Abstract
Often presenting as medical emergencies, nervous system infections can be diagnostically challenging. Knowledgeable utilization of neuroimaging modalities and the understanding of characteristic imaging findings facilitate early diagnosis and treatment. In the first part of this two-part review, we address common and unique diagnostic imaging features of bacterial and parasitic nervous system infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nervous system (NS) infections are associated with significant global morbidity and mortality. For individual patients, they can have devastating consequences and considerable impact on quality of life. Neuroimaging can aid prompt diagnosis and facilitate effective treatment of NS infections. In the first part of this two-part review, we focus on neuroimaging characteristics of selected bacterial and parasitic NS infections.
Computed tomography (CT) and magnetic resonance imaging (MRI) are the mainstays in imaging of NS infections. CT is highly sensitive for detecting hemorrhage, mineral deposits, and bony defects. MRI, however, is superior in most other respects. In NS infections, MRI can delineate edematous areas with increased vascular permeability and can show ischemia, necrosis, and pus. Infection may cause interruption of the blood brain barrier (BBB), which can appear as abnormal enhancement on postcontrast T1-weighted (T1W) MR sequences. MR thus further assists in the diagnosis of infection of either the brain parenchyma (cerebritis), the meninges (meningitis), or both. The T2 fluid-attenuated inversion recovery (FLAIR) sequence suppresses CSF hyperintensity and highlights meningeal inflammation by distinguishing the hyperintense signal due to inflammation from the hypointense signal of cerebrospinal fluid in the sulcal and cisternal spaces. The short tau inversion recovery (STIR) sequence helps identify spinal or soft tissue inflammation by suppressing the hyperintense signal from fat. Both cytotoxic edema resulting from the infection and the hypercellularity of abscesses restrict the free movement of water molecules. This change in water diffusivity is seen in diffusion-weighted imaging (DWI) sequences on MRI. Vasculopathy, vascular occlusion, vascular dissections, and aneurysms can be appreciated noninvasively using either CT or MR angiography. MR spectroscopy can sometimes help differentiate infectious from neoplastic processes such as in tuberculomas and other space-occupying infections easily confused with tumors.
Incorporating neuroimaging into clinical practice can facilitate rapid diagnosis of life-threatening NS infections and reduce leading global disabilities due to NS infections such as hearing loss and vision problems [1]. In addition to diagnosing NS infections, imaging is essential in clarifying the etiology of clinical deterioration, in prognostication, in assessment of treatment efficacy, as well as in planning neurosurgical interventions when required. The first part of this two-part review will focus on the main imaging findings involving infections of particular regions of the central nervous system (CNS) and the characteristic imaging findings of selected bacterial and parasitic infections. The second part of this two-part review focuses on neuroimaging of fungal and viral NS infections.
Pyogenic Meningitis
A life-threatening emergency, bacterial meningitis requires immediate initiation of antibiotics and, when possible, prompt diagnostic CSF sampling. Notwithstanding, meningitis remains a diagnostic challenge due to variable clinical presentations depending on the microorganism, stage of infection, and immune status of the patient. A further complication is the unavailability of proper investigational modalities in resource-poor settings. History, physical exam, and identification of risk factors are crucial in determining the need for imaging prior to safe performance of lumbar puncture (LP). Focal neurologic deficits, papilledema, a reduced level of consciousness (Glasgow coma scale <11), new-onset seizures within a week of presentation, as well as a history of stroke, mass lesions, or CNS infections in the immune-compromised setting all necessitate head imaging prior to LP [2]. Absence of the above clinical features correlates with normal CT imaging in up to 97 % of cases. CT findings that comprise contraindications to performing an LP include flattened or effaced cortical gyri, narrowed sulci, loss of gray and white matter differentiation, effacement of the basal cisterns or of the fourth ventricle, compressed ventricles, noncommunicating hydrocephalus, parafalcine or cerebellar tonsillar herniation, and lateral displacement of midline structures [3]. Given the serious sequelae of delaying diagnosis and treatment of bacterial meningitis, the absence of head imaging should never delay the LP when clinical suspicion for herniation is low [4, 5].
In early pyogenic meningitis, CT and MR imaging may be normal. With disruption of the BBB, however, imaging becomes abnormal. Meningitis is associated with edema and potential compression of the ventricular system, leading to transient ventricular dilatation, widening of subarachnoid spaces along the interhemispheric fissures, and enhancement of the leptomeninges on contrast-enhanced CT or MRI [6]. CT without contrast may show ventricular dilatation and effaced sulci or basal cisterns occasionally with inflammatory debris seen as increased CSF density [5]. Precontrast T1W MRI may show isointense exudates obliterating the basal cisterns, which will appear hyperintense on T2W images. The T2-FLAIR sequence distinguishes leptomeningeal enhancement from hyperintense CSF with increased protein content. In cases of endarteritis obliterans, venous thrombophlebitis, and other thromboembolic events, cytotoxic edema can be evident as reduced diffusivity on the DWI sequence [7••]. While communicating hydrocephalus is the most common complication of meningitis, other complications include ventriculitis, infarction, cerebritis, abscess, myelitis, and subdural empyema formation. The most common abnormal findings on cranial imaging in adults with pneumococcal meningitis, for instance, are hypodense lesions on unenhanced CT that raise suspicion for brain infarction (17–30 %), brain swelling (20–29 %), and hydrocephalus (5–16 %) [8].
Cerebritis and Abscess
When infection proceeds along the perivascular spaces into the brain parenchyma, it produces cerebritis. On imaging, cerebritis is characterized by focal areas of vascular congestion, petechial hemorrhage, and edema. Cerebritis proceeds in four stages: early cerebritis, late cerebritis, early capsule formation, and late capsule formation. In the early phase of cerebritis, CT shows an ill-defined area of low attenuation with surrounding mass effect, while MRI shows T1 hypointensity and T2 hyperintensity with little or no contrast enhancement. DWI is the key diagnostic sequence, showing marked diffusion restriction resulting from cytotoxic edema and from highly cellular pus [7••]. As the immune system attempts to contain the area of infection, peripheral encapsulation and central liquefactive necrosis ensue. Abscess capsules tend to form peripherally and are often somewhat thinner on the side proximal to the ventricle. Encapsulation initially demonstrates three characteristic imaging findings on contrast-enhanced CT: (1) a hypodense, nonenhancing, necrotic center; (2) a faintly hyperdense, enhancing rim; and (3) surrounding hypodense, nonenhancing edema. T1W MRI shows similar hypointensity at the center of the abscess, surrounded by an isointense or mildly hyperintense rim, and a peripheral region of hypointense edema. T2W MRI shows a moderately hyperintense center surrounded by a relatively hypointense rim and hyperintense surrounding edema [9]. Once the abscess is fully formed, it has a well-defined, complete capsular ring that enhances strongly and uniformly with contrast on both CT and MR imaging. Surrounding edema may subside somewhat with these changes. The most common causative bacteria are Streptococcus and Staphylococcus species. Fungi, parasites, and mycobacteria are found in less than 2 % of cases [10].
Ventriculitis
Ventriculitis, referring to inflammation of the ependymal lining of internal CSF spaces, is a frequently lethal complication encountered in up to 30 % of cases of meningitis. Staphylococcus and Enterobacter species are the most common culprits in postcranial surgery patients [11]. Chronic inflammation of the ependyma may cause ependymal cell overgrowth, septation, or segmental dilatation of ventricles and manifests radiographically as periventricular hyperintensities and “entrapped” ventricles, best seen on T2W MRI sequences. There is also a prominent enhancement of the involved ependyma following contrast administration (Fig. 1). Intraventricular septations and adhesions can be diagnosed by cranial ultrasonography in neonates. The hallmark of ventriculitis is the presence of irregular purulent debris in the dependent portions of the ventricles with associated restricted diffusion. Periventricular calcification is very rare in pyogenic ventriculitis compared to viral infections [7••, 12].
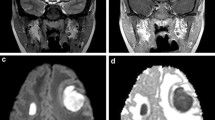
Neuroimaging of selected infections. Sagittal (a) and axial (b) T1-weighted MRI of the thoracic spine following intravenous administration of gadolinium. There is extensive abnormal epidural enhancement and a space-occupying mass that deforms the spinal cord, most prominent at the T4-T6 vertebral levels (arrows) from a patient with pyogenic spinal epidural abscess. c Coronal T1-weighted MRI following intravenous administration of gadolinium demonstrating left lateral ventricular enhancement (arrow) in bacterial ventriculitis. d Sagittal CT of the brain showing multifocal calcifications without surrounding edema in a patient with calcific neurocysticercosis. e, f From a patient with a degenerating neurocysticercosis lesion. e An axial T1-weighted MRI following intravenous administration of gadolinium showing a left temporal rim-enhancing lesion (arrow), and f an axial CT of the brain showing onset of calcification of the same lesion (arrow)
Subdural Empyema
Bacterial infection can track via retrograde thrombophlebitis into the calvarial emissary veins and from there to the subdural space, causing subdural empyema [13]. This purulent extra-axial fluid collection is an uncommon complication of meningitis and is seen in roughly 15 % of all extra-axial fluid collections [7••]. Due to proximity of the brain parenchyma to the rich venous network in the subdural space, empyema often requires combined medical and surgical treatment [14]. CT can show a lentiform collection that is slightly denser than CSF, but the location near the cranial vault limits the diagnostic accuracy of CT, especially for empyema in the posterior cranial fossa [13]. MRI can help differentiate purulent from sterile effusions, the latter of which are a more common complication of meningitis and often resolve spontaneously over weeks to months. Contrast enhancement of the dura on T1W MRI and restricted diffusion on DWI are key in differentiating empyema from nonpurulent extra-axial collections. Other imaging characteristics are similar to those seen in pyogenic abscess [15].
Pyogenic Spinal Infection
Pyogenic infections of the spine have a broad spectrum of clinicoradiological manifestations varying from discitis to osteomyelitis and from epidural to intramedullary abscesses. Discordance between symptoms and extent of infection, as well as the inability to identify the exact pathologic process from physical exam and history alone, make imaging invaluable in identifying the severity of infection and in assessing the urgency of medical or surgical intervention. Common Gram-positive organisms infecting the spine are Staphylococcus spp., Streptococcus spp. including pneumococcus, and Enterococcus spp., while common Gram-negative offenders are Escherichia coli, Salmonella spp., Pseudomonas aeruginosa, and Klebsiella pneumoniae [16]. Within 2 weeks of ongoing infection, there is 30–40 % of vertebral bone matrix loss, and at that time, abnormalities on plain X-ray films become apparent. Therefore, X-ray is not sensitive early in infection. Vertebral end plate erosion is the most reliable sign in acute infection followed by vertebral body lysis, involvement of the disk space, and loss of disk height. Chronic infection can lead to frank spinal deformities, such as unnatural kyphosis or scoliosis. Although MR is the imaging modality of choice, combined labeled leukocyte and technetium-99m sulfur colloid marrow imaging is equally accurate in the diagnosis of complicated osteomyelitis [17]. The more cost-effective and readily available technique, however, is CT, which enables visualization of bony sclerosis and end plate irregularity commonly seen with the initial loss of trabecular bone architecture. Subsequently, soft tissue replacement of the bone, erosive changes at the end plate, and direct inoculation of the disk space can be seen on CT. Contrast-enhanced CT is usually obtained when MR is contraindicated, since epidural venous plexus enhancement can help highlight the degree of mass effect on the thecal sac. Myelography can be used if the thecal sac is not well visualized on contrast-enhanced CT [18].
Early on, vertebral body bone marrow edema is hypointense on T1W and hyperintense on T2W MRI. The addition of gadolinium shows enhancement of the disk space, the adjacent vertebral bodies (particularly the end plates), and occasionally the epidural and paravertebral soft tissues. Hyperintensity on T2W MRI and loss of the internuclear cleft (a central, transverse T2W MRI hypointense band in the intervertebral disk) are reliable signs of early discitis. End plate erosion distinguishes pyogenic spondylodiscitis from the Modic type I reaction of noninfectious degenerative disk disease. Gallium scanning can sometimes be used to monitor response to treatment [18].
Spinal Epidural Abscess
Spinal epidural abscess is a medical emergency, as it can lead to spinal cord compression with long-term sequelae or death. Staphylococcus aureus is the causative organism in 70 % of patients. MRI, the imaging modality of choice, shows an extra-axial purulent collection as isointense or hypointense on T1W images and hyperintense on T2W images. The collection usually partially enhances with contrast (Fig. 1) and restricts diffusion on DWI [19].
Infectious Myelitis
Infectious myelitis has many causes that vary by geographic location. Most common in developed countries is direct infection and/or indirect inflammation of the cord parenchyma mediated by a virus. Intramedullary abscesses or vascular complications from surrounding osteomyelitis can also occur in bacterial infection. MRI is the mainstay of diagnostic imaging for any type of spinal cord inflammation. Nonvascular myelitis typically appears as a region of hyperintensity on T2W MRI and iso- to hypointensity on T1W MRI in a nonvascular distribution with variable enhancement and parenchymal expansion. While hemorrhage is uncommon in pyogenic myelitis, it can be seen in myelitis due to certain viruses [18].
CNS Tuberculosis
Tuberculosis (TB) is one of the leading infectious causes of death worldwide. CNS TB can cause significant morbidity and mortality, especially among children and HIV-infected patients. Clinically, CNS TB manifests as meningitis, tuberculoma, or tuberculous abscess [20]. Even though tuberculous meningitis (TBM) is often described as a subacute or chronic form of meningitis, it can present acutely [21]. Accurate diagnosis can be challenging due to early nonspecific symptoms and the low yield of CSF smear microscopy and culture. In patients with no focal neurological deficits, survival approaches 70 % with early initiation of treatment. To facilitate effective early treatment, combining early imaging with other diagnostic testing is essential in preventing long-term disability and death [20].
The pathophysiology of TBM typically involves reactivation of dormant bacilli in a granulomatous focus in or near the meninges with dissemination of bacilli into the subarachnoid space. Days to weeks later, meningitis ensues, causing an intense inflammatory response potentially complicated by vasculitis, CSF flow obstruction, infarction, aneurysm formation, hemorrhage, and/or cranial nerve entrapment. While gadolinium-enhanced MR is slightly more sensitive than contrast-enhanced CT, both are valuable in identifying certain features specific to CNS tuberculosis. The most common imaging features include hydrocephalus, basal exudates, and meningeal enhancement. Exudates are usually isointense or hyperintense to CSF on T1W and T2W MRI. Vascular complications can lead to infarcts, which are commonly seen in the basal ganglia, since the lenticulostriate arteries are preferentially involved. Infarcts can also be seen in the thalami, internal capsules, and anterior and middle cerebral artery (MCA) territories. Magnetic resonance arteriography may show vessel narrowing in the distal internal cerebral artery (ICA) and proximal MCA segments. Tuberculomas are round to oval masses of varying sizes, are solitary or multiple, and are seen in 10–20 % of the infected population in the United States but with much higher incidence in endemic areas. Tuberculomas are commonly located at gray-white matter junctions in the cerebral cortex and basal ganglia [22]. T1W MRI may show iso- to hypointense granulomas with occasional hyperintense rings. T2WI can show variable intensity depending on the stage of the granuloma and whether the center is solid or liquid in cases of caseating granulomas. Noncaseating granulomas are usually T2 hyperintense. Caseating granulomas with solid centers are T2 hypointense, and caseating granulomas with liquid-necrotic centers are T2 hyperintense. Gadolinium-enhanced T1WI show homogenous enhancement in noncaseating granulomas. Caseating granulomas, on the other hand, will have peripheral rim enhancement [23]. On MR spectroscopy, TB abscesses show prominent lipid peaks at 0.9, 1.3, 2.0, and 2.8 ppm and lactate peaks with absent amino acid resonances [24]. Diffusion tensor imaging and white matter tractography may in some cases help differentiate a tuberculoma from a demyelinating or destructive lesion, with displacement of fiber tracts in the former and truncation of fiber tracts in the latter [25]. HIV-infected individuals present later in the course of TB infection with subtle nonspecific manifestations like fever and altered mental status [26]. In countries with high TB incidence, TBM is most commonly seen in children, while in lower TB transmission settings, most cases of TBM occur in adults [21].
Spinal tuberculosis is seen in 10 % of tuberculous meningitis patients [27]. Vertebral body osteomyelitis with secondary nerve impingement is the most common presentation and usually involves the thoracic more than the lumbar and cervical regions [28]. MRI in Pott disease reveals vertebral T1 hypointensity and T2 hyperintensity with contrast enhancement, progressing to vertebral body collapse and subsequent cord compression. TB less commonly leads to intramedullary or intradural extramedullary tuberculomas, granulomatous myeloradiculitis, and spinal artery vasculitis with accompanying spinal cord infarction. Tuberculomas have contrast-enhancing T1 hypointense rims with high T2 signal centrally, whereas granulomatous myeloradiculitis may show contrast enhancement and thickening of the meninges and spinal roots [29]. Subarachnoid nodules, clumping of the cauda equina nerve roots, and CSF loculations are some of the other typical features [28].
Neurosyphilis
Neurosyphilis, seen in 5–10 % of infected patients when left untreated, is not limited to any particular stage of syphilis and manifests as a spectrum of disease [30]. While early neurosyphilis affects the meninges, CSF spaces, and blood vessels, late neurosyphilis preferentially affects the brain and spinal cord parenchyma [31]. Neuroimaging is increasingly used in differentiating syphilitic vasculitis from other vasculitides, as well as in early diagnosis of neurosyphilis, and in monitoring treatment response. Early neurosyphilis causes asymptomatic or symptomatic meningitis and meningovasculitis. Imaging at this stage may show lepto- or pachymeningeal thickening and ventricular dilatation. Meningovascular disease is an infectious arteritis involving medium- to small-sized arteries, which can lead to thrombosis, ischemia, and infarction. Imaging thus shows cortical and subcortical infarcts in the basal ganglia and middle cerebral artery territories on DWI and T2-FLAIR images. Multiple infarcts of different ages, intracerebral or subarachnoid hemorrhage, and impaired cerebral perfusion on perfusion MR imaging are other characteristic findings of syphilitic vasculitis. Angiography may demonstrate focal segmental arterial narrowing, focal narrowing with adjacent dilatation, or complete occlusion of the basilar, proximal anterior cerebral, middle cerebral, and/or supraclinoid carotid arteries. Focal meningeal inflammation may lead to syphilitic gummas, which are areas of inflammation that present as mass lesions contiguous with the leptomeninges, blood vessels, or brain parenchyma. Neuroimaging may show focal areas of enhancement adjacent to the pachymeninges with a “dural tail” and parenchymal edema, often indistinguishable from primary or metastatic brain tumors, meningiomas, or sarcoidosis. CT may show multiple low-density areas involving both gray and white matter with linear nonhomogenous enhancement. MRI is superior to CT in differentiating these possibilities and shows a leptomeningeal gyriform pattern of enhancement. Other possible findings include bilateral mesial temporal T2 hyperintensities; nonspecific white matter lesions involving the frontal and temporal lobes, the hippocampus, and periventricular areas; and the involvement of optic and vestibular nerves. In late-stage disease, parenchymal atrophy is the most common imaging finding [7••, 30].
Isolated involvement of the spine by syphilis is rare in the present era with a fivefold lower incidence than cerebrospinal syphilis and syphilitic meningomyelitis. However, tabes dorsalis (posterior column and nerve root involvement), hypertrophic pachymeningitis, gummas of the spinal cord with possible resulting compressive myelopathy, spinal syphilitic vasculitis with resulting infarction, and syphilitic osteitis (or syphilitic aortitis) are all clinical manifestations occasionally encountered [32]. When neurosyphilis affects the spinal cord, spinal imaging may show nonspecific nodular or linear enhancement with diffuse T2 hyperintensities on MRI, pachymeningeal enhancement, or atrophy, depending on the type of involvement [33].
Lyme Neuroborreliosis
Lyme neuroborreliosis (LNB) is caused by CNS infestation with the vector-borne spirochete Borrelia. CNS involvement is seen in 10–15 % of patients with disseminated disease in the first few weeks to months after infection. Two thirds of affected patients develop a lymphocytic meningitis, about half develop a painful radiculitis, and fewer develop cranial neuropathies [34]. Nonspecific imaging findings most frequently reported are cranial nerve enhancement (mainly of the third, fifth, and seventh cranial nerves) although a clinical correlation between multiple enhancing cranial nerves and neurologic symptoms is often lacking. Therefore, an incidental finding of cranial nerve enhancement on MRI is not pathognomonic for Lyme, and a diagnosis of Lyme neuroborreliosis should only be pursued in the appropriate clinical context.
Facial neuropathy (3–5 %) and meningitis (1 %) are the most common manifestations of neuroborreliosis in pediatric populations. In children, the clinical course is both milder and shorter. MR findings in pediatric Lyme infection include the presence of prominent Virchow-Robin spaces, T2 hyperintense white matter lesions with occasional enhancement, and pial or cranial nerve enhancement. This abnormal enhancement typically resolves with adequate antibiotic treatment [35].
When there is spinal involvement, imaging may show a variable combination of intramedullary lesions and leptomeningeal or spinal nerve root enhancement, as seen in other infectious and autoimmune processes. T2W spinal MRI may show diffuse or multifocal hyperintensities. Given these nonspecific imaging findings, accurate diagnosis depends on additional confirmatory serologic testing, as well as the appropriate history and clinical context [36]. Table 1 illustrates the most common CT and MR imaging findings, the most important diagnostic clues, the imaging modality of choice, and the radiographic differential diagnosis for all the bacterial neurologic infections described above.
Parasitic Diseases
Cerebral Malaria
Cerebral involvement occurs in 2 % of those affected with malaria and is life-threatening. Even with appropriate treatment, malarial infection is lethal in 25 % of patients with CNS involvement [37]. The neuropathogenesis of cerebral malaria is thought to be related to cytoadherence of parasitized erythrocytes causing hyperviscosity, vascular occlusion, and leakage of cerebral capillaries and venules. This results in diffuse cerebral edema, widespread small, ring-shaped hemorrhages located in the subcortical white matter of the cerebral hemispheres, and in cerebral infarcts [38]. Early diagnosis is crucial for optimizing outcomes as cerebral malaria rapidly progresses to coma or death. Without prompt treatment, survival is associated with devastating neurological sequelae [38].
Cytotoxic edema due to ischemic injury appears as focal or diffuse hyperintensities on T2WI and is most commonly seen in the centrum semiovale, corpus callosum, thalamus, and insular cortex and less commonly in the pons, medulla, and cerebellum [39]. Corresponding restricted diffusion is not reliably present in these areas. The most common imaging findings reflect cerebral edema and hemorrhagic infarctions [37]. Magnetic resonance spectroscopy (MRS) can measure lactate, a key indicator of the severity of pathology related to cerebral malaria. The presence of a lactate peak is an important independent predictor of poor outcome [40].
Neurocysticercosis
Neurocysticercosis is the single most common cause of acquired epilepsy in the developing world [41]. The CNS is involved in 60–90 % of patients with cysticercosis [42]. An accurate diagnosis requires neuroimaging. While CT is the best screening imaging modality, MRI is more sensitive in diagnosing parenchymal cysts and detecting lesions in the brain stem, ventricular system, and subarachnoid spaces [43]. Imaging features of neurocysticercosis are dependent on location (parenchymal, subarachnoid, intraventricular, and spinal), stage of parasite evolution (vesicular, colloid, granular, and calcified), host immune response, and possible complications (infarction, vasculitis, and basal leptomeningitis). CT typically shows discrete hyperdense 1–10 mm nodular lesions with or without peripheral edema or enhancement (see Fig. 1) [41]. Parenchymal cysts are usually 5–20 mm in diameter and evolve from a singular scolex to a rim-enhancing and eventually to a calcified lesion after involution. Cysts are usually located near the cortical gray-white matter interface. Less commonly, parenchymal lesions can also be found in the brain stem and cerebellum. Extraparenchymal lesions appear as space-occupying lesions in the ventricles or subarachnoid spaces. In the Sylvian fissure, these cysts can become extremely large and, when located in the third or fourth ventricle, can produce obstructive hydrocephalus. The presence of a scolex in a cyst is a diagnostic criterion for diagnosis of definite neurocysticercosis [41].
The vesicular stage consists of small, rounded, cystic lesions that are well demarcated from the surrounding brain parenchyma, have an eccentric internal nodule (scolex), have little or no perilesional edema, have no abnormal enhancement after contrast administration, and have a pathognomonic “hole-with-dot” appearance [43]. Cyst fluid intensity is similar to CSF on T1WI and T2WI and may be somewhat hyperintense on T2-FLAIR [44]. Scolices are iso- to hyperintense on T1WI and T2WI [42]. The high burden of these lesions can make the brain parenchyma have the appearance of “Swiss cheese.” In contrast, the colloidal stage is usually characterized by ill-defined rim-enhancing lesions with surrounding edema but only rarely with visualization of a scolex. T1WI may show hyperintense cystic fluid also seen on T2WI, and surrounding edema in the colloidal stage may be seen as surrounding T2W hyperintensity [43,42]. DWI may highlight the presence of a scolex as a hyperintense nodule with diffusion restriction within the vesicle that is iso- to mildly hyperintense compared to CSF [45•]. In the absence of scolex visualization, the radiographic differential diagnosis includes tuberculomas, pyogenic brain abscesses, mycotic granulomas, and primary or metastatic brain tumors [41]. The granular stage shows similar characteristics as the colloidal stage but may have a more intensely enhancing cyst wall and more surrounding edema; given the variability in host response, however, this is difficult to quantify, and there is a substantial overlap. The final calcified stage typically consists of completely mineralized 2–10 mm lesions. Among MRI sequences, susceptibility-weighted imaging is useful in identifying the calcified cysticerci, as other sequences may miss this finding [43]. Of note, cysts at different stages of development and resolution of lesions after antiparasitic treatments are a major diagnostic criteria for neurocysticercosis [41]. MR spectroscopy of cyst fluid may show lactate, acetate, succinate, and other amino acids also found in pyogenic abscesses, but unlike in abscesses, restricted diffusion on DWI is not seen in neurocysticercosis lesions [44].
Different stages of parasite development are also encountered in extraparenchymal disease. Subarachnoid (cisternal) forms are seen in 2–3 % of infected patients [41]. Subarachnoid cysts can attain a larger diameter (up to 60 mm) and become multilobulated, resembling a bunch of grapes [41, 45•]. These cysts are typically located in the Sylvian fissure or basal cisterns, where they behave as space-occupying lesions and are referred to as the “racemose” form of neurocysticercosis. Cysts in cortical sulci tend to remain small [43]. Hydrocephalus and leptomeningeal enhancement are commonly seen due to both inflammatory blockage of CSF flow as well as fibrous arachnoiditis. When vasculitis occurs as a complication of neurocysticercosis, arteriography may demonstrate segmental narrowing or occlusion of major intracranial arteries, with the middle and posterior cerebral arteries most commonly affected [45•]. Multiple vessel involvement is seen in 50 % of cases and infarcts related to arteritis occur in 2–12 % of patients.
Intraventricular lesions are seen in 10–20 % of patients with neurocysticercosis. The most common site of intraventricular cysts is in the fourth ventricle (53 %), followed by the third ventricle (27 %), the lateral ventricle (11 %), and the cerebral aqueduct (9 %) [45•]. High-resolution, highly T2-weighted MR sequences, such as constructive interference in the steady state (e.g., CISS), are highly sensitive for detecting intraventricular cysts [44]. Cysticercal involvement of the spine is rare, with lesions in the subarachnoid space being more common than lesions in the spinal cord itself. Approximately 5 % of patients with basal cisternal leptomeningitis also have spinal cord lesions. Therefore, spinal MRI is indicated when basal leptomeningeal inflammation is encountered and the patient presents with myelopathy. The MRI appearance of cysts in the spinal subarachnoid space is similar to that of cysticercosis involving other CSF containing spaces. Overall, spinal cord lesions are seen in only 1 % of affected patients [44].
Myocysticercosis
Intramuscular cysticercosis has nonspecific, if any, clinical manifestations, and diagnosis can be difficult since lipoma, neurofibroma, fibroma, and muscle abscess may have similar radiographic appearance. High-resolution ultrasonography (US) can demonstrate the intramuscular cyst as a homogenous, hypoechoic lesion with an eccentric hyperechoic scolex. US is a convenient, easily available, and cost-effective method for detecting superficially located cysts. CT may reveal well-defined lesions with a central hypodensity. MRI, however, not only shows the stage of the lesion but also highlights the extent of inflammation in the surrounding muscle and soft tissue [46]. Calcified myocysticercosis can be seen as radiographic hyperdensities on plain films or CT scan. Case reports show involvement of various muscles throughout the body, predominantly muscles in the face and neck including temporalis, masseter, extraocular, mylohyoid, sternocleidomastoid, pterygoid, paravertebral, pharyngeal, and femoral muscles [47]. Diagnosis of myocysticercosis by identification of cysticerci in the extracranial musculature can facilitate early treatment when neuroimaging is equivocal [47].
Neuroschistosomiasis
Schistosomiasis affects 240 million people worldwide [48]. CNS involvement is caused by migration of adult worms followed by in situ oviposition or by massive embolization of eggs through retrograde venous flow in the Batson venous plexus. Brain lesions occur more frequently in Schistosoma japonicum-infected patients, whereas Schistosoma mansoni- or Schistosoma hematobium-infected patients more often develop myelitis, but these are not mutually exclusive [49, 38]. Acute schistosomal encephalopathy presents with edema and multifocal, small, contrast-enhancing lesions in the frontal, parietal, and occipital lobes, as well as in the brain stem. While the pathogenesis of schistosomal encephalopathy remains to be elucidated, multiple cerebral infarctions, particularly at arterial border zones, have also been reported [49]. Encephalitis may present with a tumor-like mass on CT appearing as a hyperdense lesion of unclear (possibly hemorrhagic) etiology surrounded by a hypodense halo of edema. MRI shows foci of T1 hypointensity and T2 hyperintensity with contrast enhancement. A characteristic MR imaging pattern is a large mass consisting of multiple intensely enhancing nodules, sometimes with areas of linear enhancement. The central linear enhancement surrounds multiple enhancing punctate nodules, forming an “arborized” appearance. This, however, is a nonspecific imaging finding that can be seen in granulomatous CNS infections, as well as several other conditions. Antihelminthic treatment usually causes symptomatic improvement within 6 weeks with complete resolution of the abnormal imaging findings within 6 months [45•].
Spinal schistosomiasis is the best-known form of neuroschistosomiasis. It is classified into three forms: medullary (spinal cord involvement), myeloradicular (spinal cord with nerve root involvement), and conus-cauda equina syndrome (inferior cord and nerve roots involved) [49]. MRI is very sensitive but nonspecific. Hyperintensity on T2WI with isointense lesions on T1W imaging, enlargement of the spinal cord (typically lower cord and conus medullaris), thickening of spinal roots (especially in the cauda equina), and a heterogeneous pattern of contrast enhancement (nodular, peripheral, or linear radicular) on T1WI are the most common findings [49, 45•]. An arborized appearance is also possible. CT myelography is a less sensitive method that might be normal or show enlargement of the spinal cord, partial or complete block of CSF flow in the spinal canal, and irregular thickening of nerve roots [49].
Cerebral Toxoplasmosis
Toxoplasmosis is an opportunistic protozoal infection typically found in immunocompromised patients with a history of HIV infection or bone marrow transplantation. Waning cell-mediated immunity leads to activation of latent “encysted bradyzoites” and liberation of free tachyzoites [45•]. Neuroimaging can aid in the diagnosis and abrogate the need for lesion biopsy. MRI is the most sensitive and thallium 201 SPECT is the most specific imaging technique available.
Cerebral toxoplasma lesions are usually multiple (85 %), but they can also be solitary (15 %). On unenhanced CT, lesions appear hypodense with surrounding edema and mass effect. Postcontrast imaging may show uniform enhancement, nodular enhancement, or rim enhancement. MRI lesions typically contain three zones with corresponding T2WI and T2-FLAIR findings: (1) a central zone of coagulative necrosis appearing as a central hyperintensity, (2) an intermediate zone of hypervascularity and accumulated inflammatory cells with tachyzoites causing a hypointense rim, and (3) a peripheral zone of vasogenic edema and encysted parasites causing a surrounding region of hyperintensity [45•, 50]. Postcontrast T1 images show rim enhancement of the intermediate inflammatory zone. A capsule is not present. An eccentric (asymmetric) target sign is an insensitive but highly suggestive sign of toxoplasmosis; it is present in 30 % of cases. The target sign is characterized by a small eccentric nodule along the wall of the enhancing rim. It is typically located in the basal ganglia (75–85 %), in the thalamus, at the corticomedullary junction, and in the brain stem. Toxoplasmosis sometimes involves the corpus callosum with a “butterfly” pattern similar to the radiographic appearance of glioblastoma multiforme or primary CNS lymphoma [45•]. Key features, such as a subcortical location, multiplicity of lesions, an asymmetric “target sign,” and evidence of intralesional blood products, help distinguish toxoplasmosis lesions from CNS lymphoma and other CNS tumors. Of note, patients with AIDS and patients who have undergone bone marrow transplantation may not be able to produce a normal inflammatory response, leading to the absence of edema and mass effect and the absence of enhancement [45•]. Therefore, the clinical response to antiparasitic treatment is a key diagnostic feature of CNS toxoplasmosis.
Echinococcosis
Echinococcosis is a zoonotic infection caused by the larval stage of the tapeworms Echinococcus granulosus and Echinococcus alveolaris causing cystic echinococcosis (CE) and alveolar echinococcosis (AE), respectively. Cerebral involvement is seen in 1–3 % of infected patients and can be primary (ranging from single to multiple simple typical cysts to complicated atypical cysts) or secondary, the latter of which is usually multifocal (either from dissemination or local rupture of a primary cyst) and indicates a worse prognosis [51]. CE produces a cystic lesion with mass effect and compression of surrounding structures. CT or MRI will demonstrate a unilocular, fluid-filled cyst with a well-defined border, minimal enhancement, and perifocal edema. T2-weighted sequences help define liquid cyst content and may reveal daughter cysts and septae.
In contrast, AE comprises single or multiple multilobulated, vesicular, grapelike, or nodular cerebral lesions with infiltrative growth, irregular borders, robust contrast enhancement, and perifocal edema with occasional calcifications and necrosis [52]. The territory of the middle cerebral artery is most commonly involved, whereas the pons, ventricular system, cerebral aqueduct, subarachnoid space at the cerebellopontine angle, and the intracranial epidural space are less frequently involved [51].
Musculoskeletal involvement is seen in 1–4 % of cases. Like with myocysticercosis, ultrasound serves as an important diagnostic tool for superficially located intramuscular cysts. It typically shows thick concentric hypoechoic walls with multiple echogenic foci due to hydatid sand. This is described as a “snow storm” appearance. MRI, however, serves the purpose of differentiating hydatid cysts from other etiologies. T2 hypointensity is considered highly specific for a hydatid cyst along with a “scroll appearance” describing a ruptured endocyst with coiled margins [53].
Chagas Disease
Chagas disease, caused by Trypanosoma cruzi, is a vector-transmitted tropical disease and a leading cause of stroke in Latin America [54]. Nervous system involvement manifests as encephalitis, meningoencephalitis, pseudotumoral mass lesions, mild sensorimotor peripheral neuropathy, and ischemic stroke depending on the immune status of the host and the stage of disease. Neuroimaging is helpful for diagnostic confirmation when a space-occupying lesion, or chagoma, is suspected in the acute phase or in reactivation of chronic disease. CT may show a single, supratentorial, nodular lesion in the parietofrontal lobes that enhances robustly with contrast. T1-weighted MRI shows hypointense lesions that enhance, and are surrounded by extensive hyperintense areas on T2-weighted images signifying mass effect. This pattern is also seen in cerebral toxoplasmosis, among many other space-occupying CNS lesions [55]. As such, in endemic regions, concurrent toxoplasma and trypanosoma intracranial infections are a diagnostic challenge. Worsening neurologic symptoms on antitoxoplasma treatment in endemic areas is highly suggestive of trypanosoma infection in HIV-positive patients [56]. Cardioembolic ischemic stroke involving the middle cerebral artery territory from chagasic cardiomyopathy is one of the most common features of CNS involvement in chronic infection [57]. Extracranial vessel atheroembolism and lacunar infarctions are other mechanisms of stroke in chagasic patients. Subclinical infarctions are detected in 18 % [54]. Table 2 illustrates the most common CT and MRI findings, the most important diagnostic clues, imaging modality of choice, and radiographic differential diagnosis for the parasitic neurologic infections described above.
Conclusion
Neuroimaging is a powerful tool in the fight against neurologic infectious diseases. Its expeditious use could potentially lessen the global impact and individual morbidity and mortality of NS infection. CT is cost-effective and often readily available, rendering it an important tool in emergent, and resource-limited settings. MRI is superior in detecting nearly all nervous system infections and, when available, is the preferred modality for clinicoradiographic and clinicopathologic correlation. Thorough understanding, judicious utilization, and accurate interpretation of imaging studies, used in conjunction with epidemiologic, clinical, and objective data, facilitate rapid and accurate diagnosis and streamline timely treatment that can be life-saving and disability-limiting in bacterial and parasitic infections of the nervous system.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO methods and data sources for global burden of disease estimates 2000–2011 [database on the Internet]. Department of Health Statistics and Information Systems WHO, Geneva 2013. Available from: http://www.who.int/healthinfo/statistics/GlobalDALYmethods.pdf?ua=1. Accessed 4 May 2014
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267–84.
van Crevel H, Hijdra A, de Gans J. Lumbar puncture and the risk of herniation: when should we first perform CT? J Neurol. 2002;249(2):129–37.
Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345(24):1727–33. doi:10.1056/NEJMoa010399.
van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354(1):44–53.
Castillo M. Magnetic resonance imaging of meningitis and its complications. Top Magn Reson Imaging. 1994;6(1):53–8.
Mohan S, Jain KK, Arabi M, Shah GV. Imaging of meningitis and ventriculitis. Neuroimaging Clin N Am. 2012;22(4):557–83. doi:10.1016/j.nic.2012.04.003. This review thoroughly summarizes pathophysiology of characteristic findings and advances in neuroimaging of various infectious causes of acute and chronic meningitis. It also illustrates differentiating imaging features of noninfectious causes of meningitis.
Weisfelt M, de Gans J, van der Poll T, van de Beek D. Pneumococcal meningitis in adults: new approaches to management and prevention. Lancet Neurol. 2006;5(4):332–42. doi:10.1016/S1474-4422(06)70409-4.
Brain Abscess. CONTINUUM: lifelong learning in neurology. 2002;8(3, Infectious Disease):38–47.
Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology. 2014;82(9):806–13. doi:10.1212/wnl.0000000000000172.
Fukui MB, Williams RL, Mudigonda S. CT and MR imaging features of pyogenic ventriculitis. AJNR Am J Neuroradiol. 2001;22(8):1510–6.
Jorens PG, Voormolen MH, Robert D, Parizel PM. Imaging findings in pyogenic ventriculitis. Neurocrit Care. 2009;11(3):403–5. doi:10.1007/s12028-009-9263-3.
Ferreira NP, do Otta GM, Amaral LL, da Rocha AJ. Imaging aspects of pyogenic infections of the central nervous system. Top Magn Reson Imaging. 2005;16(2):145–54.
Beckham JD, Tyler KL. Neuro-intensive care of patients with acute CNS infections. Neurotherapeutics. 2012;9(1):124–38. doi:10.1007/s13311-011-0086-5.
Sarrazin JL, Bonneville F, Martin-Blondel G. Brain infections. Diagn Interv Imaging. 2012;93(6):473–90. doi:10.1016/j.diii.2012.04.020.
Stabler A, Reiser MF. Imaging of spinal infection. Radiol Clin North Am. 2001;39(1):115–35. doi:10.1016/S0033-8389%2805%2970266-9.
Love C, Palestro CJ. Radionuclide imaging of inflammation and infection in the acute care setting. Semin Nucl Med. 2013;43(2):102–13. doi:10.1053/j.semnuclmed.2012.11.003.
Go JL, Rothman S, Prosper A, Silbergleit R, Lerner A. Spine infections. Neuroimaging Clin N Am. 2012;22(4):755–72. doi:10.1016/j.nic.2012.06.002.
Wang VY, Chou D, Chin C. Spine and spinal cord emergencies: vascular and infectious causes. Neuroimaging Clin N Am. 2010;20(4):639–50. doi:10.1016/j.nic.2010.07.006.
Chin JH, Mateen FJ. Central nervous system tuberculosis: challenges and advances in diagnosis and treatment. Curr Infect Dis Rep. 2013. doi:10.1007/s11908-013-0385-6.
Van TT, Farrar J. Tuberculous meningitis. J Epidemiol Community Health. 2014;68(3):195–6. doi:10.1136/jech-2013-202525.
Tuberculous Meningitis. CONTINUUM: lifelong learning in neurology. 2002;8(3, Infectious Disease):27–37.
DeLance AR, Safaee M, Oh MC, Clark AJ, Kaur G, Sun MZ, et al. Tuberculoma of the central nervous system. J Clin Neurosci. 2013;20(10):1333–41. doi:10.1016/j.jocn.2013.01.008.
Patkar D, Narang J, Yanamandala R, Lawande M, Shah GV. Central nervous system tuberculosis: pathophysiology and imaging findings. Neuroimaging Clin N Am. 2012;22(4):677–705. doi:10.1016/j.nic.2012.05.006.
Lyons JL, Neagu MR, Norton IH, Klein JP. Diffusion tensor imaging in brainstem tuberculoma. J Clin Neurosci. 2013;20(11):1598–9. doi:10.1016/j.jocn.2013.01.003.
Zunt JR, Baldwin KJ. Chronic and subacute meningitis. Contin (Minneap Minn). 2012;18(6):1290–318. doi:10.1212/01.CON.0000423848.17276.21.
Ranganathan N, Hogarth K. Tuberculous meningitis. BMJ Case Rep. 2013;2013. doi:10.1136/bcr-2013-009412
Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59(3):167–87. doi:10.1016/j.jinf.2009.06.011.
Cho TA, Vaitkevicius H. Infectious myelopathies. Contin (Minneap Minn). 2012;18(6):1351–73. doi:10.1212/01.CON.0000423851.63017.2a.
Czarnowska-Cubala M, Wiglusz MS, Cubala WJ, Jakuszkowiak-Wojten K, Landowski J, Krysta K. MR findings in neurosyphilis—a literature review with a focus on a practical approach to neuroimaging. Psychiatr Danub. 2013;25 Suppl 2:S153–7.
Marra CM. Update on neurosyphilis. Curr Infect Dis Rep. 2009;11(2):127–34.
Berger JR. Infectious myelopathies. Contin (Minneap Minn). 2011;17(4):761–75. doi:10.1212/01.CON.0000403794.13291.3d.
Tsui EY, Ng SH, Chow L, Lai KF, Fong D, Chan JH. Syphilitic myelitis with diffuse spinal cord abnormality on MR imaging. Eur Radiol. 2002;12(12):2973–6. doi:10.1007/s00330-001-1244-7.
Halperin JJ. Lyme disease: a multisystem infection that affects the nervous system. Contin (Minneap Minn). 2012;18(6):1338–50. doi:10.1212/01.CON.0000423850.24900.3a.
Hildenbrand P, Craven DE, Jones R, Nemeskal P. Lyme neuroborreliosis: manifestations of a rapidly emerging zoonosis. AJNR Am J Neuroradiol. 2009;30(6):1079–87. doi:10.3174/ajnr.A1579.
Hattingen E, Weidauer S, Kieslich M, Boda V, Zanella FE. MR imaging in neuroborreliosis of the cervical spinal cord. Eur Radiol. 2004;14(11):2072–5. doi:10.1007/s00330-004-2300-x.
Rasalkar DD, Paunipagar BK, Sanghvi D, Sonawane BD, Loniker P. Magnetic resonance imaging in cerebral malaria: a report of four cases. Br J Radiol. 2011;84(1000):380–5. doi:10.1259/bjr/85759874.
Roman GC. The neurology of parasitic diseases and malaria. Contin (Minneap Minn). 2011;17(1):113–33. doi:10.1212/01.CON.0000394678.13115.ad.
Sakai O, Barest GD. Diffusion-weighted imaging of cerebral malaria. J Neuroimaging. 2005;15(3):278–80.
Looareesuwan S, Laothamatas J, Brown TR, Brittenham GM. Cerebral malaria: a new way forward with magnetic resonance imaging (MRI). Am J Trop Med Hyg. 2009;81(4):545–7. doi:10.4269/ajtmh.2009.07-0411.
Del Brutto OH, Rajshekhar V, White Jr AC, Tsang VC, Nash TE, Takayanagui OM, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57(2):177–83.
Chang KH, Han MH. MRI of CNS parasitic diseases. J Magn Reson Imaging. 1998;8(2):297–307.
Del Brutto OH. Neurocysticercosis. Contin (Minneap Minn). 2012;18(6):1392–416. doi:10.1212/01.CON.0000423853.47770.90.
Castillo M. Imaging of neurocysticercosis. Semin Roentgenol. 2004;39(4):465–73.
Abdel Razek AA, Watcharakorn A, Castillo M. Parasitic diseases of the central nervous system. Neuroimaging Clin N Am. 2011;21(4):815–41. doi:10.1016/j.nic.2011.07.005. This review describes imaging features of common parasitic intracranial and spinal infections along with their epidemiological, clinical and pathophysiological characteristics.
Rastogi S, Arora P, Devi P, Wazir SS, Kapoor S. Importance of ultrasonography and magnetic resonance imaging in diagnosis of cysticercosis of temporalis muscle mimicking temporal space infection. Contemp Clin Dent. 2013;4(4):504–8. doi:10.4103/0976-237X.123059.
Shoji H, Hirai T, Shirakura T, Takuma T, Okino T, Wakatsuki Y, et al. A case of cysticercosis with multiple lesions in the brain and femoral muscles. Kansenshogaku Zasshi. 2013;87(5):608–12.
Schistosomiasis. World Health Organisation. 2014. http://www.who.int/schistosomiasis/en/. Accessed 4 May 2014.
Ferrari TC, Moreira PR. Neuroschistosomiasis: clinical symptoms and pathogenesis. Lancet Neurol. 2011;10(9):853–64. doi:10.1016/S1474-4422(11)70170-3.
Akgoz A, Mukundan S, Lee TC. Imaging of rickettsial, spirochetal, and parasitic infections. Neuroimaging Clin N Am. 2012;22(4):633–57. doi:10.1016/j.nic.2012.05.015.
Turgut AT, Altin L, Topcu S, Kilicoglu B, Aliinok T, Kaptanoglu E, et al. Unusual imaging characteristics of complicated hydatid disease. Eur J Radiol. 2007;63(1):84–93.
Stojkovic M, Junghanss T. Cystic and alveolar echinococcosis. Handb Clin Neurol. 2013;114:327–34. doi:10.1016/B978-0-444-53490-3.00026-1.
Ghonge NP, Rajan S, Aggarwal B, Sahu AK. Imaging of ruptured endocyst in an isolated intramuscular hydatid cyst—the Scroll appearance. J Radiol Case Rep. 2012;6(8):17–21. doi:10.3941/jrcr.v6i8.739.
Carod-Artal FJ. American trypanosomiasis. Handb Clin Neurol. 2013;114:103–23. doi:10.1016/B978-0-444-53490-3.00007-8.
Di Lorenzo GA, Pagano MA, Taratuto AL, Garau ML, Meli FJ, Pomsztein MD. Chagasic granulomatous encephalitis in immunosuppressed patients computed tomography and magnetic resonance imaging findings. J Neuroimaging. 1996;6(2):94–7.
Yoo TW, Mlikotic A, Cornford ME, Beck CK. Concurrent cerebral American trypanosomiasis and toxoplasmosis in a patient with AIDS. Clin Infect Dis. 2004;39(4):e30–4. doi:10.1086/422456.
Carod-Artal FJ, Vargas AP, Horan TA, Nunes LG. Chagasic cardiomyopathy is independently associated with ischemic stroke in Chagas disease. Stroke. 2005;36(5):965–70.
Compliance with Ethics Guidelines
Conflict of Interest
Pooja Raibagkar and Martha Neagu have no conflicts of interest. Joshua Klein served as a board member for the American Society of Neuroimaging; received an Associate Editor stipend from the Journal of Neuroimaging; received honoraria from the American Academy of Neurology and honoraria from the Neurological Society of Pune, India; received payment for manuscript preparation from the AAN Continuum journal; and received royalties from the “Adams and Victor’s Principles of Neurology” textbook, 10th edition (McGraw-Hill). Jennifer Lyons received honoraria from Springer for working as a section editor.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Central Nervous System Infections
Rights and permissions
About this article
Cite this article
Raibagkar, P., Neagu, M.R., Lyons, J.L. et al. Imaging in Neurologic Infections I: Bacterial and Parasitic Diseases. Curr Infect Dis Rep 16, 443 (2014). https://doi.org/10.1007/s11908-014-0443-8
Published:
DOI: https://doi.org/10.1007/s11908-014-0443-8