Subjects were considered to be immunized against pneumococci if they received PPV23 within 5 years before the study. Throughout the study, pneumococcal vaccination status was defined as a time-varying variable and subjects were considered vaccinated 14 days after vaccine administration [22]. At the start of the study, 8891 (33 %) received PPV23 in the past 5 years, 6179 (23 %) received vaccination more than 5 years ago, and 12,044 (44 %) were never vaccinated. After the study began, 2390 (9 %) received vaccination, including 2355 with “prime” vaccination, and 35 with revaccination. The primary outcomes were hospitalization for CAP and all-cause mortality.
Multivariate analysis of the whole study population demonstrated no benefit for PPV23 in any outcome measure [22]. The study population was also stratified by immunological status (i.e., immunocompetent vs. immunocompromised), influenza vaccine status (vaccinated vs. non-vaccinated), nursing home residence at time of enrolling into the study, and by receipt of PPV23 after enrolling into the study. Of these analyses, only the analysis of the 2390 subjects who were vaccinated after the study started demonstrated that PPV23 significantly reduced the risk of pneumococcal CAP compared to unvaccinated individuals. However, none of the stratified analyses found that PPV23 significantly reduced the risk for all-cause CAP or death from CAP compared to the unvaccinated (Table 1) [22].
PPV23 does not produce lasting immunologic memory, and the duration of its protective effects are poorly characterized [20]. To examine any effects of its duration of protection, the investigators performed two sensitivity analyses. In the first one, all subjects who received PPV23 at any time before the study started were classified as “immunized” and compared to those who were never vaccinated throughout the study [22]. No protective effects for PPV23 vaccination in any outcome measure analyzed were found [22]. The second sensitivity analysis compared the 8891 subjects vaccinated within 5 years before the study to the 12,044 who were never vaccinated before the study [22]. PPV23 was found to significantly protect against non-bacteremic pneumococcal CAP, pneumococcal CAP, and all-cause CAP [22].
Although the CAPAMIS Study demonstrated protective benefits for PPV23 against several forms of CAP among elderly patients, its results should be interpreted cautiously. First, the effects were detected only in subgroup analyses and were modest (<50 %) in many instances [22]. Secondly, the definitions of the “nonvaccinated” in the main analysis and various subgroup analyses included patients who were at some point vaccinated either well before the start of the study (i.e., more than 5 years) or after the study started. This made it hard to interpret the findings.
Thirdly, the estimates from the sensitivity analysis comparing those vaccinated within 5 years before the study and those never vaccinated before the study may have underestimated the effect of prior vaccination in the previous 5 years. It is likely in some of the 251 vaccinated subjects who experienced CAP after the study began that more than 5 years had elapsed since their prior vaccination. On the other hand, the group classified as “nonvaccinated” prior to the study actually included 2355 individuals who received vaccination after the study started.
Fourthly, although propensity scores were used in the adjustment, they were estimated based only on the baseline characteristics and did not take into account any changes in the other risk factors occurring after the study started. In addition, there was no report of whether balance in the baseline characteristics was achieved after this adjustment [22]. Lastly, the urinary antigen test used for diagnosis is not very sensitive, thus some of the pneumonia hospitalizations in this study may have actually been undiagnosed pneumococcal pneumonia [19].
This study, illustrates the challenge inherent in determining PPV23’s effectiveness in preventing non-invasive pneumococcal pneumonia or all-cause CAP among the elderly. The duration of immunity following PPV23 vaccination is heterogeneous and not long lasting [20]. Thus, a protective effect may be obscured depending on how the immunization status is defined for those who have not been vaccinated recently [22].
The Israel Study
This study from Israel identified invasive pneumococcal disease cases using ICD-9 codes for pneumococcal meningitis or sepsis diagnosis, or free text search terms of “pneumococcal” with “sep-” or “bacter-” from hospital discharge and outpatient records within a month of discharge or pneumococcal infection diagnosis from laboratory results [23]. CAP patients identified only in outpatient records were excluded. Participants who received PPV23 in an outpatient clinic between 14 days and 5 years prior to the first invasive pneumococcal disease or hospital-treated pneumonia event were considered immunized [23]. Control subjects were randomly selected according to birth year, sex, and risk score (low, moderate, and high risk), which was based on criteria determined by ACIP for PPV23 immunization among adults [23]. A propensity score for likelihood of vaccination was included as a covariate in the base case models to further adjust for potential confounding [23]. Separate analyses were conducted for invasive pneumococcal disease and hospital-treated pneumonia events. Vaccine effectiveness for PPV23 was assessed using multivariable conditional logistic regression. Subgroup analyses examined sicker subpopulations by age group (65–74 years vs ≥75 years) and risk group (low vs moderate/high) matched to controls via propensity score for vaccination [23].
In the primary unadjusted and adjusted analyses, PPV23 protected against the development of invasive pneumococcal disease, but not against hospital-treated pneumonia (Table 1). In subgroup analyses by age group, PPV23 protected against invasive pneumococcal disease and development of hospital-treated pneumonia in elderly patients aged 65–74 years, but did not protect those 75 years of age or older [23]. In subgroup analyses by risk groups, PPV23 protected against invasive pneumococcal disease in elderly at moderate to high risk of disease but did not significantly protect elderly at low risk [23].
The CAPO Study
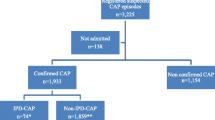
As part of the CAPO cohort study, a total of 2688 hospitalized elderly patients with CAP were identified for the analyses, of which 1724 were males and 964 were females. CAP was defined as a new pulmonary infiltrate within 24 h of hospitalization in the presence of an existing sign or symptom of pneumonia, with no history of hospitalization during the two weeks prior to admission [24]. Pneumococcal CAP cases were patients with CAP and S. pneumoniae identified from the blood, bronchoalveolar lavage, sputum, or by urinary antigen test [24]. Controls were patients with CAP without S. pneumoniae identified in any clinical sample [24]. A patient was considered vaccinated with PPV23 prior to CAP hospitalization if it was documented in their medical record [24]. This study found that prior PPV23, vaccination protected the elderly from hospitalizations due to pneumococcal CAP [24]. This investigation also suggests the effect may be gender-related as PPV23 protected elderly females, but not males from hospitalization due to pneumococcal CAP [24]. While this difference may be immunologically plausible, given the focus of the study (i.e., effect on hospitalizations), any gender-related difference should be viewed in terms preventing the severity of infection, rather than the infection itself. Also, all subjects in the study were hospitalized with CAP. If PPV23 also reduces hospitalizations due to non-pneumococcal CAP, the findings may exaggerate the effect of PPV23 on pneumococcal CAP hospitalizations.
Pneumococcal Conjugated Vaccines
Until recently, studies had only demonstrated that PCV7 and PCV13 provided an indirect benefit to the elderly through herd protection resulting from the incorporation of these vaccines into routine childhood vaccination efforts since 2000 [2, 4, 16–21]. However, the large parallel group, randomized, placebo-controlled, double-blind trial known as The Community-Acquired Pneumonia Immunization Trial in Adults (CAPITA) has now been published [15]. This study from the Netherlands sought to address the efficacy of PCV13 in preventing vaccine-type CAP (i.e., CAP with either a positive vaccine-type urinary antigen test or vaccine-type positive blood or sterile site culture), non-bacteremic and non-invasive vaccine-type CAP (i.e., CAP with a positive urinary antigen test, but negative blood and site cultures), vaccine-type invasive pneumococcal disease (i.e., CAP in which the sterile site positive), all-cause CAP, and mortality in the elderly [15]. To participate, subjects had to be immunocompetent and pneumococcal vaccine naïve. Vaccine-type and all-cause CAP were defined by a clinical definition of CAP, positive blood or sterile site culture, or a positive urinary antigen, and an independent verification of a chest radiograph consistent with pneumonia [15]. Seasonal influenza vaccination was provided to patients if indicated.
During the study, 84,496 persons were enrolled, of which 42,240 received PCV13 [15]. In this placebo-controlled study, PCV13 was effective in preventing vaccine-type pneumococcal CAP, bacteremic CAP, non-bacteremic pneumococcal CAP, and vaccine-type invasive pneumococcal disease, but not all-cause CAP, and its efficacy persisted for at least 4 years (Table 1) [15]. Although the CAPITA produced encouraging results, clinicians and policy makers must understand it was performed in a single country, with a relatively homogeneous population, among which the incidence of pneumococcal disease was low [15]. With the herd protective effects from childhood vaccination efforts that have already been realized among the elderly, the success of a strategy to vaccinate the elderly with PCV13 will partly depend on the percentage of CAP caused by PCV13 serotypes in this population. If vaccine serotypes are responsible for a small proportion of CAP or invasive pneumococcal disease in the elderly, such a strategy may produce a smaller than anticipated benefit for this population [15, 16, 18]. In recommending the use of PCV13 in the elderly, the ACIP took into consideration the indirect effects of the pneumococcal conjugate vaccines seen among the elderly to date and stipulated that the recommendation be revisited in 2018 [17, 21].
The Influence of Influenza Vaccines on CAP Outcomes in Elderly
Influenza-associated bacterial infections such as CAP are well-recognized and much of the mortality attributed to seasonal and pandemic influenza is actually from secondary bacterial pneumonia, particularly pneumococcal CAP [6]. Animal models suggest influenza vaccination may indirectly prevent secondary pneumococcal infection by preventing the primary viral infection and excess pneumococcal carriage [25]. However, quality evidence demonstrating that seasonal influenza vaccine is effective in preventing such bacterial coinfection, hospitalization due to influenza, or CAP, and mortality among the elderly is lacking. The influence of seasonal influenza vaccine alone on CAP-related outcomes among the elderly is difficult to ascertain. Most data regarding influenza vaccine effectiveness are from observational studies and are prone to biases, particularly selection bias due to many confounding variables for CAP, influenza, and hospitalization found among elderly [6, 26, 27]. Selection bias in observational studies that assess seasonal influenza vaccine effectiveness in the elderly can be difficult to mitigate, even if conventional meta-analysis methods are used [26].
Our search found no studies since 2014 evaluating the influence of seasonal influenza vaccination on CAP outcomes among the elderly. However, recently, an 8-year population-based study of laboratory-confirmed influenza among adults aged 20 years and older evaluated whether receipt of the same-season influenza vaccine was associated with reduced risk of hospital admission within 14 days after onset of influenza illness [27]. Influenza vaccination status was determined prospectively using a current internet-based registry that was used by all vaccination providers serving the population and captured 95 % of all influenza vaccinations [27]. Adults who received influenza vaccine 14 or more days prior to the onset of illness were classified as vaccinated [27]. Hospital admission dates, discharge diagnoses, and outpatient diagnosis were identified for 2 weeks post onset of influenza using a combined electronic medical record. Antiviral drug use was captured and defined as a prescription for specific antivirals within 2 weeks of symptom onset for those treated as outpatients (i.e., not hospitalized) and between symptom onset and hospital admission for persons who were hospitalized [27]. Acute care hospital admission occurring within 14 days of influenza symptom onset was the primary outcome of interest [27]. To mitigate potential confounding, investigators assessed a variety of covariates (e.g., age, gender, antiviral prescription, specific high-risk medical conditions, year, and influenza type/subtype) and used ICD-9 codes to classify patients as “high risk” (certain medical conditions were present (e.g., cancer, cardiovascular disease, diabetes, pulmonary, etc.) with at least one visit during a recent 12-month period). In addition, to minimize confounding by indication for vaccination, a propensity score regression adjustment was used [27]. In eight influenza seasons, nearly 5000 adults with acute respiratory illness seeking medical care were enrolled [27]. Laboratory-confirmed influenza occurred in approximately 28 % of persons, and the majority (73 %) was due to type A infection [27]. Influenza vaccination was more common among the elderly, women, and those with comorbidities classified as “high risk” [27]. Among patients with laboratory-confirmed influenza, influenza vaccination was not associated with a decreased risk of hospitalization following onset of influenza.
This study did not use 65 as an age threshold, but approximately 24 % of patients with laboratory-confirmed influenza were older than 60 years of age [27]. Hospitalization due to influenza is not common in healthy young adult populations. Among the 158 hospitalizations during the 8 years studied, 79 (50 %) were for laboratory-confirmed influenza, of which 48 (61 %) were in patients 60 years of age or older. In addition, during the study, 236 vaccinated patients, 60 years of age or older, developed laboratory-confirmed influenza, of which 35 (15 %) of these patients ultimately were hospitalized. This study demonstrated that influenza vaccination provides only moderate benefit against influenza hospitalization and that any protection it provides is likely due to the primary prevention of influenza illness [27]. However, age-based analyses were not reported, and this study did not evaluate the influence of influenza vaccination on pneumonia or specifically CAP outcomes among elderly patients [27].
The Influence of Pneumococcal and Influenza Co-vaccination on CAP Outcomes in Elderly
Determining the sole influence of pneumococcal and influenza on CAP outcomes among elderly patients is difficult due to varying study designs and outcome measures. However, vaccination is regarded as the most effective means to prevent both influenza and pneumococcal pneumonia [28]. A search of the published literature since 2014 identified two studies that examined the pneumococcal and influenza co-vaccination on CAP outcomes in the elderly. One was a multicenter, prospective cohort study from the Republic of Korea which examined the effectiveness of influenza and pneumococcal vaccination alone or in combination to prevent pneumonia and hospitalization following an influenza-like illness [28]. Another was a retrospective cohort study from the USA that examined the association of prior pneumococcal and/or influenza vaccination with inpatient outcomes among elderly veterans admitted for CAP.
The Korean Study
This study was conducted in 10 hospitals during the 2013–14 influenza season, using an emergency department-based surveillance system to identify influenza-like illness criteria in patients 19 years of age or older. A bedside rapid influenza detection system was used and confirmatory tests were performed on specimens via PCR testing [28]. Influenza-like illness was defined as sudden onset of fever (≥38 °C) with at least one respiratory symptom (e.g., cough, sore throat, or nasal symptoms) [28]. CAP was defined as chest radiograph evidence of acute pulmonary infiltrate consistent with pneumonia within 48 h after admission confirmed with findings on clinical examination and acquisition of the pulmonary infection outside the hospital setting [28]. At the start of the study period, elderly in the Republic of Korea had been immunized with the PPV23 as part of their national immunization program for approximately 6 months, and overall vaccine coverage rates were estimated to reach about 40 % by the end of 2013 [28].
During the season studied, patients with influenza-like illness were enrolled in the surveillance system, and vaccination records were available in 2217 (98 %). Patients with pneumonia were much older and had more underlying medical diseases than those without pneumonia; 72.9 % of patients with pneumonia were 65 years or older [28]. Surprisingly, the pneumococcal vaccination rate was higher in cases with pneumonia [28]. Overall, this study found that influenza vaccination reduced the risk of pneumonia development and hospitalization in patients with influenza-like illness who visited the emergency department, but pneumococcal vaccination did not demonstrate significant preventive effectiveness [28]. Although not defined, “old age” was an independent risk factor for pneumonia development and hospitalization [28]. An age-stratified analysis of vaccine effectiveness showed that influenza vaccine was effective in preventing pneumonia (60 %) and hospitalization (55 %) in elderly individuals, but pneumococcal vaccination was not effective. In elderly patients, receiving both vaccines was not synergistic for preventing pneumonia or hospitalization among those who visited the emergency department for influenza-like illnesses [28].
This study suggests the influenza vaccine may reduce post-influenza pneumonia and hospitalization by its primary protective effect against influenza itself and by preventing complications and reducing disease severity in elderly adults and those with comorbidities. However, it does not really address the influence of pneumococcal vaccines, or both vaccines together on the CAP outcomes among the elderly, as the results pertaining to influence of pneumococcal vaccine should be interpreted very cautiously [28]. First, inclusion of PPV23 in the national immunization program for elderly individuals had only been in place approximately 6 months prior to the start of the study period. Although the estimated PPV23 coverage rate of this national program was approximately 40 % 1 month after the beginning of the study period, the actual coverage rate associated with the program during the study period reached only about 10 % [28]. Thus, the extent of pneumococcal vaccine coverage in the community was low. Second, of the patients in this study who had been administered a pneumococcal vaccine, 94.4 % received the PPV23, and although the number of patients older than 75 years of age is not reported, the efficacy of this vaccine declines after this age threshold [16, 28]. Lastly, in this study, 70 patients with influenza-like illness also had pneumonia, of which 26 (37 %) were infected by pneumococcus [28]. However, of these 26, only 4 received pneumococcal vaccine [28]. Among these 70 patients, 69 (99 %) were ultimately hospitalized, of which 51 were elderly, and the lone case that was not hospitalized was younger than 65 years old [28]. Although the exact number of vaccinated elderly with an influenza-like illness who developed pneumococcal pneumonia was not reported, it is likely that a vast majority were not vaccinated with PPV23.
The US Study of Veterans
The study identified all elderly veterans admitted to any Veterans Affairs hospitals for CAP from October 1, 2002 to September 30, 2003 [29]. The primary outcomes were length of stay and inpatient mortality, and the secondary outcomes were respiratory complications and any bacteremia identified via the diagnosis field of discharge records [29]. To separate any possible independent or additive effects influenza vaccine may contribute to improved CAP outcomes in hospitalized elderly patients, this study stratified patients into four subgroups based on vaccine receipt: pneumococcal vaccine alone, influenza vaccine alone, both vaccines, or neither vaccines [29].
All patients were elderly on the day of the first admission (i.e., index admission) and had at least one outpatient visit to a Veterans Affairs facility each year during the 5 years prior to the index admission [29]. Using ICD-9 codes, inpatient admissions for pneumonia were defined based on the principal diagnosis of non-viral pneumonia [29]. The principal diagnosis was defined as the reason for the admission [29]. Only those patients admitted directly or through a Veterans Affairs outpatient clinic were included in the analysis, those transferred from another hospital, skilled nursing facility, intermediate care facility, or another healthcare facility were excluded [29]. The primary outcomes were length of stay (LOS) and inpatient mortality [29].
This study examined a period prior to the advent of PCV13 and when the use of PCV7 had only been incorporated into childhood vaccination efforts several years prior. Thus, patients in this study had received only PPV23, and the significant impact of herd effects of PCV7 had yet to fully materialize in this population [30]. According to a multivariate analysis, no significant effect of prior PPV23 alone, influenza vaccine alone, or both vaccines on the risk of inpatient mortality was detected relative to those who had no record of receiving either vaccine prior to hospitalization. However, receiving both vaccines was associated with a shorter LOS relative to receiving one of the vaccines alone or having no record of receiving either vaccine [29]. When vaccinated and unvaccinated elderly veterans hospitalized for CAP were matched using propensity scores, prior receipt of PPV23 alone was found to significantly reduce the risk of developing bacteremia due to any cause, but it was not statistically significantly associated with any other outcome of interest [29]. Whether influenza vaccine was received during influenza season did not affect the findings [29].
The primary limitation to this study was the use of a population that is nearly exclusively male. Recent data suggest that PPV23 protects elderly females, but not males from hospitalization due to pneumococcal CAP [24]. Thus, this study is not generalizable to females because in males, PPV23 may not attenuate the severity of infection as much as it may among females [24]. The values for LOS and bacteremia in this study were similar to what other investigators at the time observed, and during the period captured by this study, the significant herd effects of PCV7 among the elderly had yet to materialize [30]. Thus, the results of this study provide evidence, which is not confounded by herd immunity effects, that giving pneumococcal and seasonal influenza vaccines can have a beneficial effect on CAP outcomes among a group of elderly patients. Nonetheless, the results of this study may not apply in contemporary practice in the presence of the herd immunity from PCV7 and PCV13 use in children and use of both PPV23 and PCV10 in elderly.
Conclusion
Recent studies somewhat clarify the influence of influenza and pneumococcal vaccines on CAP outcomes among elderly patients. Data published since 2014 demonstrate the influence of PPV23 on various types of CAP are consistent with its variable and relatively short lasting immune response. The influence of PPV23 on various types of CAP and its outcomes may depend upon how many years have elapsed since the patient received this vaccine. Moreover, these data demonstrate the protection PPV23 provides against invasive pneumococcal disease, and hospitalizations due to CAP are confined to specific subsets of elderly patients particularly those who are moderately ill or less than 75 years of age and elderly women. In contrast, recent data on PCV13 demonstrates increasingly broad protection among the elderly against a variety of forms of CAP in pneumococcal vaccine naïve patients. The influence of this vaccine on CAP outcomes in this population may only be limited by the successful establishment of herd immunity among the elderly resulting from PCV7 and then PCV13 use in childhood immunization efforts.
Recent data suggest influenza vaccination provides only moderate protection against influenza hospitalization and does so primarily by preventing influenza illness. Although, there are animal data that suggest the prevention of influenza would limit the amount of a key substrate pneumococcus needs in the pathogenesis of CAP, prospective human studies replicating these data are lacking. Lastly, although the combined use of PPV23 and influenza vaccine may shorten length of stay in hospitalized elderly with CAP, it is uncertain if that benefit would be realized today in the presence of herd immunity.
References
Centers for Disease Control and Prevention 2013 “Deaths: final data for 2013.” http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf accessed July 23, 2015.
Park H, Adeyemi AO, Rascati KL. Direct medical costs and utilization of health care services to treat pneumonia in the United States: an analysis of the 2007–2011 medical expenditure survey. Clin Ther. 2015;37:1466–76.
Weil-Oliviera C, Gaillat J. Can the success of pneumococcal conjugate vaccines for the prevention of pneumococcal diseases in children be extrapolated to adults? Vaccine. 2014;32:2022–6.
Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–27.
Fry AM, Kim IK, Reed C, et al. Modeling the effect of different vaccine effectiveness estimates on the number of vaccine-prevented influenza-associated hospitalizations in older adults. Clin Infect Dis. 2014;59:406–9.
Campigotto A, Mubareka S. Influenza-associated bacterial pneumonia; managing and controlling infection on two fronts. Expert Rev Anti Infect Ther. 2015;13:55–68.
Christopolou I, Roose K, Ibañez LI, Saelens X. Influenza vaccines to control influenza-associated bacterial infection: where do we stand? Expert Rev Vaccines. 2015;14:55–67.
McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014;12:252–62.
Siegel SJ, Roche AM, Weiser JN. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe. 2014;16:55–67.
Smith SB, Ruhnke GW, Weiss CH, Waterer GW, Wunderink RG. Trends in pathogens among patients hospitalized for pneumonia from 1993 to 2011. JAMA Intern Med. 2014;174:1837–9.
Liapikou A, Polverino E, Cilloniz C, et al. Worldwide perspective of nursing home-acquired pneumonia compared to community-acquired pneumonia. Respir Care. 2014;59:1078–85.
Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20 Suppl 5:45–51.
Curcio D, Cane' A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis. 2015;37:30–5.
Shea KM, Edelsberg J, Weycker D, et al. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis 2014. doi: 10.1093/ofid/ofu024. eCollection 2014
Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25.
Weinberger DM, Shapiro ED. Pneumococcal conjugate vaccines for adults reasons for optimism and for caution. Hum Vaccine Immunother. 2014;10:1334–6.
Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822–5.
Fedson DS. Preventing non bacteremic pneumococcal pneumonia in older adults. Hum Vaccine Immunother. 2014;10:1322–30.
Klugman KP. A tale of 2 pneumococcal vaccines. Clin Infect Dis. 2014;58:925–7.
Payeras A, Villoslada A, Garau M, Salvador MN, Gallegos MC. Evolution of pneumococcal infections in adult patients during a four-year period after vaccination of a pediatric population with 13-valent pneumococcal conjugate vaccine. Int J Infect Dis. 2015;33:22–7.
Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15:301–9.
Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged ≥60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis. 2014;58(7):909–17.
Leventer-Roberts M, Feldman BS, Brufman I, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive disease and hospital-treated pneumonia among people aged ≥65 years: a retrospective case-control study. Clin Infect Dis. 2015;60:1472–80.
Wiemken TL, Carrico RM, Klein SL, et al. The effectiveness of the polysaccharide pneumococcal vaccine for the prevention of hospitalizations due to Streptococcus pneumoniae community-acquired pneumonia in the elderly differs between the sexes: results from the Community-Acquired Pneumonia Organization (CAPO) international cohort study. Vaccine. 2014;32:2198–203.
Mina MJ, Klugman KP. The role of influenza in the severity and transmission of respiratory bacterial disease. Lancet Respir Med. 2014;2:750–63.
Darvishan M, Gefenaite G, Turner RM, et al. After adjusting for bias in meta-analysis seasonal vaccine remains effective in community dwelling elderly. J Clin Epidemiol. 2014;67:734–44.
McLean HQ, Meece JK, Belongia EA. Influenza vaccination and risk of hospitalization among adults with laboratory confirmed influenza illness. Vaccine. 2014;32:453–7.
Song JY, Lee JS, Wie SH, et al. Prospective cohort study on the effectiveness of influenza and pneumococcal vaccines in preventing pneumonia development and hospitalization. Clin Vaccine Immunol. 2015;22:229–34.
Li C, Gubbins PO, Chen GJ. Prior pneumococcal vaccination and influenza vaccinations and in-hospitalization outcomes for community-acquired pneumonia in the elderly veterans. J Hosp Med. 2015;10:287–93.
Grijalva CG, Nuorti JP, Arbogast PG, et al. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet. 2007;7(369(9568)):1179–86.
Compliance with Ethics Guidelines
Conflict of Interest
Chenghui Li has worked as a consultant for eMaxHealth. Paul Gubbins has no conflicts.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Respiratory Infections
Rights and permissions
About this article
Cite this article
Gubbins, P.O., Li, C. The Influence of Influenza and Pneumococcal Vaccines on Community-Acquired Pneumonia (CAP) Outcomes Among Elderly Patients. Curr Infect Dis Rep 17, 49 (2015). https://doi.org/10.1007/s11908-015-0505-6
Published:
DOI: https://doi.org/10.1007/s11908-015-0505-6