Abstract
Purpose of Review
Radiation-associated valvular disease (RAVD) is characterized by late valvular manifestations following radiation exposure to the mediastinum. Review of current guidelines was performed to examine best practices to reduce risk and optimize outcomes in this patient population.
Recent Findings
Early and consistent screening and comprehensive and careful planning are critical in managing RAVD. Due to long latency periods, serial screening and targeted evaluation of risk factors are essential to early detection. Varying and complex presentations of RAVD require an integrated team of experienced specialists equipped with multimodality imaging-based screening protocols to stratify risk, plan intervention, and evaluate treatment response.
Summary
Patients with valvular manifestations associated with radiation therapy call for an individualized plan of care involving longitudinal multimodality imaging-based screening and experienced decision-making regarding timing and strategy of intervention to improve patient outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiation-associated cardiac disease (RACD) represents a late manifestation of radiation therapy (XRT) for various thoracic malignancies, including breast cancer, Hodgkin’s and non-Hodgkin’s lymphoma, and lung and esophageal cancer [1•]. While advances in radiation and chemotherapeutic regimens over the past several decades have resulted in vastly improved long-term survival among patients with thoracic malignancies, this increased longevity has come at the cost of an increasing prevalence of RACD [2, 3].
Although the acute cardiac manifestations of XRT have been recognized since the beginning of the twentieth century when high-dose, wide-field mediastinal XRT was the norm, the longer-term cardiac implications have only come to light in recent years. This is due to a significant latency period between XRT and the cardiac manifestations of RACD [4]. Radiation-associated cardiac disease carries substantial healthcare implications, with an absolute risk of cardiac morbidity and mortality of 2% at 5 years and 23% at 20 years compared with non-irradiated patients [5]. In light of the heightened awareness of the cardiotoxic effects of XRT, modern radiation delivery techniques utilize a variety of techniques to mitigate the deleterious cardiac effects of therapy. These include provisions such as respiratory gating techniques, including deep inspiratory breath-holds and activated breath control, shielding techniques, and using treatment algorithms utilizing narrow tangential beams [6]. The extent to which these cardioprotective techniques minimize the incidence of RACD remains to be seen due to the significant lag between XRT and the development of RACD. Furthermore, current clinical practice is largely shaped by antiquated radiation delivery practices.
Although RACD covers a spectrum of cardiac disease processes (coronary, aortic, great vessel, myocardial) and can involve any cardiac structure, radiation-associated valvular heart disease (RAVD) is the most common manifestation of RACD [7]. Management of valvular disease is challenging in patients with RACD due to concomitant involvement of other cardiac structures, and the high morbidity and mortality associated with surgical intervention [8••]. In this article, we will review the clinical and imaging manifestations of RAVD and outline our approach to diagnosis and management.
Molecular Mechanisms and Risk Factors
The adverse cardiac implications of XRT are largely mediated by damage to endothelial cells. This is due to the fact that cardiac myocytes are fairly resistant to the toxic effects of radiation because of their postmitotic state, while endothelial cells are sensitive to radiation damage. Radiation therapy results in an inflammatory cascade initiated by the formation of reactive oxygen species with a subsequent increase in the production of nuclear factor-kappa beta. Ultimately, this results in downregulation of nitric oxide synthesis and increased expression of proinflammatory cytokines, adhesion molecules, and matrix metalloproteases [9]. Among patients with RAVD, XRT is believed to result in higher expression of osteogenic factors, including alkaline phosphatase, osteopontin, runt-related transcription factor 2, and bone morphogenic protein 2 by valvular interstitial cells which result in a phenotypic shift from a myofibroblast- to an osteoblast-like cell [10].
A dose-response relationship exists between XRT and RACD, and the volume of cardiac tissue exposed to radiation is the greatest determinant for the development of RACD [11]. Smoking and cardiometabolic disturbances such as hypertension, hyperlipidemia, and diabetes amplify and hasten the endothelial damage brought about by XRT [12]. Younger age at the time of XRT and concurrent use of cardiotoxic chemotherapeutic agents such as anthracyclines and HER-2 receptor antagonists also increase the risk of RACD [13]. The risk factors for RACD are summarized in Table 1.
Valvular Manifestations
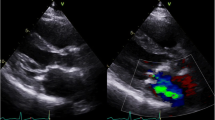
Valvular heart disease is present in as many as 81% of patients with RACD [14]. The left-sided valves are involved to a far greater extent than right-sided valves, and valvular manifestations may be either stenotic or regurgitant. Radiation may result in damage to valve leaflets, including fibrotic thickening, retraction, and calcification, and perivalvular structures including the annulus, subvalvular apparatus, and aortomitral curtain (AMC, Fig. 1). Indeed, thickening and calcification of the AMC is a hallmark feature of RAVD and is an independent predictor of adverse longer-term outcomes in these patients [15].
The latency period from XRT to the development of clinically significant RAVD is typically one to two decades following therapy [16]. Compared with patients treated with XRT within 10 years, those treated more than two decades ago are at substantially increased risk for aortic regurgitation (60% vs. 4%), mitral regurgitation (52.1% vs. 26.3%), aortic stenosis (16% vs. 0%), and tricuspid regurgitation (4% vs. 0%) [17].
Screening and Diagnosis
Early and consistent screening is key in RAVD. Prior to XRT, all patients should undergo a comprehensive transthoracic echocardiogram (TTE), in addition to a complete cardiovascular history and examination. Patients should be followed clinically at least annually thereafter. The development of any new signs or symptoms of cardiopulmonary disease should prompt repeat TTE.
Among patients without signs or symptoms of cardiopulmonary disease, screening should begin roughly 10 years after XRT and every 5 years thereafter [18]. Patients with at least one risk factor for RAVD (Table 1) undergoing anterior or left-sided XRT are considered high risk, and screening should commence 5 years after XRT at 5-year intervals following this.
Patients with at least one risk factor for RACD undergoing anterior or left-sided XRT are considered high risk, and initial screening TTE should be performed approximately 5 years after XRT and at 5-year intervals thereafter [4]. Modifiable risk factors such as hypertension, hyperlipidemia, obesity, and smoking should be addressed given the synergism between traditional cardiac risk factors and XRT in increasing the risk of adverse cardiac outcomes [8••]. Our approach to screening and diagnosing RAVD is summarized in Fig. 2.
Multimodality Imaging
Echocardiography is the most common imaging modality used in screening, diagnosing, and monitoring RAVD [18]. Features of RAVD on TTE include biventricular systolic and diastolic dysfunction, involvement of multiple valves, wall motion abnormalities, prominent calcification, and pericardial disease. One should be cognizant of the TTE features of constrictive physiology when RAVD is suspected, including biatrial enlargement, an early diastolic septal bounce, pericardial thickening and calcification, and plethora of the inferior vena cava and hepatic veins with increased respiratory variation [1•]. The earliest manifestation of RAVD involves progressive valvular retractions and regurgitation, which typically occurs within the first 10 years following treatment [8••]. This is followed by progression to fibrotic thickening, calcification, and stenosis, which occurs ~ 2 decades after XRT [1•,18]. Again, progressive thickening and calcification of the AMC is a hallmark feature [15]. Although TTE provides sufficient information for screening and diagnosis and to inform management in the vast majority of cases, transesophageal echocardiography (TEE) may provide further diagnostic fidelity. However, one must be cautious when performing TEE in RACD due to the possibility of XRT-associated esophageal injury.
Multidetector computed tomography (MDCT) is also commonly utilized in RAVD to evaluate for aortic, valvular, myocardial, and pericardial calcification. Among patients with RAVD undergoing surgical evaluation, pre-operative MDCT is used to assess for aortic calcification to determine feasibility of aortic cross-clamping and cannulation. In patients with RAVD undergoing redo surgical procedures, pre-operative MDCT provides useful information. Extensive fibrosis and adhesions may preclude median sternotomy and require a nonsternotomy or transcatheter approach [19]. Moreover, valve repair may be prohibited by severe valvular or perivalvular calcification [8••]. Radiation-associated pulmonary fibrosis adversely impacts outcomes and should be assessed on MDCT [20]. When transcatheter interventions are being considered, a 4-dimensional MDCT is essential in assessing annular shape and size and the iliofemoral vasculature [21].
Cardiac magnetic resonance is not routinely recommended in patients with RAVD. However, it can provide a more refined assessment of valvular function using transvalvular gradients and regurgitation volumes.
Management
Radiation-associated valvular disease is a complex disease that requires integrated care from a team of cardiologists, imaging specialists, interventionalists, and cardiothoracic surgeons [22]. Timing and strategy of surgical intervention for RAVD necessitate an experienced team equipped with comprehensive multimodality screening protocols to stratify risk, plan intervention, and evaluate treatment response. Our approach to the management of RAVD is shown in Fig. 3.
Simplified approach to managing RAVD. AS, aortic stenosis; AR, aortic regurgitation; MS, mitral stenosis; MR, mitral regurgitation; CAD, coronary artery disease; AVR, aortic valve replacement; MVR, mitral valve replacement; CABG, coronary artery bypass graft; TAVR, transcatheter aortic valve replacement; PCI, percutaneous coronary intervention; TMVR, transcatheter mitral valve replacement
Although no medication has been approved for the treatment of RACD, limited data from animal models suggest that certain therapies may have cardioprotective effects [23]. For instance, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have been shown to attenuate XRT-induced cardiotoxicity [24]. Statins and melatonin may have similar cardioprotective effects [25, 26]. Recombinant human neuregulin-1β may reduce mitochondrial dysfunction in the early phase following XRT and reduce fibrosis and cardiomyocyte hypertrophy at later stages [27]. However, there is currently a paucity of prospective, randomized controlled trials assessing the efficacy of these therapies in human subjects.
Among patients with severe symptomatic RAVD, the choice of valvular intervention is challenging and often informed by the extent to which other cardiopulmonary structures are concomitantly involved. Moreover, surgical intervention in RAVD carries a high morbidity and mortality, with a long-term mortality rate of approximately 45% in those undergoing surgery on a single valve and 61% in those undergoing surgery on multiple valves, compared with 13 and 17%, respectively, in patients without a history of XRT [28]. Consequently, comprehensive pre-operative evaluation with TTE, MDCT, coronary angiography, and pulmonary function testing is essential.
Many patients with RAVD have concomitant disease involving other valves, the pericardium, coronary arteries, conduction system, and myocardium. Furthermore, surgical intervention in patients with RAVD is frequently complicated by intrathoracic fluid retention as a result of XRT-induced lymphatic dysfunction, and this has major implications on postoperative quality of life. Redo surgery in RACD is associated with significant morbidity and mortality [29••]. Consequently, many experts believe that surgical intervention should be considered later than normal in the course of disease, so the operation addresses all valve issues, and a re-operation is avoided. Many patients will not return to a near-normal quality of life following intervention, and management of patient expectations is important. Moreover, postoperative recuperation is oftentimes protracted as a result of poor wound healing, recurrent pleural effusions, and conduction system disturbances.
Pre-operative planning with respect to cannulation, cross-clamping, and management of valvular and fibrous skeleton calcification is imperative [30]. In general, thin and patchy aortic calcification on MDCT will allow for safe aortic clamping. In instances where the calcification is denser and more circumferential, plans should be made for circulatory arrest and ascending aorta replacement. The cardiac surgeon should be comfortable removing or working around areas of calcification [8••].
In general, valve replacement is preferred to valve repair given the high failure rates resulting from ongoing XRT-associated valvular changes after repair, including thickening, restriction, and calcification [31]. In patients with disease involving multiple valves, replacement of both the aortic and mitral valve is recommended to avoid subsequent reoperation, even if only 1 of the valves has moderate disease. Multi-valve surgery can be complicated by severe calcification across the aortomitral curtain, which can jeopardize a safe valve replacement if aggressive debridement of calcium leaves little healthy tissue to anchor and seal the valve. Double-valve replacements are very common when radiation fibrosis leads to shrinkage of the aortic and mitral annuli. The Commando operation takes both scenarios into account by attaching a patch of autologous or bovine pericardium to the tissues formed by the left atrium, mitral annulus, aortomitral curtain, aortic annulus, and aortic valve. This operation allows for the sealing of 2 prosthetic valves and increased valve size for physiological hemodynamics [8••].
When choosing the valve prosthesis, there are several factors to consider. Younger patients undergoing valve replacement for RAVD are at higher risk for reoperation, so mechanical protheses are generally recommended [32]. Older patients and patients with comorbidities that may prevent them from taking lifelong anticoagulants fare better with bioprostheses. Bioprostheses also allow for subsequent valve-in-valve transcatheter intervention as a second operation.
Our group has previously studied the longer-term outcomes of patients with RAVD undergoing mitral valve surgery, surgical aortic valve replacement, and transcatheter aortic valve replacement. Among 146 patients with RAVD requiring mitral valve (MV) surgery, MV repair was performed in 23%, while 58% underwent bioprosthetic MV replacement, and 19% underwent mechanical MV replacement. A total of 55% required repair or replacement of another valve and 21% were reoperations. We found that 51% of these patients died during a median follow-up of 1.6 years with an annualized mortality of ~ 18% per year. While a higher Society of Thoracic Surgeons (STS) score was associated with increased mortality on multivariable models, the type of MV surgery was not [33•].
We also studied longer-term outcomes of surgical aortic valve replacement (SAVR) in patients with RAVD. Among 172 patients with RAVD undergoing SAVR, death occurred in 48% during a mean follow-up of 6 years, compared with 7% in an age- and gender-matched control population (p < 0.001) [34]. We subsequently found that the rate of progression of aortic stenosis in RAVD is similar to the rate observed in control patients. However, we found that a significantly higher proportion of patients with RAVD underwent SAVR (80% vs. 50%, p < 0.01) at a far shorter time from the initial TTE 2.9 ± 1.6 years vs. 4.1 ± 2.4 years (p < 0.01) [35].
Due to the high morbidity and mortality in RAVD, there has been a great deal of enthusiasm surrounding the potential role for transcatheter aortic valve replacement (TAVR) in this patient population. In theory, TAVR could mitigate several issues encountered during surgical valve replacement, including XRT-induced fibrosis and adhesions, and restrictive lung disease. However, TAVR is oftentimes not optimal in RAVD due to extensive ascending aortic calcification, extensive AMC calcification with extension into the anterior mitral leaflet, and a high prevalence of conduction system disease. We previously compared the outcomes of 98 patients with RAVD undergoing TAVR with 172 patients with RAVD undergoing SAVR and demonstrated an in-hospital, 1- and 2-year survival of 96%, 91%, and 86% in the TAVR cohort compared with 96%, 86%, and 80% in the SAVR group [36]. Zhang et al. compared the outcomes of 55 patients with RAVD undergoing TAVR with further 55 patients undergoing SAVR and found that TAVR was associated with lower adjusted 30-day mortality, postoperative atrial fibrillation, and shorter hospitalization compared with SAVR [37]. TAVR should be performed in accordance with consensus guidelines, [21, 38] and TAVR may be considered the default strategy in patients with RAVD in whom transfemoral access can be performed, and in the absence of complicating factors such as multivalvular disease, advanced coronary artery disease, or excessive risk for coronary obstruction or annulus rupture.
Conclusions
Radiation-associated valvular disease is an increasingly recognized late consequence of mediastinal radiation. Knowledge of the potential for cardiotoxicity following a long latency period is crucial in order for appropriate multimodality imaging-based screening to be conducted. Among patients requiring percutaneous or surgical intervention, individualized timing and technique are critical, and, as such, these patients should be managed at high-volume centers with experience in managing radiation-associated valvular disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Desai MY, Jellis CL, Kotecha R, Johnston DR, Griffin BP. Radiation-associated cardiac disease: a practical approach to diagnosis and management. JACC Cardiovasc Imaging. 2018;11:1132–49 Provided a comprehensive overview of the disease and pragmatic approaches to diagnosis and management.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Donnellan E, Phelan D, Mccarthy CP, Collier P, Desai M, Griffin B. Radiation-induced heart disease: a practical guide to diagnosis and management. Cleve Clin J Med. 2016;83:914–22.
Donnellan E, Jellis CL, Griffin BP. Radiation-associated cardiac disease: from molecular mechanisms to clinical management. Curr Treat Options Cardiovasc Med. 2019;21(5):22.
Galper SL, Yu JB, Mauch PM, Strasser JF, Silver B, LaCasce A, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117:412–8.
Emami B, Lyman J, Brown A, Cola L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22.
Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–73.
•• Desai MY, Windecker S, Lancellotti P, et al. Prevention, diagnosis, and management of radiation-associated cardiac disease: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;74:905–27 A landmark scientific statement on radiation heart disease from a panel of experts.
Hayashi T, Morishita Y, Kubo Y, et al. Long-term effects of radiation dose on inflammatory markers in atomic bomb survivors. Am J Med. 2005;79:129–36.
Nadlonek NA, Weyant MJ, Yu JA, Cleveland JC Jr, Reece TB, Meng X, et al. Radiation induces osteogenesis in human aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2012;144:1466–70.
Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ, Allen AM, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76:S77–85.
Amromin GD, Gildenhorn HL, Solomon RD, Nadkarni BB, Jacobs ML. The synergism of X-irradiation and cholesterol-fat feeding on the development of coronary artery lesions. J Atheroscler Res. 1964;4:325–34.
Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366:399–408.
Tamura A, Takahara Y, Mogi K, Katsumata M. Radiation-induced valvular disease is the logical consequence of irradiation. Gen Thorac Cardiovasc Surg. 2007;55:53–6.
Desai MY, Wu W, Masri A, Popovic ZB, Agarwal S, Smedira NG, et al. Increased aorto-mitral curtain thickness independently predicts mortality in patients with radiation-associated cardiac disease undergoing cardiac surgery. Ann Thorac Surg. 2014;97:1348–55.
Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid , subclavian , and coronary artery disease treated with radiation therapy. Jama. 2003;290:2831–387.
Heidenreich PA, Hancock SL, Lee BK, Mariscal CS, Schnittger I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42:743–9.
Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14(8):721–40.
Kamdar AR, Meadows TA, Roselli EE, Gorodeski EZ, Curtin RJ, Sabik JF, et al. Multidetector computed tomographic angiography in planning of reoperative cardiothoracic surgery. Ann Thorac Surg. 2008;85:1239–45.
Desai MY, Karunakaravel K, Wu W, Agarwal S, Smedira NG, Lytle BW, et al. Pulmonary fibrosis on multidetector computed tomography and mortality in patients with radiation-associated cardiac disease undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2014;148:475–81.
Otto CM, Kumbhani DJ, Alexander KP, et al. ACC expert consensus decision pathway for transcatheter aortic valve replacement in the management of adults with aortic stenosis: a report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol. 2017;2017(69):1313–46.
Lancellotti P, Suter TM, López-Fernández T, Galderisi M, Lyon AR, van der Meer P, et al. Cardio-oncology services: rationale, organization, and implementation. Eur Heart J. 2019;40:1756–63.
Donis N, Oury C, Moonen M, Lancellotti P. Treating cardiovascular complications of radiotherapy: a role for new pharmacotherapies. Expert Opin Pharmacother. 2018;19:431–42.
Van Der Veen SJ, Ghobadi G, De Boer RA, et al. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol. 2015;114:96–103.
Haydont V, Bourgier C, Pocard M, Lusinchi A, Aigueperse J, Mathe D, et al. Pravastatin inhibits the rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res. 2007;13:5331–40.
Gürses I, Özeren M, Serin M, Yücel N, Erkal HŞ. Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol Res Pract. 2014;210:863–71.
Gu A, Jie Y, Sun L, Zhao S, Mingyan E, You Q. RhNRG-1β protects the myocardium against irradiation-induced damage via the ErbB2-ERK-SIRT1 signaling pathway. PLoS One. 2015;10:e0137337.
GHARAGOZLOO F, CLEMENTS IP, MULLANY CJ. Use of the internal mammary artery for myocardial revascularization in a patient with radiation-induced coronary artery disease. Mayo Clin Proc. 1992;67(11):1081–4.
•• Wu W, Masri A, Popovic ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127:1476–84 Highlighted the high risk nature of open heart surgery in patients with radiation heart disease.
Desai MY, Cremer PC, Schoenhagen P. Thoracic aortic calcification: diagnostic, prognostic, and management considerations. JACC Cardiovasc Imaging. 2018;11:1012–26.
Crestanello JA, McGregor CGA, Danielson GK, et al. Mitral and tricuspid valve repair in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2004;78:826–31.
Wu W, Masri A, Popovic ZB, Smedira NG, Lytle BW, Marwick TH, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127:1476–85.
• Donnellan E, Alashi A, Johnston DR, et al. Outcomes of patients with mediastinal radiation-associated mitral valve disease undergoing cardiac surgery. Circulation. 2019;140:1288–90 The first study to investigate outcomes of mitral valve interventions in these patients.
Donnellan E, Masri A, Johnston DR, et al. Long-term outcomes of patients with mediastinal radiation-associated severe aortic stenosis and subsequent surgical aortic valve replacement: a matched cohort study. J Am Heart Assoc. 2017;6:e005396.
Donnellan E, Griffin BP, Johnston DR, Popovic ZB, Alashi A, Kapadia SR, et al. Rate of progression of aortic stenosis and its impact on outcomes in patients with radiation-associated cardiac disease: a matched cohort study. JACC Cardiovasc Imaging. 2018;11:1072–80.
Donnellan E, Krishnaswamy A, Hutt-Centeno E, Johnston DR, Aguilera J, Kapadia SR, et al. Outcomes of patients with mediastinal radiation-associated severe aortic stenosis undergoing transcatheter aortic valve replacement. Circulation. 2018;138(16):1752–4.
Zhang D, Guo W, Al-Hijji MA, et al. Outcomes of patients with severe symptomatic aortic valve stenosis after chest radiation: transcatheter versus surgical aortic valve replacement. J Am Heart Assoc. 2019;8:e012110.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;2017(38):2739–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Valvular Heart Disease
Rights and permissions
About this article
Cite this article
Xu, S., Donnellan, E. & Desai, M.Y. Radiation-Associated Valvular Disease. Curr Cardiol Rep 22, 167 (2020). https://doi.org/10.1007/s11886-020-01411-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-020-01411-0