Abstract
Purpose of Review
This paper explores how the Environmental Exposure Unit (EEU) experimental model can be used to further our understanding of pharmacotherapies and immunotherapies for the treatment of allergic rhinitis (AR).
Recent Findings
EEUs are used increasingly for the study of combination therapies, immunotherapies, and novel AR treatments. A combined antihistamine/corticosteroid nasal spray formulation was seen to have a faster onset of action relative to the therapies individually in the Environmental Exposure Chamber. House dust mite sublingual immunotherapy tablets are both safe and efficacious as evaluated by the Vienna Challenge Chamber. The Kingston EEU found that a novel peptide-based immunotherapy approach to be effective in reducing grass pollen-induced AR. Lastly, nasal filters were determined to reduce seasonal AR symptoms, given out-of-season in the Denmark Environmental Exposure Unit.
Summary
EEUs are controlled, replicable models that provide valuable insight into the efficacy, onset and duration of action, and dose-related impacts of AR therapeutics, with direct clinical relevance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allergic rhinitis (AR) is an inflammatory disease of the nasal mucosa triggered by allergen exposure in sensitized patients. It is the most common form of chronic rhinitis and manifests clinically as rhinorrhea (runny nose), sneezing, nasal congestion, nasal itch, and is often accompanied by conjunctivitis with symptom of itchy, watery, and red/burning eyes [1]. While not a life-threatening condition, AR imposes a significant socioeconomic burden on patients and their families, impacting sleep, productivity, and overall quality of life [2, 3]. AR has been associated with comorbid conditions, including asthma, sleep apnea, and atopic dermatitis [2] and carries a global incidence of ~ 15–25% [4•]. The pharmacologic management of AR includes antihistamines, intranasal corticosteroids, antileukotrienes, and allergen immunotherapy [5]. The utility of Environmental Exposure Units (EEUs) as a reliable clinical model for AR is threefold. In essence, EEUs can further the understanding of drug pharmacokinetics, the onset of action, and the efficacy of established and proposed therapies. This review aims to assess recent advancements in AR drug therapeutics using the EEU model.
Historical and Technical Overview of EEU Models
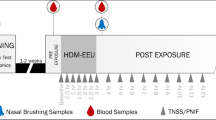
EEUs are among a collection of experimental models for the study of AR, including but not limited to phase 3 efficacy outpatient studies and outdoor park studies. A standard phase 3 efficacy study makes use of the natural environment for allergen exposure and is typically used to evaluate the efficacy of AR medications [6]. They rely on patient compliance with the treatment regimen and for daily recording of symptoms. This model allows the effect of medications to be observed in large numbers of participants for an extended timespan. However, there are many disadvantages to this model, including weather conditions that affect local pollen counts, varying pollen exposure between participants due to lifestyle differences, and reliability of participant-reported data [7]. Similarly, these studies typically have to be multicentre to achieve the proper statistical power [8]. Park studies make use of the outdoor environment and natural pollen seasons to expose participants. While this model ensures that the level of pollen exposure is similar among participants, that the treatment drug is taken appropriately, and that participants effectively record their symptoms, there remain variables that are unable to be controlled. These include weather conditions, pollen season timings, and concurrent exposure to other allergens, which may skew the results. The EEU model overcomes the challenges faced with both phase 3 efficacy studies and park studies because these custom-designed facilities allow researchers to control temperature, allergen concentration, humidity, and air quality. They are validated to ensure efficacy and consistency and are proven specific and reproducible models for investigating AR therapies [9••].
The Vienna Challenge Chamber (VCC; Vienna, Austria) is the longest-standing controlled allergen challenge facility, first described in 1987 [10]. It features Burkard pollen traps to measure allergen concentrations, with ceiling vents that circulate the air [8]. Soon after, the Environmental Exposure Unit (EEU; Kingston, Canada) was initially developed to assess the biological effects of formaldehyde foam insulation exposure and was then converted to study AR in up to 140 participants simultaneously [11]. Through a highly efficient ventilation system, fresh outdoor air is filtered and circulated through the Unit. A custom-engineered laser-aided system is responsible for the release of allergen to a single point of delivery, while directional fans propel and evenly distribute it throughout the EEU [7]. Seven Rotorod impact samplers and microscopy are used to continually verify room allergen concentration during challenge sessions. Other facilities located in North America include the Biogenics Research Chamber (San Antonio, TX), the Environmental Exposure Chamber (EEC; Mississauga, Canada), the Allergen Challenge Theatre™ (Ottawa, Canada), and the BioCube (Andover, MA) [12,13,14,15,16]. Denmark and Germany are home to an Environmental Exposure Unit (EEU; Denmark) and the Fraunhofer Environmental Challenge Chamber (ECC; Hannover, Germany), respectively [8, 17]. The latter features filtered air and a pollen feeding system, with concentrations measured by a laser particle counter and Rotorod samplers [17]. Japan is home to the Environmental Challenge Chamber (ECC; Chiba, Japan), built in 2008, as well as the Osaka allergen challenge chamber (Osaka, Japan) [18, 19].
Antihistamines
Antihistamines (AHs) are the first-line therapy for mild-moderate AR and provide relief by inversely agonizing the H1-receptor, meaning they suppress its activity [20]. AHs fall under two broad classes: first generation and newer generation. Newer-generation AHs are safe and effective, without the anticholinergic and sedative effects presented by first-generation AHs [21••, 22•]. The Kingston EEU investigated the efficacy of loratadine, astemizole, terfenadine, and cetirizine, a set of newer-generation AHs, in relieving AR symptoms [23]. It was found that terfenadine and cetirizine ranked higher than astemizole and loratadine in both efficacy and onset of action. Day et al. further investigated the effect of cetirizine, loratadine, and placebo in a larger sample size, following ragweed pollen exposure in the Kingston EEU [24]. Again, cetirizine had the more rapid onset of action of 1 h, whereas loratadine was 3 h. The same group replicated the study few years after and yielded the same concluding results, demonstrating the highly reproducible nature of the Kingston EEU [25].
Levocetirizine is an R-enantiomer of the racemic cetirizine. This drug has demonstrated its antihistaminergic and low sedative effects in many studies [26•, 27]. The effect of levocetirizine in comparison with loratadine was assessed in both seasonal and perennial AR patients in the VCC following exposure to either grass pollen or house dust mite, respectively [28]. Stübner et al. found that both medications were more efficacious than placebo; however, levocetirizine had a more rapid onset of action. In comparison with desloratadine, the active and potent metabolite found in loratadine, Day et al. found that levocetirizine had an earlier onset of action and greater symptom improvement [29, 30]. In the VCC, levocetirizine has been seen to have a longer duration of action than fexofenadine [31].
Fexofenadine is an active metabolite in terfenadine and is more effective at controlling rhinorrhea and sneezing than the latter [32]. It has been evaluated in the Kingston EEU for efficacy, onset, and duration of action, and has been compared with well-established AHs, such as cetirizine. Fexofenadine was efficacious and safe for use at multiple doses (60 and 120 mg) with an average onset of 60 min, though it was less effective than cetirizine at reducing seasonal AR symptoms [33,32,35]. Investigations in the VCC confirmed such findings, reporting that both bilastine and cetirizine had longer durations of action than fexofenadine [36]. The fact that unrelated EEUs, each with their own unique ventilation and allergen distribution systems, present similar findings when investigating the same medications attests to the reliability of these facilities and the validity of the results.
Antihistamines can be administered as oral or intranasal medication. The efficacy and onset of action of an azelastine nasal spray were evaluated in the Kingston EEU compared with oral doses of loratadine and cetirizine against seasonal AR [37]. While all three drugs were associated with nasal symptom reduction, the effect of intranasal azelastine was noticed as early as 15 min, compared with at least 60–75 min post-dose for cetirizine and loratadine. Further analysis showed that loratadine tablets had an onset of action of 75 min for nasal and ocular symptomatic control [38••]. These findings also support previously established findings by the VCC that the azelastine nasal spray was more efficacious than desloratadine tablets in seasonal AR [39]. Olopatadine, another intranasal AH, was assessed for efficacy in seasonal AR in the EEC and was found to produce clinically relevant and statistically significant symptom improvement compared with placebo [40].
Preventative measures, such as prophylactic treatments, have also been studied in EEUs. The Chiba EEC evaluated the efficacy of an 8-day administration of levocetirizine (prophylactically), a single dose on day 8 or an 8-day placebo followed by allergen exposure on day 9 [41]. The prophylactically administered levocetirizine showed no superior efficacy to the single dose. In the VCC, patients given a daily 10-mg dose of rupatadine, an AH that is also a platelet-activating factor antagonist, experienced less severe symptoms than placebo [42]. These studies reveal insights into the nature of AHs, that a prophylactic regimen may confer some protective effects. While further research is required, such findings from EEU studies may direct clinical practice.
Antileukotrienes
Antileukotrienes (leukotriene receptor antagonists; LTRAs) antagonize cysteinyl leukotriene-1 receptors to inhibit the activity of cysteinyl leukotrienes, which mediate inflammation in AR [43]. The efficacy of montelukast, an LTRA, was assessed with levocetirizine, a newer-generation AH, in the Kingston EEU [44]. Patel et al. found that while both drugs showed significant symptomatic improvements compared with placebo, levocetirizine provided longer-lasting relief with higher patient satisfaction. A similar study in the EEC also found that the 5-mg dose of levocetirizine was more efficacious than a 10-mg dose of montelukast [45]. EEUs prove to be a useful model to compare and evaluate the efficacy and clinical relevance of pharmacologic agents that act on independent immunologic pathways.
Nasal Corticosteroids
In addition to antihistamines, intranasal corticosteroids (INCS) are also considered first-line therapy for AR, classed as the best option for both children and adults [46, 47••]. The first evaluation of an INCS in the Kingston EEU provided revolutionary results. It was formerly believed that the onset of action for INCS drugs was 3–7 days, though the investigation by Day et al. demonstrated that within 10-h post-administration, triamcinolone acetonide produced a marked reduction in nasal congestion [48, 49]. The Kingston EEU also tested intranasal budesonide and found the onset of action to be 7 h [50]. The same group also evaluated the onset of action of a ciclesonide nasal spray in the treatment of seasonal AR, though found no clinically significant differences in TNSS between placebo during the 12-h study period, besides at hour 6 [51]. The EEC further assessed ciclesonide and determined it to have a 6-h onset [52].
A novel nasal spray formulation, S0597, was studied for its efficacy and safety, in the Fraunhofer Environmental Challenge Chamber, compared with placebo [53]. Participants were given one of three twice-daily doses (200 μg/day, 400 μg/day, or 800 μg/day) for a 15-day evaluation period. All three treatment conditions produced significant symptom improvement compared with placebo, with the 800 μg/day resulting in the greatest improvement. The Kingston EEU performed a similar study with lower doses (50 μg, 200 μg, or 400 μg) and found, again, that all drug concentrations were well-tolerated and resulted in significantly reduced AR symptoms for participants following ragweed pollen exposure [54].
The efficacy of INCSs has also been evaluated in comparison with AHs. With an 8-h allergen exposure period in the EEC, azelastine nasal spray ranked superior, in terms of faster onset of action and symptom reduction, to both placebo and mometasone post-allergen exposure [55]. Olopatadine also mirrored the previous findings, being superior in total nasal symptom score (TNSS) reduction and a more rapid onset of action than both placebo and mometasone [56]. Despite AHs displaying more significant results in these studies, it is important to take into consideration that INCSs have longer onsets of action than AHs, hence would display symptom-modifying effects at a later timepoint. INCS have been determined to be more effective than AHs and have a greater prophylactic benefit [57]. The Chiba EEC evaluated the 7-day prophylactic daily administration of mometasone in comparison with fexofenadine before allergen challenge on day 8 [58]. Allergen-induced symptoms persisted for up to 3 days, during which, the mometasone group experienced significantly reduced TNSS. This suggests that the prophylactic use of INCSs may have a longer-lasting effect that is not present with AHs. Fluticasone furoate, another intranasal glucocorticoid, was studied in the VCC. With TNSS as the primary endpoint, a daily 8-day course of fluticasone furoate (200 mcg) was found to be more effective in comparison with placebo [59]. Likewise, mometasone provided significant symptomatic relief when a daily dose (200 mcg) was taken for 8 days, with a 6-h onset of action and > 24-h duration of action [60].
Combination Therapies
The drugs previously discussed are well-established and have proven efficacy against AR symptoms. Kingston EEU studies have shown that when used in combination, the benefits can be more significant. The Kingston EEU evaluated the onset of action for an AH/LTRA combination (loratadine/montelukast) and found that it reduced total symptoms and nasal congestion after 75 min post-dose, with limited adverse events [61]. Interestingly, a similar investigation in the VCC noted the same improvement in nasal symptoms compared with placebo except the onset of action was determined to be 105 min [62].
Despite the effectiveness of AHs in regulating AR symptoms as previously discussed, they provide minimal relief for nasal congestion. Sympathomimetic drugs, such as pseudoephedrine, promote vasoconstriction in the nasal mucosa, resulting in decongestion [63]. A cetirizine/pseudoephedrine combination therapy was determined by the Fraunhofer Institute to be more efficacious than when the drugs were used individually [64]. The VCC investigated the safety and efficacy of an oral cetirizine/pseudoephedrine formulation compared with a budesonide nasal spray [65], with reduction of nasal congestion being the primary endpoint. It was found that cetirizine/pseudoephedrine provided more immediate symptom relief following allergen exposure, though budesonide was more effective long-term than the former. Pseudoephedrine was also evaluated in the VCC in relation to phenylephrine, another sympathomimetic agent, and was found to provide superior nasal symptom relief [66]. Subsequently, the Kingston EEU investigated the efficacy of phenylephrine in comparison with loratadine-montelukast in relieving nasal congestion [67]. Berkowitz et al. found that the AH/LTRA combination was more effective and resulted in fewer adverse events than both the phenylephrine and placebo experimental groups. The combination of fexofenadine/pseudoephedrine was evaluated in (the now decommissioned) Atlanta allergy exposure unit and determined to be safe and efficacious (compared with placebo), with a 45-min onset of action [68].
While efficacious, agents that combine antihistamines and sympathomimetics come with various adverse events, including headaches and insomnia [69]. Typical AHs target the H1-receptor. It is thought that antagonists of H3-receptors, rather than H1-receptors, may present a better safety profile with similar decongestion activities. The efficacy of combined H1/H3 receptor antagonists was well-tolerated with either oral or intranasal administrations; however, only the intranasal formula significantly reduced nasal symptoms after a continuous 3-day schedule [70]. The Kingston EEU was used to evaluate the effect of an H1/H3 combination (fexofenadine/ PF-03654764) compared with a combined H1/sympathomimetic (fexofenadine/pseudoephedrine) drug [71••]. While the dual antihistamine formulation improved symptoms compared with placebo, it was not superior to the H1/sympathomimetic combination, and rather resulted in more adverse events. Although a potential line of treatment, EEU studies have been important in evaluating the safety profile of H3-receptor antagonists.
Intranasal antihistamines have also been combined with intranasal corticosteroids in hopes of combining their therapeutic profiles. A formulation of azelastine and fluticasone propionate in a single nasal spray (MP-AzeFlu) was determined to produce superior effects than either of the pharmacotherapies individually [72••]. The EEC determined its onset of action to be 5 min whereas sequential monotherapies of an AH and INCS took 150 min. Moreover, MP-AzeFlu was more efficacious [73]. A different solubilized INAH/INCS combination nasal spray (azelastine + budesonide) was also evaluated by the EEC in comparison with solubilized budesonide alone and respective suspension-type comparators [74]. The combination therapy was more efficacious, with a longer-lasting effect, than the other products. Interestingly, the solubilized formulation allowed for a more rapid onset of action and greater duration of action.
Immunotherapy
The EEU model is also conducive for the study of allergen specific immunotherapies (AITs). AIT is a disease-modifying treatment method that aims to grant patients long-term relief from their AR symptoms [75]. It is often prescribed when pharmacotherapies insufficiently address patients’ AR symptoms. The conventional method of administering AIT is subcutaneously (SCIT) by injection. The Kingston EEU evaluated the efficacy of a 2-year SCIT treatment in ragweed-allergic participants in comparison with allergic participants not receiving AIT and healthy controls [76•]. After a 3-h allergen exposure session, Donovan et al. observed that SCIT recipients experienced significantly reduced symptoms compared with the positive controls [76•].
As a newer AIT approach, sublingual immunotherapy (SLIT) has gained traction as it is safer and more convenient than SCIT. A series of house dust mite (HDM) SLIT investigations have been undertaken recently using the EEU model. The safety and efficacy of SLIT tablets using HDM extracts were studied in the EEC. The participants took one of three daily doses (500IR, 300IR, or 100IR) of HDM-SLIT tablet or placebo for 6 months. All three treatment doses were found to be well-tolerated, with on average, 70% of participants receiving AIT reporting reactions at the site of application [77]. A dose-response relationship was observed, with the 500IR SLIT tablet resulting in the greatest symptom reduction [78]. The VCC was used to explore the efficacy and onset of action of two doses (6 developmental unit (DU) and 12 DU) of MK-8237, an HDM SLIT tablet, taken by HDM-allergic participants for 24 weeks [79]. While both doses were well-tolerated with no serious adverse events reported, the 12 DU HDM SLIT tablet had greater efficacy than both the 6 DU dose and placebo, with an onset of action of 8 weeks.
SLIT treatments for pollen-induced AR have also been explored. The VCC evaluated the efficacy and onset of action of a 5-grass-pollen tablet [80]. Repeated grass pollen allergen challenges revealed that the SLIT tablet provided allergic participants with significant symptom relief as from the first month of treatment, with a sustained effect for up to 4 months. Finally, the Kingston EEU evaluated whether a Timothy grass SLIT tablet approved for grass pollen-induced AR would have an effect on birch pollen-induced AR symptoms [81]. While the treatment was well-tolerated using a once daily dosage for 4 months, symptom occurrences with the SLIT group were comparable with placebo. Given these results, it is likely that AIT treatments provide allergen-specific benefits. Clearly, EEUs provide clinically relevant insights into the efficacy, onset of action, and dose effects of immunotherapy treatments for AR.
Peptide-based immunotherapy (PIT) is a novel AIT approach though not commercially available. A study in the Kingston EEU evaluated the efficacy of a grass allergen PIT comprised of 7 T cell epitopes [82]. The participants received either eight 6 nmol biweekly doses (8x6Q2W), four 12 nmol doses every 4 weeks (4x12Q4W), or eight 12 nmol biweekly doses (8x12Q2W). The 8x6Q2W group experienced significantly reduced TNSS compared with the placebo. The EEC investigated an ultrashort course (4 injections given pre-seasonally) PIT regimen featuring ragweed pollen extract [83••]. Compared with placebo, the treated group experienced significantly decreased TNSS, demonstrating the efficacy of pre-seasonal AIT for seasonal AR. A similar study used a peptide antigen treatment against cat allergy in the EEC [84]. A PIT schedule of four 6 nmol weekly doses was more efficacious than eight 3 nmol biweekly doses, and the treatment effects persisted for up to 1 year since the initiation of treatment.
Novel Therapies
EEUs can be used to explore novel experimental therapies. The VCC evaluated the efficacy of OC000459, an antagonist to chemoattractant receptor-homologous molecules (CRTH2) which had previously shown promise for allergic disease [85, 86]. Compared with placebo, participants given the antagonist experienced reduced nasal symptoms. A follow-up study was completed by the Fraunhofer ECC, comparing three twice-daily doses (50 mg, 200 mg, and 400 mg) of the oral CRTH2 antagonist with a once-daily 200 μg fluticasone propionate nasal spray and once-daily 10 mg oral montelukast [86]. Following a 6-h out-of-season ECC pollen exposure, the 200-mg dose of the CRTH2 antagonist was seen to be more efficacious at reducing seasonal AR symptoms.
Another alternative to conventional AR treatment involves nasal filters. The Denmark EEU conducted a study of the efficacy of Rhinix™ nasal filters in seasonal AR [87]. They reduced daily sneezing and daily TNSS, though the maximum TNSS values were not significantly greater than those experienced by placebo. They were nonetheless well-tolerated by the participants and resulted in no adverse events.
Conclusions
The EEU is a reproducible and unique model for the study of AR therapies. Unlike phase 3 efficacy trials and park studies which rely on the natural environment, EEUs allow for the control of study variables, such as humidity, temperature, and allergen distribution. AR symptoms generated in EEUs are comparable with natural pollen seasons which allows for allergen-specific research to continue even outside of the natural season. EEU studies have made important strides in our understanding of the efficacy, onset and duration of action, and pharmacodynamic properties of anti-allergic therapies, including antihistamines, nasal corticosteroids, combination therapies, and allergen-specific immunotherapies. These models support the development and testing of new therapies and provide clinically relevant insights for clinicians in the treatment of allergic rhinitis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lakhani N, North M, Ellis A. Clinical manifestations of allergic rhinitis. J Allergy Ther. 2012;01. https://doi.org/10.4172/2155-6121.S5-007.
Keith PK, Desrosiers M, Laister T, Schellenberg RR, Waserman S. The burden of allergic rhinitis (AR) in Canada: perspectives of physicians and patients. Allergy, Asthma Clin Immunol. 2012;8. https://doi.org/10.1186/1710-1492-8-7.
Meltzer EO, Gross GN, Katial R, Storms WW. Allergic rhinitis substantially impacts patient quality of life: findings from the Nasal Allergy Survey Assessing Limitations. J Fam Pract. 2012;61.
• Passali D, Cingi C, Staffa P, Passali F, Muluk NB, Bellussi ML. The international study of the allergic rhinitis survey: outcomes from 4 geographical regions. Asia Pac Allergy. 2018;8. https://doi.org/10.5415/apallergy.2018.8.e7. This is a cross-sectional, a questionnaire survey concerning AR was completed across different countries in Asia, Europe, the Americas, and Africa. The prevalence of AR was reported to be 15%-25%.
Small P, Keith PK, Kim H. Allergic rhinitis. Allergy, Asthma Clin Immunol. 2018;14:51.
Day JH, Ellis AK, Rafeiro E, Ratz JD, Briscoe MP. Experimental models for the evaluation of treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2006;96:263–78.
Ellis AK, North ML, Walker T, Steacy LM. Environmental exposure unit: a sensitive, specific, and reproducible methodology for allergen challenge. Ann Allergy Asthma Immunol. 2013;111:323–8.
Day JH, Horak F, Briscoe MP, Canonica GW, Fineman SM, Krug N, et al. The role of allergen challenge chambers in the evaluation of anti-allergic medication: an international consensus paper. Clin Exp Allergy Rev. 2006;6:31–59.
•• Ellis AK, Jacobs RL, Tenn MW, et al (2019) Clinical standardization of two controlled allergen challenge facilities: the Environmental Exposure Unit and the Biogenics Research Chamber. Ann Allergy, Asthma Immunol 122:639-646.e2. This article demonstrates equivalent results between 2 CACFs, one located in Kingston, Canada and the other in San Antonio, USA in a double-blind,placebo-controlled, crossover intervention trial.
Horak, F. and SJ (1987) The Vienna challenge chamber (VCC)—a new method for allergen exposition tests. Wiener Klin Wochenschrift 99:509–510.
Pross HF, Day JH, Clark RH, Lees REM. Immunologic studies of subjects with asthma exposed to formaldehyde and urea-formaldehyde foam insulation (UFFI) off products. J Allergy Clin Immunol. 1987;79:797–810.
A pilot study evaluating the signs and symptoms of seasonal allergic rhinoconjunctivitis following exposure in the Allergen BioCube - Full Text View - ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/study/NCT00985075. Accessed 31 Mar 2020.
Ramirez DA, Jacobs RL, Andrews CP. Juniperus asheii (mountain cedar) pollen utilized as an antigen in the biogenics chamber: comparison of natural and controlled exposures. J Allergy Clin Immunol. 2011;127:AB19–9.
Jacobs RL, Ramirez DA, Andrews CP. Validation of the biogenics research chamber for Juniperus ashei (mountain cedar) pollen. Ann Allergy Asthma Immunol. 2011;107:133–8.
Rønborg SM, Mosbech H, Johnsen CR, Poulsen LK. Exposure chamber for allergen challenge the development and validation of a new concept. Allergy. 1996;51:82–8.
Kelly S, Yang J, Perrins R, Karsh J, Yang WH. Technical evaluation of an allergen Challenge Theatre™. Allergy, Asthma Clin Immunol. 2014;10:A22.
Krug N, Loedding H, Hohlfeld JM, Larbig M, Buckendahl A, Badorrek P, et al. Validation of an environmental exposure unit for controlled human inhalation studies with grass pollen in patients with seasonal allergic rhinitis. Clin Exp Allergy. 2003;33:1667–74.
Hamasaki S, Okamoto Y, Yonekura S, Okuma Y, Sakurai T, Iinuma T, et al. Characteristics of the Chiba environmental challenge chamber. Allergol Int. 2014;63:41–50.
Ito K, Terada T, Yuki A, Ichihara T, Hyo S, Kawata R, et al. Preliminary study of a challenge test to the patients with Japanese cedar pollinosis using an environmental exposure unit. Auris Nasus Larynx. 2010;37:694–9.
Baroody FM, Naclerio RM. Antiallergic effects of H1-receptor antagonists. Allergy Eur. J. Allergy Clin. Immunol. Suppl. 2000:17–27.
•• Fein MN, Fischer DA, O’Keefe AW, Sussman GL. CSACI position statement: newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria. Allergy, Asthma Clin Immunol. 2019;15:61. A CSACI position statement presenting that newer generation H1-antihistamines are safer than first-generation H1-antihistamines and should be the first-line antihistamines for the treatment of allergic rhinitis and urticaria.
• Kawauchi H, Yanai K, Wang DY, Itahashi K, Okubo K. Antihistamines for allergic rhinitis treatment from the viewpoint of nonsedative properties. Int J Mol Sci. 2019;20. https://doi.org/10.3390/ijms20010213. This article evaluates the non-sedative effects of antihistamines in AR treatment, recommending non-brain-penetrating antihistamines as first-line therapy of mild AR.
Day JH, Briscoe MP, Clark RH, Ellis AK, Gervais P. Onset of action and efficacy of terfenadine, astemizole, cetirizine, and loratadine for the relief of symptoms of allergic rhinitis. Ann Allergy Asthma Immunol. 1997;79:163–72.
Day JH, Briscoe M, Widlitz MD. Cetirizine, loratadine, or placebo in subjects with seasonal allergic rhinitis: effects after controlled ragweed pollen challenge in an environmental exposure unit. J Allergy Clin Immunol. 1998;101:638–45.
Day JH, Briscoe M, Rafeiro E, Chapman D, Kramer B. Comparative onset of action and symptom relief with cetirizine, loratadine, or placebo in an environmental exposure unit in subjects with seasonal allergic rhinitis: confirmation of a test system. Ann Allergy Asthma Immunol. 2001;87:474–81.
• Snidvongs K, Seresirikachorn K, Khattiyawittayakun L, Chitsuthipakorn W. Sedative effects of levocetirizine: a systematic review and meta-analysis of randomized controlled studies. Drugs. 2017;77:175–86. This study investigates the sedative effects of levocetirizine compared to placebo, demonstrating modest effects, though not significantly different fromother second-generation antihistamines.
Tillement J-P, Testa B, Brée F. Compared pharmacological characteristics in humans of racemic cetirizine and levocetirizine, two histamine H1-receptor antagonists. Biochem Pharmacol. 2003;66:1123–6.
Stübner P, Zieglmayer R, Horak F. A direct comparison of the efficacy of antihistamines in SAR and PAR: randomised, placebo-controlled studies with levocetirizine and loratadine using an environmental exposure unit - the Vienna Challenge Chamber (VCC). Curr Med Res Opin. 2004;20:891–902.
Geha RS, Meltzer EO. Desloratadine: a new, nonsedating, oral antihistamine. J Allergy Clin Immunol. 2001;107:751–62.
Day JH, Briscoe MP, Rafeiro E, Ratz JD. Comparative clinical efficacy, onset and duration of action of levocetirizine and desloratadine for symptoms of-seasonal allergic rhinitis in subjects evaluated in the Environmental Exposure Unit (EEU). Int J Clin Pract. 2004;58:109–18.
Horak F, Zieglmayer PU, Zieglmayer R, Kavina A, Lemell P. Levocetirizine has a longer duration of action on improving total nasal symptoms score than fexofenadine after single administration. Br J Clin Pharmacol. 2005;60:24–31.
Terrien MH, Rahm F, Fellrath JM, Spertini F. Comparison of the effects of terfenadine with fexofenadine on nasal provocation tests with allergen. J Allergy Clin Immunol. 1999;103:1025–30.
Day JH, Briscoe MP, Rafeiro E, Hewlett D, Chapman D, Kramer B. Randomized double-blind comparison of cetirizine and fexofenadine after pollen challenge in the Environmental Exposure Unit: duration of effect in subjects with seasonal allergic rhinitis. Allergy Asthma Proc. 2004;25:59–68.
Day JH, Briscoe MP, Rafeiro E, Ratz JD, Ellis AK, Frankish CW, et al. Comparative efficacy of cetirizine and fexofenadine for seasonal allergic rhinitis, 5-12 hours postdose, in the environmental exposure unit. Allergy Asthma Proc. 2005;26:275–82.
Day JH, Briscoe MP, Welsh A, Smith JN, Clark A, Ellis AK, et al. Onset of action, efficacy, and safety of a single dose of fexofenadine hydrochloride for ragweed allergy using an environmental exposure unit. Ann Allergy Asthma Immunol. 1997;79:533–40.
Horak F, Zieglmayer P, Zieglmayer R, Lemell P. The effects of bilastine compared with cetirizine, fexofenadine, and placebo on allergen-induced nasal and ocular symptoms in patients exposed to aeroallergen in the Vienna Challenge Chamber. Inflamm Res. 2010;59:391–8.
Ellis AK, Zhu Y, Steacy LM, Walker T, Day JH. A four-way, double-blind, randomized, placebo controlled study to determine the efficacy and speed of azelastine nasal spray, versus loratadine, and cetirizine in adult subjects with allergen-induced seasonal allergic rhinitis. Allergy, Asthma Clin Immunol. 2013;9. https://doi.org/10.1186/1710-1492-9-16.
•• Tenn MW, Steacy LM, Ng CC, Ellis AK. Onset of action for loratadine tablets for the symptomatic control of seasonal allergic rhinitis in adults challenged with ragweed pollen in the Environmental Exposure Unit: a post hoc analysis of total symptom score. Allergy, Asthma Clin Immunol. 2018;14:5. In this article, the onset of action of loratadine tablets was determined to be 75 mins was investigtaed in the Environmental Exposure Unit for seasonal AR.
Horak F, Zieglmayer UP, Zieglmayer R, Kavina A, Marschall K, Munzel U, et al. Azelastine nasal spray and desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opin. 2006;22:151–7.
Patel P, Roland PS, Marple BF, Benninger PJ, Margalias H, Brubaker M, et al. An assessment of the onset and duration of action of olopatadine nasal spray. Otolaryngol Head Neck Surg. 2007;137:918–24.
Yonekura S, Okamoto Y, Yamamoto H, Sakurai T, Iinuma T, Sakurai D, et al. Randomized double-blind study of prophylactic treatment with an antihistamine for seasonal allergic rhinitis. Int Arch Allergy Immunol. 2013;162:71–8.
Stuebner P, Horak F, Zieglmayer R, Arnáiz E, Leuratti C, Pérez I, et al. Effects of rupatadine vs placebo on allergen-induced symptoms in patients exposed to aeroallergens in the Vienna Challenge Chamber. Ann Allergy Asthma Immunol. 2006;96:37–44.
Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36:689–703.
Day JH, Briscoe MP, Ratz JD. Efficacy of levocetirizine compared with montelukast in subjects with ragweed-induced seasonal allergic rhinitis in the environmental exposure unit. Allergy Asthma Proc. 2008;29:304–12.
Patel P, Patel D. Efficacy comparison of levocetirizine vs montelukast in ragweed sensitized patients. Ann Allergy Asthma Immunol. 2008;101:287–94.
Trangsrud AJ, Whitaker AL, Small RE. Intranasal corticosteroids for allergic rhinitis. Pharmacotherapy. 2002;22:1458–67.
•• Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J Allergy Clin Immunol. 2017;140:950–8. The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines present recommendations to support patients, their caregivers, and health care providers in choosing the optimal AR treatment, which might improve patients' quality of life and school and work productivity.
Mabry RL. Corticosteroids in the management of upper respiratory allergy: the emerging role of steroid nasal sprays. Otolaryngol Head Neck Surg. 1992;107:855–9 discussion 859-60.
Day JH, Briscoe MP, Rafeiro E, Ellis AK, Pettersson E, Åkerlund A. Onset of action of intranasal budesonide (Rhinocort aqua) in seasonal allergic rhinitis studied in a controlled exposure model. J Allergy Clin Immunol. 2000;105:489–94.
Day JH, Buckeridge DL, Clark RH, Briscoe MP, Phillips R. A randomized, double-blind, placebo-controlled, controlled antigen delivery study of the onset of action of aerosolized triamcinolone acetonide nasal spray in subjects with ragweed-induced allergic rhinitis. J Allergy Clin Immunol. 1996;97:1050–7.
Study using the Environmental Exposure Unit (EEU) to assess the onset of action of ciclesonide, applied as a nasal spray in the treatment of seasonal allergic rhinitis (BY9010/M1-407) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00659503?term=BY9010%2FM1&cond=Seasonal+Allergic+Rhinitis&draw=2&rank=5. Accessed 7 Apr 2020.
A study of ciclesonide nasal spray in patients 18 years and older with seasonal allergic rhinitis (BY9010/M1-413) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00384475?term=M1&cond=allergic+rhinitis&draw=2&rank=10. Accessed 15 Apr 2020.
Badorrek P, Hohlfeld JM, Krug N, Joshi A, Raut A. Efficacy and safety of a novel nasal steroid, S0597, in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2015;115:325–329.e1.
Ellis AK, Steacy LM, Joshi A, Bhowmik S, Raut A. Efficacy of the novel nasal steroid S0597 tested in an environmental exposure unit. Ann Allergy Asthma Immunol. 2016;117:310–7.
Patel P, D’Andrea C, Sacks HJ. Onset of action of azelastine nasal spray compared with mometasone nasal spray and placebo in subjects with seasonal allergic rhinitis evaluated in an environmental exposure chamber. Am J Rhinol. 2007;21:499–503.
Patel D, Garadi R, Brubaker M, Conroy PJ, Kaji Y, Crenshaw K, et al. Onset and duration of action of nasal sprays in seasonal allergic rhinitis patients: Olopatadine hydrochloride versus mometasone furoate monohydrate. Allergy Asthma Proc. 2007;28:592–9.
Van Cauwenberge P, Van Hcecke H, Bousquet J (2005) Allergic rhinitis and its impact on asthma. In: Pediatr. Nasal Sinus Disord. CRC Press, pp 402–422.
Yamamoto H, Yonekura S, Sakurai D, Katada K, Inamine A, Hanazawa T, et al. Comparison of nasal steroid with antihistamine in prophylactic treatment against pollinosis using an environmental challenge chamber. Allergy Asthma Proc. 2012;33:397–403.
Zieglmayer P, Zieglmayer R, Bareille P, Rousell V, Salmon E, Horak F. Fluticasone furoate versus placebo in symptoms of grass-pollen allergic rhinitis induced by exposure in the Vienna Challenge Chamber. Curr Med Res Opin. 2008;24:1833–40.
Salapatek AM, Patel P, Gopalan G, Varghese ST. Mometasone furoate nasal spray provides early, continuing relief of nasal congestion and improves nasal patency in allergic patients. Am J Rhinol Allergy. 2010;24:433–8.
Day JH, Briscoe MP, Ratz JD, Ellis AK, Yao R, Danzig M. Onset of action of loratadine/montelukast in seasonal allergic rhinitis subjects exposed to ragweed pollen in the Environmental Exposure Unit. Allergy Asthma Proc. 2009;30:270–6.
Horak F, Zieglmayer P, Zieglmayer R, Lemell P. Onset of action of loratadine/montelukast in seasonal allergic rhinitis patients exposed to grass pollen. Arzneimittel-Forschung/Drug Res. 2010;60:249–55.
Johnson DA, Hricik JG. The pharmacology of α-adrenergic decongestants. Pharmacother J Hum Pharmacol Drug Ther. 1993;13:110S–5S.
Badorrek P, Dick M, Schauerte A, Hecker H, Murdoch R, Luettig B, et al. A combination of cetirizine and pseudoephedrine has therapeutic benefits when compared to single drug treatment in allergic rhinitis. Int J Clin Pharmacol Ther. 2009;47:71–7.
Zieglmayer UP, Horak F, Toth J, Marks B, Berger UE, Burtin B. Efficacy and safety of an oral formulation of cetirizine and prolonged-release pseudoephedrine versus budesonide nasal spray in the management of nasal congestion in allergic rhinitis. Treat Respir Med. 2005;4:283–7.
Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Yao R, Staudinger H, et al. A placebo-controlled study of the nasal decongestant effect of phenylephrine and pseudoephedrine in the Vienna Challenge Chamber. Ann Allergy Asthma Immunol. 2009;102:116–20.
Day JH, Briscoe MP, Ratz JD, Danzig M, Yao R. Efficacy of loratadine-montelukast on nasal congestion in patients with seasonal allergic rhinitis in an environmental exposure unit. Ann Allergy Asthma Immunol. 2009;102:328–38.
Berkowitz RB, Woodworth GG, Lutz C, Weiler K, Weiler J, Moss M, et al. Onset of action, efficacy, and safety of fexofenadine 60 mg/pseudoephedrine 120 mg versus placebo in the Atlanta allergen exposure unit. Ann Allergy Asthma Immunol. 2002;89:38–45.
Chervinsky P, Nayak A, Rooklin A, Danzig M. Efficacy and safety of desloratadine/pseudoephedrine tablet, 2.5/120 mg two times a day, versus individual components in the treatment of patients with seasonal allergic rhinitis. Allergy asthma Proc. 2005;26:391–6.
Daley-Yates P, Ambery C, Sweeney L, Watson J, Oliver A, McQuade B. The efficacy and tolerability of two novel H 1/H 3 receptor antagonists in seasonal allergic rhinitis. Int Arch Allergy Immunol. 2012;158:84–98.
•• North ML, Walker T, Steacy LM, Hobsbawn BG, Allan RJ, Hackman F, et al. Double blind randomized crossover trial of PF-03654764+fexofenadine in the environmental exposure unit (EEU). Allergy, Asthma Clin Immunol. 2014;10:A68. This double blind randomized crossover trial of PF-03654764+fexofenadine in the Environmental Exposure Unit found improved TNSS compared toplacebo, with insignificant side effects.
•• Roca-Ferrer J, Pujols L, Pérez-González M, Alobid I, Callejas B, Vicens-Artés S, et al. Superior effect of MP-AzeFlu than azelastine or fluticasone propionate alone on reducing inflammatory markers. Allergy, Asthma Clin Immunol. 2018;14:86. This study found superior clinical efficacy with MP-AzeFlu than azelastine or fluticasone propionate alone in reducing inflammatory markers.
Bousquet J, Meltzer EO, Couroux P, Koltun A, Kopietz F, Munzel U, et al. Onset of action of the fixed combination intranasal azelastine-fluticasone propionate in an allergen exposure chamber. J Allergy Clin Immunol Pract. 2018;6:1726–1732.e6.
Salapatek AM, Lee J, Patel D, D’Angelo P, Liu J, Zimmerer RO, et al. Solubilized nasal steroid (CDX-947) when combined in the same solution nasal spray with an antihistamine (CDX-313) provides improved, fast-acting symptom relief in patients with allergic rhinitis. Allergy Asthma Proc. 2011;32:221–9.
Moote W, Kim H. Allergen-specific immunotherapy. Allergy, Asthma Clin Immunol. 2011;7:S5.
• Donovan JP, Buckeridge DL, Briscoe MP, Clark RH, Day JH. Efficacy of immunotherapy to ragweed antigen tested by controlled antigen exposure. Ann Allergy Asthma Immunol. 1996;77:74–80. Ragweed antigen immunotherapy was evaluated in a controlled ragweed allergen exposure showed significantly reduced symptoms of ragweed-allergic rhinitis with no significant effect on ocular symptoms.
Roux M, Viatte A, Zeldin RK. Safety of a sublingual tablet of house dust mite allergen extracts in an environmental exposure chamber study. J Allergy Clin Immunol. 2015;135:AB266.
Roux M, Yang WH, Viatte A, Cadic VZR. Efficacy of house dust mite sublingual tablets in an environmental exposure chamber study of patients with house dust mite-associated allergic rhinitis | Cochrane Library. Allergy Eur J allergy Clin Immunol. 2013;68:5–6.
Nolte H, Maloney J, Nelson HS, et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J Allergy Clin Immunol. 2015;135:1494–1501.e6.
Horak F, Zieglmayer P, Zieglmayer R, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009;124:471–7 477.e1.
Ellis AK, Tenn MW, Steacy LM, Adams DE, Day AG, Walker TJ, et al. Lack of effect of Timothy grass pollen sublingual immunotherapy tablet on birch pollen–induced allergic rhinoconjunctivitis in an environmental exposure unit. Ann Allergy, Asthma Immunol. 2018;120:495–503.e2.
Ellis AK, Frankish CW, O’Hehir RE, Armstrong K, Steacy L, Larché M, et al. Treatment with grass allergen peptides improves symptoms of grass pollen–induced allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2017;140:486–96.
•• Patel P, Holdich T, Fischer Von Weikersthal-Drachenberg KJ, Huber B (2014) Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol 133:121–9.e1–2. This study demonstrated that an ultrashort course of ragweed immunotherapy is efficacious in reducing allergy symptoms in patients with seasonal AR and that it is well tolerated.
Patel D, Couroux P, Hickey P, Salapatek AM, Laidler P, Larché M, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–9.e1–7.
Horak F, Zieglmayer P, Zieglmayer R, et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy Eur J Allergy Clin Immunol. 2012;67:1572–9.
Krug N, Gupta A, Badorrek P, Koenen R, Mueller M, Pivovarova A, et al. Efficacy of the oral chemoattractant receptor homologous molecule on T H2 cells antagonist BI 671800 in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2014;133:414–9.
Kenney P, Hilberg O, Pedersen H, Nielsen OB, Sigsgaard T. Rhinix™ nasal filters for the treatment of allergic rhinitis: a randomized, double-blinded placebo-controlled crossover clinical trial. J Allergy Clin Immunol. 2014;133:AB186.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Ellis has participated in advisory boards for ALK Abello, AstraZeneca, Aralez, Bausch Health, Circassia Ltd., GlaxoSmithKline, Johnson & Johnson, Merck, Mylan, Novartis, Pediapharm, and Pfizer, has been a speaker for ALK, Aralez, AstraZeneca, Boerhinger-Ingleheim, CACME, Meda, Mylan, Merck, Novartis, Pediapharm, Pfizer, The ACADEMY, and Takeda. Her institution has received research grants from Bayer LLC, Circassia Ltd., Green Cross Pharmaceuticals, GlaxoSmithKline, Sun Pharma, Merck, Novartis, Pfizer, Regeneron, and Sanofi. She has also served as an independent consultant to Allergy Therapeutics, Bayer LLC, Ora Inc., and Regeneron in the past. The other authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rhinitis, Conjunctivitis, and Sinusitis
Rights and permissions
About this article
Cite this article
Hossenbaccus, L., Steacy, L.M., Walker, T. et al. Utility of Environmental Exposure Unit Challenge Protocols for the Study of Allergic Rhinitis Therapies. Curr Allergy Asthma Rep 20, 34 (2020). https://doi.org/10.1007/s11882-020-00922-8
Published:
DOI: https://doi.org/10.1007/s11882-020-00922-8