Abstract
Purpose of Review
To highlight the gender-based differences in presentation and disparities in care for women with familial hypercholesterolemia (FH).
Recent Findings
Women with FH experience specific barriers to care including underrepresentation in research, significant underappreciation of risk, and interrupted therapy during childbearing. National and international registry and clinical trial data show significant healthcare disparities for women with FH. Women with FH are less likely to be on guideline-recommended high-intensity statin medications and those placed on statins are more likely to discontinue them within their first year. Women with FH are also less likely to be on regimens including non-statin agents such as PCSK9 inhibitors. As a result, women with FH are less likely to achieve target low-density lipoprotein cholesterol (LDL-C) targets, even those with prior atherosclerotic cardiovascular disease (ASCVD).
Summary
FH is common, under-diagnosed, and under-treated. Disparities of care are more pronounced in women than men. Additionally, FH weighs differently on women throughout the course of their lives starting from choosing contraceptives as young girls along with lipid-lowering therapy, timing pregnancy, choosing breastfeeding or resumption of therapy, and finally deciding goals of care during menopause. Early identification and appropriate treatment prior to interruptions of therapy for childbearing can lead to marked reduction in morbidity and mortality. Women access care differently than men and increasing awareness among all providers, especially cardio-obstetricians, may improve diagnostic rates. Understanding the unique challenges women with FH face is crucial to close the gaps in care they experience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When Katherine Wilemon experienced severe chest pain in her mid-thirties, as a young woman she did not expect to have heart disease, nor did the medical teams who initially evaluated her and dismissed her symptoms. Despite an LDL cholesterol above the ninety-fifth percentile, she was only taken seriously after a reluctant cardiologist performed a coronary angiogram which revealed significant coronary artery disease requiring percutaneous coronary intervention. Even after the diagnosis of early-onset atherosclerotic cardiovascular disease (ASCVD), it took her an additional 2 years to be formally diagnosed with familial hypercholesterolemia (FH). This story is all too common for individuals, especially women, with FH. Katherine Wilemon recovered and founded the FH Foundation (www.thefhfoundation.org). Her story, unfortunately, highlights how women with FH are managed and brings to the forefront the missed opportunities for preventive care.
FH is an autosomal dominant genetic disorder characterized by lifelong elevations in low-density lipoprotein cholesterol (LDL-C) levels. Approximately 1 in every 200 people has FH, but the vast majority are undiagnosed [1•]. Individuals with FH are often asymptomatic until a presentation with acute coronary syndrome (ACS) or stroke. Untreated women with FH are at very high risk for early-onset atherosclerotic cardiovascular disease (ASCVD) [2]. Early and aggressive lipid-lowering therapy can markedly attenuate this risk in both women and men. Women are not only less likely be prescribed lipid-lowering therapy and initiate early statin therapy, but they are more likely to have interrupted management compared with men due to childbearing and are less likely to achieve goal LDL-C levels [3••]. Additionally, perception of lower ASCVD risk in women can be particularly deleterious for women with FH and lead to under-treatment. In this review, we highlight the gender disparities in the treatment of women with FH and provide perspectives for healthcare providers to consider while treating a young woman with FH throughout her life.
Disparities in Care and Representation of Women in FH Research
In untreated women with FH, 30% will develop ASCVD by the age of 60 years. The onset of ASCVD occurs 20 years earlier in life for women with FH than in women without FH [4]. Even after an ASCVD event, women are 10% less likely to be diagnosed with of FH upon hospital discharge compared with men [5•]. Furthermore, compared with men, women are less likely to receive appropriate guideline-based cholesterol management or have an adequate LDL-C reduction [3, 6•, 7,8,9,10]. The Cascade Screening for Awareness and Detection of Familial Hypercholesterolemia (CASCADE-FH) registry is a national registry that tracks treatment and outcomes of patients with FH in the USA [11]. In this dataset, women were 31% less likely than men to achieve LDL-C < 100 mg/dL, while women with FH and ASCVD were 25% less likely than their male counterparts to achieve LDL-C < 100 mg/dL (Fig. 1) [3••]. Women are more likely to experience statin-associated side effects than men (Fig. 1) [3••], but this is not the sole reason for this discrepancy. Implicit physician biases in treating women with ASCVD are established [12], and for women with FH, these become more dangerous, and provider and patient awareness is a key component in overcoming them.
Disparity outcomes in the CASCADE-FH registry [3]. a Differences in the reduction of LDL-C between men and women. Treated LDL-C levels are higher in women than men (< 0.001). b Statin intolerance between men and women. Women had a higher prevalence of statin intolerance (< 0.001). CASCADE-FH categorized individuals citing intolerance or allergy as a reason for not being on statins as statin intolerant. c Percentage of patients on lipid-lowering therapy by drug and gender. Women were less likely to be on statin therapy than men (< 0.001). d Achievement of LDL-C levels less than 100 mg/dL or reduction by 50% LDL-C in men versus women. Women were less likely to achieve LDL-C < 100 mg/dL (odds ratio (OR) 0.68, 95% CI, 0.57–0.82) or reduction of 50% LDL-C (OR, 0.79, 95% CI, 0.65–0.96). Adapted from CASCADE-FH registry data [3]
Representation of women in registries and trials studying FH and lipid-lowering medication is a critical to improve their outcomes and reduce gender-based disparities. Some of the important FH registries and clinical trials are detailed in Table 1. Historically, many of these studies have had an underrepresentation of women [14••, 16, 18]. The CASCADE-FH registry has been more representative, with 60% of the cohort being women. A caveat of this representation is that 79% of these women are white. Racial and ethnic disparities in the USA and in cardiovascular care are rampant today and it has even been shown that Asian and African American women are significantly less likely to achieve an LDL-C < 100 mg/dL [3••]. In the CASCADE-FH cohort, only 4.6% of the cohort identified as Black, 2.4% Hispanic, 2.3% Asian, and 3.4% other [23••]. Nevertheless, even with this small sample size, there is evidence that Blacks were less likely to be on guideline-based medical management and achieve target LDL-C levels. Therefore, while women’s representation needs to improve, the granularity of this representation is an important consideration to truly ascertain the disparities experienced by women with FH.
Diagnostics
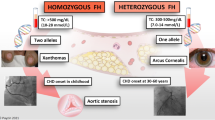
Undiagnosed FH costs 16 years of life expectancy [24] and on average, women are diagnosed with FH 4 years later than men [3••]. Although FH is a dominant genetic disorder, caused by genetic mutations in one of three genes: LDLR, APOB, and PCSK9, diagnosis is based on a combination of lipid levels, personal and family history of heart disease, physical exam findings, and genetic testing results (if available) for mutations in one of the three genes. People often have just one disease-causing variant causing heterozygous FH (HeFH). Rarely, people may inherit two disease-causing variants (homozygous or HoFH). There is a free mobile phone-based application that provides a stepwise diagnostic tool that can be used by primary care providers and cardiologists alike to simplify the diagnostic process [25].
While recommendations for routine screening for hyperlipidemia start as early as 8–10 years, less than 3% of children are screened for lipids [26]. In part, this can be explained by data showing overall poor awareness of the guidelines: Only 26% of pediatricians were knowledgeable about the 2011 NHLBI Guidelines for lipid screening [27•]. There is also a general lack of knowledge about FH across medical fields: Only 15% of general practitioners are very familiar with FH, while 61% of cardiologists and 45% of other specialists were very familiar. As young girls and women receive care from pediatricians, general practitioners, and obstetricians/gynecologists, the key to early diagnosis lies in awareness among all practitioners.
Cascade screening (family-based testing of relatives of affected individuals) is a proven effective approach to improve FH diagnosis [28]. Cascade testing can be done with lipid and/or genetic testing. Early diagnosis of young FH children by screening children at immunization visits and reverse cascade screening of their parents can widen the reach of FH screening and increase diagnostic rates [29•]. In this paper, a majority of parents supported genetic testing. Importantly cascade genetic screening has not been linked to psychological or social harm in adults or children [30]. Diagnosing children will dramatically reduce the age at which individuals are diagnosed and treated, especially benefitting women, who are likely to have interrupted management [31].
Girls and Young Adult Women
Though ASCVD is thought to be a condition that only affects adults, in patients with HoFH, major coronary events and death can be seen in early childhood [32,33,34]. Pre-adolescence being a period of minimal dietary and hormonal influences is the optimal period to discriminate between FH and non-FH based on LDL-C concentration. Following dietary interventions, a child with an LDL-C level ≥ 160–190 mg/dL has a high probability of genetically based FH [35]. Girls aged 5–19 years with FH have been shown to have higher total cholesterol, low-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol compared with boys in the same age group [36] and therefore experience a higher risk burden from a younger age.
Early statin initiation is especially important for young girls as they are more likely to see interruptions in therapy. Current guidelines call for the initiation of statins in children around age 8 if the LDL-C is > 190 mg/dL especially if there is a family history of heart disease. However, this is often not done. While pediatricians believe statins are appropriate for children and adolescents, only a minority of pediatricians initiate statins [27•]. Statins are well-tolerated in the pediatric population and studies have shown no significant risk in growth and puberty delays, transaminitis, or myopathy. Additionally, a 10-year follow-up study on the initiation of statins in pediatric patients with FH showed normalization in the development of atherosclerosis when compared with their unaffected sibling [37•]. Earlier disease control minimizes the effects of interruptions during childbearing and allows women the possibility of safely starting their families at a younger age if they choose to.
Lipid-lowering therapy should not be withheld in young women of childbearing age, but appropriate contraception must be advised to post-pubertal young girls on lipid-lowering therapy. Providing an overview of long-term care, discussing adherence strategies, time to achieve acceptable LDL levels, follow-up, and creating an environment for shared decision-making is imperative to effective treatment especially in those diagnosed young.
Contraceptive Considerations
Contraception is advised for women on all lipid-lowering therapies used in FH except for bile acid-binding resins because of teratogenicity. In patients with FH and high risk of ASCVD, a preferred method of contraception is a non-hormonal intrauterine device, followed by progesterone-only containing contraceptives including levonorgestrel-releasing intrauterine device, subcutaneous implant, depot medroxyprogesterone acetate injection, or progestin-only pill [38]. Combined hormonal contraceptive pills, patches, and rings can also be used but are classified as “theoretical or proven risks usually outweigh the advantages” [38]. In the USA, after tubal ligation, oral contraceptive pills (OCPs) are the most commonly used contraceptives. Although combined oral contraceptives are not contraindicated in FH, they might increase the risk of ASCVD events and hormone-containing contraceptives have been associated with increased triglyceride and LDL-C levels in women. While it is the provider’s responsibility to bring these risks to a women’s attention, ultimately the decision on which contraceptive method to use is a personal choice that should be made via shared decision-making between a woman and her care provider. Similarly, pre-pregnancy counseling is crucial in women with FH and these discussions should be started early in all adolescent girls with FH. These conversations should be patient-centric and include goals and expectations around pregnancy. It is important to discuss the side effects of lipid-lowering therapy should a woman become pregnant while on therapy and therefore stress the importance of adherence to her chosen contraceptive method.
Fertility and Conception
Women with FH have fertility rates similar to women without FH, in spite of a higher incidence of polycystic ovarian syndrome than the general population [39]. Importantly, while statins and other lipid-lowering medications are teratogenic, they have no reported effect on fertility. If a woman with FH is dealing with infertility, her dyslipidemia is a major determinant in her infertility management [40]. She may not be an ideal candidate for hormonal therapy and evidence from in vitro fertilization (IVF) studies indicate that dyslipidemia is associated with poorer oocyte quality, thereby effecting fecundity [41]. Women with FH may need to consider alternate strategies to deal with infertility and there is a need for more research in the area of dyslipidemia, FH, fertility, and fecundity.
Women are at higher risk of experiencing complications of FH during their reproductive years, as most cholesterol-reducing agents are contraindicated during pregnancy. Appropriately timing pregnancy in women with FH is a meaningful discussion that needs to be started early. Women with severe FH (especially those diagnosed late) may benefit from being aggressively managed in the years leading up to pregnancy to prevent pregnancy-related complications such as ACS. Improving cholesterol levels before conception not only benefits the mother but has important cardiovascular implications for her children. Elevated maternal cholesterol levels during pregnancy were associated with early fatty streak formations in fetal aortas, predisposing the fetus to atherosclerosis [42]. The FELIC Study (1999) showed that children born to mothers with hypercholesterolemia were more susceptible to atherogenic risk factors than children born to mothers with normal cholesterol levels [42]. Identifying women young allows greater flexibility in this timeline to ensure safe outcomes for both mother and child.
If a woman with FH is considering pregnancy, her partner should be tested for FH as well. Assuming her partner does not have FH, each child of a mother with HeFH will have a 50% chance of inheriting the condition. When both parents have FH, their child has a 25% chance of having HoFH and a 50% chance of having a child with HeFH. Prenatal counseling, risk discussions, and ensuring the couple is aware of these implications is an important second step. If a woman is planning to conceive after appropriate risk discussions, it is recommended to discontinue statins, ezetimibe, and niacin therapy 1–3 months before conception [43]. Apart from genetic counseling, prenatal or preimplantation genetic testing is only indicated if the parents have a homozygous child and are hoping to conceive again or if both parents have HeFH.
Women with FH commonly have healthy pregnancies, but unlike the general population, appropriate risk discussions and a thorough evaluation of their cardiovascular health may be necessary. Risk discussions for women with FH considering pregnancy vary based on the presence of cardiovascular symptoms and the progression of their atherosclerotic disease processes. Women with symptoms of cardiac disease need additional care and benefit from a multidisciplinary approach.
Pregnancy and FH
During pregnancy, LDL-C levels increase by more than 40% in the general population [44]. Women with FH show relative changes in lipids that are similar to unaffected women. However, the absolute increase is amplified in women with FH [45]. The consequences of these changes are theoretical postulations that increased cholesterol levels are associated with reduced uteroplacental perfusion [39]. Studies addressing the pregnancy outcomes and newborns of women with FH are limited, and the results can be conflicting [39, 46•].
Generally speaking, pregnancy in women with FH is relatively safe for both mothers and babies. An analysis of a registry of 2319 births of 1093 women with heterozygous FH collected between 1967 and 2006 in Norway showed that there was no increased risk of preeclampsia, gestational diabetes, or pregnancy-induced hypertension. There was also no increase in preterm delivery, low birth weight, or congenital malformations [39, 47]. Despite reassuring data, it is important to consider each pregnancy on an individual basis, especially in women with FH. The presence of cardiovascular symptoms in these women before pregnancy must be taken into consideration, as they may become exacerbated during pregnancy. These include coronary artery disease, cerebrovascular disease, and aortic stenosis. Coordinating care in such instances with a cardio-obstetric expert may improve outcomes, and that being said, coronary artery disease is a known problem in pregnancy and is among the top causes of pregnancy-associated cardiac death [48].
Apart from the effects of the FH alone, the inability to remain on cholesterol reduction therapy during pregnancy and lactation makes managing FH in the peripartum period challenging (Table 2). Cohort studies examining birth outcomes in statin exposure during pregnancy have not shown increased risk. A multicenter, observational prospective controlled study analyzed 249 pregnancies in women exposed to statins during the first trimester. They found no difference in the rate of major congenital disabilities (4.1% versus 2.7% OR 1.5; 95% CI 0.5–4.5, P = 0.43) [59], but data shows an increased risk of miscarriage in patients exposed to statins during pregnancy. The lack of prospective cohorts, however, leads to no conclusive evidence of statin safety in pregnancy. Although newer data suggest that statin therapy during pregnancy is not associated with fetal risk [60,61,62], avoiding use during pregnancy and breastfeeding is still standard practice [63, 64]. PCSK9 inhibitors have not been established to be safe and are also not recommended for use during pregnancy, though observational studies are ongoing [65].
The only FDA-approved lipid-lowering agents in pregnant women are Colesevelam and Mipomersen, and the latter of the two is no longer on the market [43, 66, 67]. Lipoprotein apheresis is rarely used but is an alternate option approved for controlling hypercholesterolemia in pregnant women who have homozygous or severe FH [68, 69]. Ideally, women with FH would have stopped all other lipid-lowering therapy 1–3 months before conception. If they are still on any medications, it is essential to stop them immediately, and they should not be resumed in women planning to breastfeed. Women who have been exposed can be considered for enrollment in registries that are investigating the effects of these exposures, and as randomized control trials in this population are unethical, these observational studies remain an essential source of information on managing women with FH during pregnancy and peripartum. In women whose FH has been managed earlier in life, interruption of therapy is often safe during pregnancy.
Breastfeeding
There are no definitive guidelines on the management of FH in women that are breastfeeding. Research examining the concentration of lipid-lowering agents excreted in breast milk and its effect on a breastfed infant is limited (Table 2). Lipid-lowering regimens have the same limitations as they do in pregnancy. Bile acid resins are the only lipid-lowering therapy not systemically absorbed and can be continued during lactation. Two significant choices need to be considered; maintain lactation for breastfeeding and delay resuming FH treatment, or forego breastfeeding, and resume aggressive therapy. Risks versus benefits for the woman and breastfed infant should be discussed. Ultimately, the decision should be made by the mother, with the clinician supporting and providing guidance. For women who have completed their family planning, long-term contraception strategies and long-acting injectables such as PCSK9 inhibitors can be considered once breastfeeding is stopped.
Menopausal Women with FH
In a study of a UK cohort of HeFH patients, mean LDL-C in men versus women 20–39 years old was 292 mg/dL and 276 mg/dL, while in men and women aged 60–74 years old, LDL-C was 228 mg/dL versus 272 mg/dL [70]. Per this study, menopausal women with FH had similar LDL values to premenopausal women and higher levels compared with men in the same age group. Menopausal women with FH may suffer the consequences of high LDL-C from delayed diagnosis or due to interruptions in management they may have faced during childbearing. These women now have an opportunity for an aggressive and uninterrupted approach to management. Some women attain menopause as early as forty and with appropriate care can significantly improve their life expectancy and quality of life. There are no specific guidelines for LDL-C management in postmenopausal patients. The necessity of medication escalation, reduction, or cessation should be made via shared decision-making between patient and provider based on their specific goals.
Hormonal therapy is not indicated for primary or secondary prevention of coronary artery disease and is especially not advisable for women with FH. When treatment is necessary for the relief of postmenopausal symptoms, preparations associated with lower thrombotic risk, such as patches and creams, are preferable [71]. As postmenopausal women with FH fall under a high-risk category, non-hormonal therapies, such as gabapentin or duloxetine, should be actively considered. These drugs are an alternative for treating vasomotor symptoms that do not increase coronary artery disease risk.
Caring for Women with FH Throughout Their Life
FH affects a woman in every stage of her life, from adolescence to menopause. Each stage represents a new set of unique challenges that must be addressed, from the selection of birth control to family planning (Fig. 2). Intensive management in the years before puberty if diagnosed as a child could be potentially lifesaving and has been shown to improve outcomes [37•]. If the diagnosis is made after the onset of puberty, initiation of lipid-lowering therapy and birth control as soon as possible is imperative. Regular assessments of adherence to lipid-lowering therapy and contraception are encouraged, while also discussing family planning as most medications should to be interrupted months before conception. Goals of care discussions and shared decision-making are paramount as patients may have to make difficult decisions that might lead to personal dilemmas, such as breastfeeding, or the desire for hormonal therapy.
Overview of screening and management of FH in (a) women and young adult (b) children and adolescents. Adapted from Lloyd-Jones DM et al. [72]; Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute, Pediatrics [73]; de Ferranti et al. [74]
The recommended goal for LDL-C is less than 100 mg/dL for patients with FH, with a minimal acceptable standard of a reduction of at least 50% from pretreatment. Follow-up should include LDL-C monitoring at regular intervals. While statins are the mainstay of disease prevention in FH, women were 40% less likely than men to be on a statin and if on a statin were 40% less likely than men to be on a high-intensity statin (Fig. 1) [3••]. Additionally, 40–75% of patients discontinue their statin therapy within 1 year of initiation, with higher rates for women than men [75]. Women were also more likely to have rejected or unfilled PCSK9i prescriptions [76••]. The discrepancies in prescription could be the lack of perceived risk for premature disease or due to misconceptions regarding fertility, pregnancy considerations, or higher reported statin adverse effect rates among women. When a woman with FH is not on appropriate statin or lipid-lowering therapy, her provider must initiate a discussion to investigate the cause. Additionally, stepwise escalation of lipid-lowering agents should be implemented, especially in patients who are on sub-optimal first-line statin therapy due to adverse effects. Women are more likely to experience statin-associated side effects than men [3••] and it is appropriate to re-challenge these women following appropriate discussions. This is accomplished by restarting current statin with every other day dosing, restarting on the lowest dose possible or a lower intensity statin, and slowly titrating until the highest tolerable dose is established. Newer agents have been shown to reduce the level of LDL-C in women significantly. In the CLEAR Harmony clinical trial, bempedoic acid, an ATP citrate lyase inhibitor, reduced LDL-C by 19.2 mg/dL or 18.1% (95% CI, − 20.0 to − 16.1%; P < .001) when used in combination with a maximally tolerated moderate- or high-intensity statin, with the most significant reduction in LDL-C observed in women [21••]. The use of bempedoic acid was also associated with lower incidences of muscle-associated statin sensitivity [21••, 77, 78].
Standard risk calculators are not appropriate for FH patients, and titration of medication is based on risk factors, coronary heart disease, and LDL-C goals. Women with FH are less likely than men to achieve goal LDL-C (Fig. 1) [3••]. For women that are still not at their goal LDL-C on the highest tolerated regimen of statin, add a second lipid-lowering agent, such as ezetimibe. It is also appropriate to add a PCSK9 inhibitor at this point. When added to the maximum tolerated statin dose, alirocumab reduced LDL-C by 62% (P < 0.001) and reduced major cardiac events [18]. Ensuring adherence through education and risk discussions is a necessary step to reach target LDL-C levels. For patients who remain above goal, referral to a lipidologist for consideration of PCSK9i should not be delayed. The FH Foundation website has a “Find an FH Specialist” link, which allows patients to connect to specialists in their area [79]. Considering lower rates of statin use and higher LDL-C values, this is especially important in women with FH. Women and families with FH are best treated by a multidisciplinary team of cardiologists, obstetricians/gynecologists, and genetic counselors.
The Future of FH in Women
FH is a common but vastly under-diagnosed condition. Strategies to improve diagnosis, especially for women, are essential to improve their outcomes. Women can be instrumental in overseeing the wellness visits and medical care their children receive. Screening in childhood is vital as each child in an FH family has a 50% chance of inheriting the genetic defect. Despite long-established medical guidelines, established heart disease, and the hard-won diagnosis of FH, Katherine Wilemon found her pediatrician unwilling to screen her first born. Another practice performed the screening and found her daughter had FH. Cascade screening has shown great promise in countries around the world, increasing the rate of early FH diagnosis and dramatically reducing the risk of premature morbidity and mortality. Centering care around women and providing them with adequate tools and knowledge to care for themselves and their families with FH can dramatically reduce the burden of premature heart disease for future generations.
Considerations surrounding lipid-lowering therapy for women are different than men. Cardio-obstetrics allows for comprehensive care of women with unique cardiovascular conditions and may be an effective approach for managing women with FH longitudinally. Women experience interrupted management during childbearing due to teratogenicity of these medications, leading to difficulties in treating women with FH perinatally. CURE ATHERO trial proposes lowering LDL-C at a younger age via an intensive period of LDL-C/non-HDL-C/apo B reduction, with subsequent periodic retreatment every decade or so [80]. Adaptations of this approach may be viable for young girls and women. Optimizing LDL levels in the years before women with FH consider motherhood is ideal and innovative strategies to achieve this may be necessary. While this is an intermediary step, there is a need for further investigation into therapies that do not need to be discontinued with pregnancy or have incrementally beneficial effects in women in particular.
FH registries provide a unique opportunity to study women in the population to identify shortcomings. Observational studies, like the CASCADE-FH registry, highlight the disparities in both representation of non-white women and management of high LDL-C in women compared with men. Women with FH face delayed diagnosis and interrupted management, making them more likely to require escalation of therapy. Though the price of statins is relatively affordable, and ezetimibe is generic, escalation to a PCSK9i remains costly. Unfortunately, women, minorities, and individuals of low socioeconomic status are often disproportionately affected with studies showing these populations to be more likely to have rejected or unfilled prescriptions [76••]. Given the heterogeneity of the USA, it is important that granular healthcare disparity data including outcomes in non-white women and women of low socioeconomic status is available. To understand these disparities and improve minority outcomes, representation in research is of utmost importance, while education and raising awareness will lead us to address them.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Onorato A, Sturm AC. Heterozygous familial hypercholesterolemia. Circulation. 2016;133(14):e587–9 Comprehensive review on the diagnosis and treatment of familial hypercholesterolemia.
Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, et al. The agenda for familial hypercholesterolemia. Circulation. 2015;132(22):2167–92.
•• Amrock SM, et al. Health disparities among adult patients with a phenotypic diagnosis of familial hypercholesterolemia in the CASCADE-FH patient registry. Atherosclerosis. 2017;267:19–26 Provides analysis of gender and racial disparities of data obtained from the CASCADE-FH, highlighting biases that lead to later diagnosis and less aggressive treatment of familial hypercholesterolemia in the USA.
Turgeon RD, Barry AR, Pearson GJ. Familial hypercholesterolemia. Review of diagnosis, screening, and treatment. 2016;62(1):32–7.
• Mundal L, et al. Cardiovascular disease in patients with genotyped familial hypercholesterolemia in Norway during 1994-2009, a registry study. Eur J Prev Cardiol. 2016;23(18):1962–9 Describes the difference in the incidence and prevalence of cardiovascular disease between men and women with familial hypercholesterolemia in Norway.
• McSweeney JC, et al. Preventing and experiencing ischemic heart disease as a woman: state of the science. Circulation. 2016;133(13):1302–31 Comprehensive review of research related to the diagnosis and treatment of women with ischemic heart disease.
Victor BM, Teal V, Ahedor L, Karalis DG. Gender differences in achieving optimal lipid goals in patients with coronary artery disease. Am J Cardiol. 2014;113(10):1611–5.
Schoen MW, Tabak RG, Salas J, Scherrer JF, Buckhold FR. Comparison of adherence to guideline-based cholesterol treatment goals in men versus women. Am J Cardiol. 2016;117(1):48–53.
Hammond G, Mochari-Greenberger H, Liao M, Mosca L. Effect of gender, caregiver, on cholesterol control and statin use for secondary prevention among hospitalized patients with coronary heart disease. Am J Cardiol. 2012;110(11):1613–8.
Rodriguez F, Olufade T, Heithoff K, Friedman HS, Navaratnam P, Foody JAM. Frequency of high-risk patients not receiving high-potency statin (from a large managed care database). Am J Cardiol. 2015;115(2):190–5.
O’Brien EC, et al. Rationale and design of the familial hypercholesterolemia foundation CAscade SCreening for Awareness and DEtection of Familial Hypercholesterolemia registry. Am Heart J. 2014;167(3):342–349.e17.
Daugherty SL, et al. Implicit gender bias and the use of cardiovascular tests among cardiologists. J Am Heart Assoc. 2017;6(12):e006872.
Ahmad ZS, Andersen RL, Andersen LH, O’Brien EC, Kindt I, Shrader P, et al. US physician practices for diagnosing familial hypercholesterolemia: data from the CASCADE-FH registry. J Clin Lipidol. 2016;10(5):1223–9.
•• Singh, A., et al., Familial hypercholesterolemia among young adults with myocardial infarction. J Am Coll Cardiol, 2019. 73(19): p. 2439–2450. Approximately 10% of patients younger than 50 years old with a history of myocardial infarctions had familial hypercholesterolemia, stressing the importance of FH screening in young adults with ischemic heart disease.
Al-Rasadi K, et al. The gulf familial hypercholesterolemia registry (gulf FH): design, rationale and preliminary results. Curr Vasc Pharmacol. 2020;18(1):57–64.
Beliard S, et al. High burden of recurrent cardiovascular events in heterozygous familial hypercholesterolemia: the French Familial Hypercholesterolemia Registry. Atherosclerosis. 2018;277:334–40.
Rizos CV, Elisaf MS, Skoumas I, Tziomalos K, Kotsis V, Rallidis L, et al. Characteristics and management of 1093 patients with clinical diagnosis of familial hypercholesterolemia in Greece: data from the Hellenic Familial Hypercholesterolemia Registry (HELLAS-FH). Atherosclerosis. 2018;277:308–13.
Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331–40.
•• Ray KK, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022–32 Inclisiran, an siRNA molecule that targets PCSK-9, given at one or two doses, was associated with a 20% reduction of LDL at 1-year follow-up.
• Bhatt DL, et al. REDUCE-IT USA. Circulation. 2020;141(5):367–75 Trial studying the use of icosapent ethyl (icosapent ethyl provided a 30% relative risk reduction and a 2.6% absolute risk reduction in all-cause mortality (P = .004)).
•• Duell PB, et al. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: the CASCADE FH registry. Atherosclerosis. 2019;289:85–93 Women with FH were less likely to achieve LDL goals than men.
Wong B, Kruse G, Kutikova L, Ray KK, Mata P, Bruckert E. Cardiovascular disease risk associated with familial hypercholesterolemia: a systematic review of the literature. Clin Ther. 2016;38(7):1696–709.
FH diagnosis by FH foundation. April 17, 2020]; Available from: https://apps.apple.com/us/app/fh-diagnosis/id543676258.
Mihalopoulos NL, Stipelman C, Hemond J, Brown LL, Young PC. Universal lipid screening in 9- to 11-year-olds before and after 2011 guidelines. Acad Pediatr. 2018;18(2):196–9.
• de Ferranti SD, et al. Cholesterol screening and treatment practices and preferences: a survey of United States pediatricians. J Pediatr. 2017;185:99–105.e2 Lipid screening practices among the pediatric population are not ideal.
Knowles JW, Rader DJ, Khoury MJ. Cascade screening for familial hypercholesterolemia and the use of genetic testing. JAMA. 2017;318(4):381–2.
• Wald DS, et al. Child–parent familial hypercholesterolemia screening in primary care. N Engl J Med. 2016;375(17):1628–37 Routine testing of pediatric patients for hypercholesterolemia was shown to be an effective way of identifying children with familial hypercholesterolemia.
Keenan KF, Finnie RM, Simpson WG, McKee L, Dean J, Miedzybrodzka Z. Parents’ views of genetic testing and treatment of familial hypercholesterolemia in children: a qualitative study. J Community Genet. 2019;10(1):129–41.
Sturm AC, Knowles JW, Gidding SS, Ahmad ZS, Ahmed CD, Ballantyne CM, et al. Clinical genetic testing for familial hypercholesterolemia: JACC scientific expert panel. J Am Coll Cardiol. 2018;72(6):662–80.
Gautschi M, Pavlovic M, Nuoffer J-M. Fatal myocardial infarction at 4.5 years in a case of homozygous familial hypercholesterolaemia. JIMD Rep. 2012;2:45–50.
Ekici F, Özçobanoğlu S, Kardelen F. Premature coronary artery disease due to homozygous familial hypercholesterolemia in a 12-year-old girl. Balkan Med J. 2018;35(2):208–11.
Kumar AA, Shantha G, Srinivasan Y, Senthil N, Rajkumar K, Paunikar N, et al. Acute myocardial infarction in an 18 year old South Indian girl with familial hypercholesterolemia: a case report. Cases J. 2008;1(1):71.
Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36(36):2425–37.
Holven KB, et al. Sex differences in cholesterol levels from birth to 19 years of age may lead to increased cholesterol burden in females with FH. J Clin Lipidol. 2018;12(3):748–755.e2.
• Kusters DM, et al. Ten-year follow-up after initiation of statin therapy in children with familial hypercholesterolemia. JAMA. 2014;312(10):1055–7 Ten-year follow-up to a randomized controlled study showed that long-term pravastatin use was safe and effective in preventing worsening of carotid intima-media thickness in children with FH.
https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/appendixa_tables.html. Accessed 7 May 2020.
Toleikyte I, Retterstøl K, Leren TP, Iversen PO. Pregnancy outcomes in familial hypercholesterolemia. Circulation. 2011;124(15):1606–14.
Pugh SJ, Schisterman EF, Browne RW, Lynch AM, Mumford SL, Perkins NJ, et al. Preconception maternal lipoprotein levels in relation to fecundability. Hum Reprod. 2017;32(5):1055–63.
Wang S, Wang J, Jiang Y, Jiang W. Association between blood lipid level and embryo quality during in vitro fertilization. Medicine. 2020;99(13):e19665.
Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354(9186):1234–41.
Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):S1–8.
Russi G. Severe dyslipidemia in pregnancy: the role of therapeutic apheresis. Transfus Apher Sci. 2015;53(3):283–7.
Amundsen AL, Khoury J, Iversen PO, Bergei C, Ose L, Tonstad S, et al. Marked changes in plasma lipids and lipoproteins during pregnancy in women with familial hypercholesterolemia. Atherosclerosis. 2006;189(2):451–7.
• Smith CJ, et al. Maternal dyslipidemia and risk for preterm birth. PLoS One. 2018;13(12):e0209579 This study details the risks of maternal lipid profiles on infants.
Karalis DG, Hill AN, Clifton S, Wild RA. The risks of statin use in pregnancy: a systematic review. J Clin Lipidol. 2016;10(5):1081–90.
Jeejeebhoy FM, Zelop CM, Lipman S, Carvalho B, Joglar J, Mhyre JM, et al. Cardiac arrest in pregnancy. Circulation. 2015;132(18):1747–73.
Schutte AE, Symington EA, du Preez JL. Rosuvastatin is transferred into human breast milk: a case report. Am J Med. 2013;126(9):e7–8.
Product Information: LIPITOR(R) oral tablets, atorvastatin calcium oral tablets. Pfizer (Per FDA), New York, NY, Feb, 2012. Accessed on 14 Apr 2020.
Product Information: PRAVACHOL(R) oral tablets, pravastatin sodium oral tablets. Bristol-Myers Squibb Company (per FDA), Princeton, NJ, Jul, 2016. Accessed on 14 Apr 2020.
Product Information: LESCOL(R) oral capsules, fluvastatin sodium oral capsules. Novartis Pharmaceuticals Corporation, East Hanover, NY, Oct 1, 2006. Accessed on 14 Apr 2020.
Product Information: WELCHOL oral tablets, colesevelam hcl oral tablets. Daiichi Sankyo Inc, Parsippany, NJ, Jan 1, 2008. Accessed on 14 Apr 2020.
Product Information: Zetia(R), ezetimibe. Merck/Schering-Plough Pharmaceuticals, North Wales, PA, April, 2004. Accessed on 14 Apr 2020.
Patel G, King A, Dutta S, Korb S, Wade JR, Foulds P, et al. Evaluation of the effects of the weak CYP3A inhibitors atorvastatin and ethinyl estradiol/norgestimate on lomitapide pharmacokinetics in healthy subjects. J Clin Pharmacol. 2016;56(1):47–55.
Product Information: JUXTAPID(TM) oral capsules, lomitapide oral capsules. Aegerion Parmaceuticals (per manufacturer), Cambridge, MA, Dec, 2012. Accessed on 14 Apr 2020.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211616s000lbl.pdf. Accessed 9 May 2020.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202057s019lbl.pdf. Accessed 9 May 2020.
Winterfeld U, Allignol A, Panchaud A, Rothuizen LE, Merlob P, Cuppers-Maarschalkerweerd B, et al. Pregnancy outcome following maternal exposure to statins: a multicentre prospective study. Bjog. 2013;120(4):463–71.
Zarek J, Koren G. The fetal safety of statins: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2014;36(6):506–9.
Bateman BT, Hernandez-Diaz S, Fischer MA, Seely EW, Ecker JL, Franklin JM, et al. Statins and congenital malformations: cohort study. Bmj. 2015;350:h1035.
Botha TC, Pilcher GJ, Wolmarans K, Blom DJ, Raal FJ. Statins and other lipid-lowering therapy and pregnancy outcomes in homozygous familial hypercholesterolaemia: a retrospective review of 39 pregnancies. Atherosclerosis. 2018;277:502–7.
Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, de Bonis M, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–241.
Pieper PG. Use of medication for cardiovascular disease during pregnancy. Nat Rev Cardiol. 2015;12(12):718–29.
Evolocumab Pregnancy Exposure Registry. https://clinicaltrials.gov/ct2/show/NCT02957604. Accessed 17 Apr 2020.
Rutherford JD. Maternal heterozygous familial hypercholesterolemia and its consequences for mother and child. Circulation. 2011;124(15):1599–601.
Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol. 2014;171(3):309–25.
Santos RD, Gidding SS, Hegele RA, Cuchel MA, Barter PJ, Watts GF, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4(10):850–61.
Stefanutti C, et al. Toward an international consensus-integrating lipoprotein apheresis and new lipid-lowering drugs. J Clin Lipidol. 2017;11(4):858–871.e3.
Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ (Clinical research ed.), 1991. 303(6807):893–896.
France M, Rees A, Datta D, Thompson G, Capps N, Ferns G, et al. HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis. 2016;255:128–39.
Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma S, et al. 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(14):1785–822.
Expert Panel on Integrated Guidelines for Cardiovascular, H, et al. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–56.
de Ferranti SD, Steinberger J, Ameduri R, Baker A, Gooding H, Kelly AS, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation. 2019;139(13):e603–34.
Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328–50.
•• Myers KD, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404 Analysis of healthcare claims from a private dataset showed that women, minorities, and individuals of lower socioeconomic status were less likely to receive approval for PCSK9i prescriptions, which was associated with a small increase in the risk of cardiovascular events.
Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203.
Laufs U, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662.
https://thefhfoundation.org/find-fh-specialist. Accessed on 17 Apr 2020.
Robinson JG, Williams KJ, Gidding S, Borén J, Tabas I, Fisher EA, et al. Eradicating the burden of atherosclerotic cardiovascular disease by lowering apolipoprotein B lipoproteins earlier in life. J Am Heart Assoc. 2018;7(20):e009778.
Funding
J. Knowles is funded by the National Institute of Health grants (NIH U41HG009649, NIDDK P30DK116074). F. Rodriguez was funded by a career development award from the National Heart, Lung, and Blood Institute (K01 HL 144607) and the American Heart Association/Robert Wood Johnson Harold Amos Medical Faculty Development Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Fatima Rodriguez reports personal fees from HealthPals, personal fees from Janssen, The Medicines Company and personal fees from NovoNordisk outside the submitted work. Sujana Balla, Eson P. Ekpo, Katherine Wilemon, and Joshua W. Knowles declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Women and Ischemic Heart Disease
Rights and permissions
About this article
Cite this article
Balla, S., Ekpo, E.P., Wilemon, K.A. et al. Women Living with Familial Hypercholesterolemia: Challenges and Considerations Surrounding Their Care. Curr Atheroscler Rep 22, 60 (2020). https://doi.org/10.1007/s11883-020-00881-5
Published:
DOI: https://doi.org/10.1007/s11883-020-00881-5