Abstract
In this study, to arrest the volatile Bi, a two-stage rapid solid-state reaction method has been adopted to prepare Bi1−xCaxFeO3 (x = 0.2 and 0.4) ceramics. Rietveld refinement on x-ray diffraction data confirmed the single-phase nature of the prepared samples. Unit cell volume of hexagonal structured (space group; R3c) Bi1−xCaxFeO3 decreases with the increase of Ca concentrations. Temperature-dependent (250 K to 450 K) dielectric constant measurements have been performed at different frequencies (100 kHz, 300 kHz, 500 kHz, 700 kHz, 900 kHz and 1100 kHz). The dielectric constant (ɛʹ), loss tangent (tan δ) and ac-conductivity (σac) remain nearly constant for low temperatures (~250 K to ~365 K) and have shown enhancement at higher temperatures (365 K to 450 K). Higher values of ɛʹ, tan δ and σac are detected in case of Bi0.6Ca0.4FeO3 sample. The butterfly-like loop in capacitance versus voltage curves confirms a weak ferroelectric nature. Magnetic field-dependent magnetization supports the Ca doping induced weak ferromagnetic character and exchange bias effect in the Bi0.6Ca0.4FeO3 sample. The origin of high dielectric constant and weak ferromagnetic nature is briefly discussed by considering the structural perturbations and cationic interaction in the Bi1−xCaxFeO3 (x = 0.2 and 0.4) ceramics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiferroic materials are having applications in memory storage devices, multiple-state memories, and new data storage media.1,2,3 Specifically, the multiferroics of BiFeO3, BiMnO3 and YMnO3 were found to have huge scope for research due to the simultaneous existence of ferroelectricity and magnetism. BiFeO3 (BFO) is one of the best multiferroic materials showing ferroelectricity and magnetism at room temperature (300K). It is a widely studied material owing to its relatively high curie temperature (TC = 1103 K) and Neel temperature (TN = 643 K) with G-type anti-ferromagnetism.4,5 The single-phase bulk BiFeO3 exhibits a rhombohedral distorted perovskite structure with space group R3c. All of the ions along the {111}c direction are displaced relative to the ideal centrosymmetric positions, and oxygen octahedra rotate alternately clockwise and counter-clockwise about the {111}c direction. The off-centered distortion triggered by a stereochemically active Bi3+ lone pair gives rise to ferroelectric properties, whereas partially filled 3d orbitals of Fe3+ ions result in G-type antiferromagnetic ordering due to super-exchange interactions.6 The antiferromagnetic characters and large leakage current are the major issues to the practical applications of BFO.7,8 There are studies to overcome the antiferromagnetic characters and large leakage current of BFO by substituting Bi3+ with both trivalent rare-earth ions and divalent ions.2,9,10,11,12 The enhanced magnetization due to structural distortion was observed in high pressure synthesized BFO ceramics.13 Saturated ferroelectric and ferromagnetic hysteresis loops have been reported by substituting Bi3+ with La3+, Nd3+, Sm3+ and Ba2+ ions.11,12 The partial substitution of Bi3+ with La3+ and Fe3+ with Mn4+ have improved magnetic properties.14 Heterovalent doping up to 30% at Bi site in BFO has already been attempted by several researchers.15,16 However, specific doping may enrich the multiferroic properties of BFO and remove the obstacles in its practical applications. Keeping this in mind, Bi1−xCaxFeO3 (x = 0.2 and 0.4) ceramics have been synthesized using rapid two-stage solid-state reaction method, and their structural, dielectric, and magnetic properties are reported in this study.
Experimental Details
Using a rapid two-stage solid-state reaction method involving heating of the samples near the decomposition temperatures of the reagents, the bulk samples of Bi1−xCaxFeO3 (x = 0.2 and 0.4) were synthesized. This method was adopted to arrest the volatile Bi. For sample preparation, the pure form (99.99% pure, from Sigma Aldrich) of Bi2O3, Fe2O3 and CaCO3 were weighed in desired stoichiometric proportions and continuously ground for 2 h using agate mortar resulting in a fine, uniformly mixed powder. Later, the uniformly mixed powdered samples were pelletized using a pelletizer by applying a pressure of 2.5 t for 2 min. The pellets were heated from room temperature to 850°C with a uniform increment of 2°C per minute and dwelled at this temperature for 30 min in the first stage. The pellets were quenched by removing them from the furnace at the same temperature (i.e. 850°C). Consequently, the pellets are again crushed and ground using pestle and mortar for 2 h and pelletized again. In the second stage, the heating was done for 10 min at 900°C and 950°C for x = 0.2 and 0.4, respectively. The samples were quenched by removing them from the furnace at given temperatures. X-ray diffraction (XRD) measurements were performed at room temperature using a Bruker-D8 x-ray diffractometer. Cu Kα radiation with a wavelength of 1.5418 Å was used with an operating voltage of 40 kV accelerating tube current of 40 mA. XRD data were collected within the two-theta range of 20°–80°. The scan speed was 1°/min with a step of 0.02°. The temperature and frequency-dependent dielectric measurements were performed using an Agilent precision LCR meter in the temperature range 250–450 K and frequency range 100–1100 kHz. The field-dependent magnetic measurement was performed at room temperature using a vibrating sample magnetometer (VSM).
Results and Discussion
Structural Study
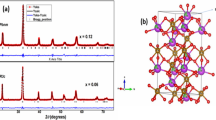
Rietveld refinement, using FULLPROF code, was applied to the XRD data collected from Bi1−xCaxFeO3 (x = 0.2 and 0.4) samples. Figure 1a–b shows the XRD pattern along with the fitted curve and difference line. The agreement between observed and calculated data suggests that Bi1−xCaxFeO3 samples are in a single phase for given concentration of Ca. The respective Wyckoff positions of Bi/Ca, Fe and O are 6a (0, 0, z), 6a (0, 0, 0), and 18b(x,y,z) in Bi1−xCaxFeO3 (x = 0.2, 0.4). Table I depicts all the parameters related to the unit cell. It is evident from Table I, that there is a decrease in the lattice parameters as well as the volume of the unit cell upon increasing the Ca doping concentration, which could be ascribed to either a smaller ionic radius of Ca2+ as compared to Bi3+ or to the formation of oxygen vacancies to neutralize/accommodate charge imbalance.17,18,19 XRD pattern reveals that the Bragg reflections are sharp for both samples. No structural phase transition has been observed as mentioned in few publications on heterovalent doped BiFeO3.20 It can be noticed that with increasing the Ca doping from x = 0.2 to x =0.4, the intensity of the peaks is decreasing. This suggests a lattice distortion in the compound because of Ca doping and is analogous to a previous report.21 Lattice strain has also been calculated using a Williamson–Hall plot (Refer to Table 1) and is found to increase with doping. It is also evidenced that the crystallite size is increasing (~39 nm to ~44 nm) with the increase in doping which reveals that substitution of Bi3+ with Ca2+ makes the crystal growth easy. This might be because volatile Bi3+(Bi2O3) is being replaced by non-volatile Ca2+(CaCO3) resulting easy crystal growth.20 The nano-range crystallite size may help in breaking the spiral spin structure and can reduce the leaky behavior of BiFeO3 system.22 The Fe-O-Fe bond angle also shrinks with the increase of Ca doping which can alter the magnetic properties of the material. Figure 2a–b displays the crystal structure in hexagonal representation. Avogadro software has been used to generate this structure. The unit cell consists of six Bi/Ca, six Fe atoms and 18 oxygen atoms. The Fe-O-Fe bond angles have been shown to govern the magnetic properties.
Dielectric Study
Figure 3a–b shows the temperature-dependent dielectric constant (ɛʹ) and Fig. 3c–d shows loss tangent (tanδ) of Bi1−xCaxFeO3 (x = 0.2 and 0.4) samples at different applied frequencies. Both the samples show high value of dielectric constant and small value of loss tangent at room temperature. In Table II, the values of dielectric constant at room temperature, remanent magnetization and maximum value of magnetization of Bi0.6Ca0.4FeO3 along with recently reported multiferroics23,24 have been presented. It is clear from the tabulated parameters that Bi0.6Ca0.4FeO3 depicts a very high value of dielectric constant in comparison to other recently reported samples. The dielectric constant (ɛʹ) and the loss tangent (tanδ) remain nearly constant for both the samples at lower temperatures due to freezing of dipoles. At higher temperatures, both rise significantly along with temperature, which might be attributed to the distorted system caused by the formation of oxygen vacancies due to Ca2+ doping. Both ɛʹ and tanδ increase significantly in higher temperature regions. This increase may be attributed to the formation of dipoles created due to oxygen vacancies resulting in a distorted system. Therefore, the fundamental idea of band conduction might be supplanted by localized sites, which are surrounded by high potential wells that cannot be transcended/exceeded by electrons. The consequences of dipole creation at the localized hopping sites result in dielectric polarization. Thus, the phenomenon of localized charge between spatially fluctuating potentials may result in conduction as well as dipolar effects.16
Upon increasing the temperature, hopping of the charge increases, resulting in more conduction and dipolar effects which increases both dielectric constant and loss tangent. Hence, the rise in the value of both parameters on increasing temperature indicates that carrier hopping plays a major part at higher temperatures in both the samples.25 The increase in loss tangent with temperature is a reflection of leakage current also which hinders its application for preparing storage devices. But a negligibly small value of loss tangent and hence leakage current at room temperature indicates a positive signal for its technological applications. However, at lower temperatures, hopping contributes a little to polarisation because of freezing of dipoles, resulting in insignificant variation in ɛʹ and tanδ with temperature. The variation is shown in the value of ɛʹ and tanδ with temperature and is obeyed at all frequencies in the measured frequency range (100–1100 kHz). Also, all these parameters decrease with an increase in frequency, indicating that at low-frequency, dipoles created because of charge defects follow the field reversal bringing about the increased value of ɛʹ and tanδ. However, on increasing the frequency, the defect-related dipoles lag behind the field, and consequently the values of ɛʹ and tanδ decrease. It is evidenced that with increasing calcium doping concentration, the values of both ɛʹ and tanδ increase by almost threefold at 450 K. This may be due to the increased oxygen vacancies created because of Ca doping leading to hopping current and dipolar effects.26 A dielectric abnormality has been noticed in the case of the Bi0.6Ca0.4FeO3 sample at 423 K in the ɛʹ versus temperature curve (see the inset of Fig. 3b), proposing some sort of ferroelectric transition, conceivably because of some structural transformation and ordering of dipoles. Because of the upper-temperature limit (450 K) of the instrument, we could not verify whether this anomaly is original or new. Further, more research is required to confirm the nature of the anomaly. Hence, to get a fine and clear view of observed peaks, the background has been subtracted for the ɛʹ vs. temperature curve at 100 kHz. It should also be noted that no such abnormality has been observed for the Bi0.8Ca0.2FeO3 sample up to 450K. For additional examination of the anomaly, variation in capacitance was recorded with respect to change in bias voltage at 420 K instead of ferroelectric hysteresis loops and is presented in Fig. 4. The butterfly-like loop in capacitance versus voltage curves are noticed which is an obvious sign of weak ferroelectric behavior in the samples.27 Hence, we can conclude that the observed anomaly is due to certain ferroelectric transitions in the samples.
AC Conductivity Study
Figure 5 displays the temperature dependence of ac-conductivity (σac) of Bi1−xCaxFeO3 (x = 0.2, 0.4) samples at selected frequencies. It is clear from the figure that conductivity increases exponentially with temperature obeying the Arrhenius equation \(\sigma_{{{\text{ac}}}} = \sigma_{o} e^{{ - E_{a} /{\text{kT}}}}\), where \(\sigma_{o}\) the pre-exponential constant and Ea is the activation energy. Also, the conductivity is increasing with the increase in frequency. This increase in the value of conductivity with an increase in temperature and frequency indicates that hopping has a significant role in both the samples.28 The value of the activation energy for both the samples has been calculated (at 1100 kHz) by finding the slope of graph ln (\(\sigma_{{{\text{ac}}}}\)) versus T−1 and results are presented in Fig. 6. The activation energy decreases while the conductivity increases as the doping is increased from 20% to 40% indicating that doping of calcium is making the conduction path easy, which might be due to an enhancement in oxygen vacancies with the increase in ca-doping. This indicates that small polaron hopping might be responsible for the σac. To identify the hopping model, ln(\(\sigma_{{{\text{ac}}}}\)) has been plotted against ln(ω) at different temperatures for both the samples and presented in Fig. 7. The slopes have been found to increase with the increase in temperature which is the signature of small polaron hopping in both samples.29,30 The figure of merit (F = \(\sigma_{{{\text{ac}}}}\)/ɛʹ) which is related to the response time has also been calculated at selected frequencies and its value has been tabulated in Table III. It is clear from the Table III that the value of F increases approximately 100 times as the Ca doping is increased from 20% to 40%. The higher value of F suggests that the material is a potential candidate for solar cell applications.
Magnetization Study
To understand the effect of Ca concentration on the magnetic properties of BiFeO3, magnetic hysteresis loops have been measured over ± 3000 Oe at room temperature (300K) for both of the samples and are presented in Fig. 8. A small M-H loop specifically in the sample doped at 40% has been observed. Non-zero values of remanent magnetization (Mr) and coercive field (Hc) are also observed (see the inset of the upper panel). This proves that at room temperature, some magnetic ordering exists in Bi0.6Ca0.4FeO3. The magnetization does not saturate up to 3000 Oe magnetic field. The presence of remanent magnetization and coercivity indicates that the antiferromagnetic ordering of BiFeO3 is transformed to some ferromagnetic ordering or canted antiferromagnetic ordering. In a previous report, the stoichiometry of various ionic species in BiFeO3 was altered to balance the charge neutrality on Ca doping which may be due to the creation of O vacancies.17 In the present case, due to the smaller radius of Ca2+ as compared to Bi3+, the structure gets slightly strained as is clear from strain values calculated from x-ray diffraction data (refer to Table I). As a result, the Fe-O-Fe bond angle (Table I) changes and resulting in enhanced magnetic properties of the doped BFO. Also, the nano-dimensional crystallite size may also suppress the spiral spin structure giving rise to enhanced magnetic properties. The asymmetric behavior in the case of the sample doped at 40% in both directions indicates exchange bias in the system contrary to the Ba-doped sample .31 The upgrading in the magnetic properties of BiFeO3 may be due the following three factors32: (i) suppression of the spiral spin structure due to small crystallite size, (ii) increase in spin canting due to the lattice strain, (iii) oxygen deficiency.31,32 Oxygen vacancies created due to Ca doping may alter the antiferromagnetic interaction into ferromagnetic interaction, i.e. there may be statistical distribution of two types of magnetic interactions in the samples, which is confirmed by the asymmetric nature of the M- H loop (see Fig. 8).
Conclusions
Single-phase polycrystalline Bi1−xCaxFeO3 (x = 0.2 and 0.4) samples have been prepared using a rapid two-stage solid-state reaction. The increase in the doping of Ca substitution in BiFeO3 (at Bi-site) does not change the parent crystal structure. However, both the lattice parameters ‘a’ and ‘c’ are found to decrease systematically with the incorporation of Ca. The εʹ, tan δ and σac were nearly invariant for the low-temperature regime (~250 K to ~365 K). Further increase in the applied temperature up to 450 K leads to significant enhancement in εʹ and tan δ which is ascribed to the oxygen defect formation. Interestingly, ε’ is significantly increased for the sample containing a higher amount of Ca while the other parameters (i.e., applied temperature and frequency) were kept the same. An increase in σac and a decrease in activation energy with an increase in Ca doping concentrations is the signature of small polaron hopping in the compound. The non-zero remanent magnetization and the existence of coercivity in the Bi0.6Ca0.4FeO3 sample indicate that the antiferromagnetic ordering of parent BiFeO3 is transformed to some ferromagnetic ordering.
References
J.F. Scott, Nat. Mater. 6, 256 (2007).
S.T. Zhang, Y. Zhang, M.H. Lu, C.L. Du, Y.F. Chen, Z.G. Liu, Y.Y. Zhu, N.B. Ming, and X.Q. Pan, Appl. Phys. Lett. 88, 162901 (2006).
Y.H. Lee, J.M. Wu, and C.H. Lai, Appl. Phys. Lett. 88, 042903 (2006).
Z.X. Cheng, X.L. Wang, K. Ozawa, and H. Kimura, Appl. Phys. Lett. 88, 132909 (2006).
Z.X. Cheng and X.L. Wang, Phys. Rev. B 75, 172406 (2007).
G.L. Yuan, S.W. Or, Y.P. Wang, Z.G. Liu, and J.M. Liu, Solid State Comm. 76, 138 (2006).
A.K. Pradhan, K. Zhang, D. Hunter, J.B. Dadson, G.B. Loiutts, P. Bhattacharya, R. Katiyar, J. Zhang, D.J. Sellmyer, U.N. Roy, Y. Cui, and A. Burger, J. Appl. Phys. 97, 093903 (2005).
M.M. Kumar and V.R. Palkar, Appl. Phys. Lett. 76, 2764 (2000).
Y.H. Lin, Q. Jiang, Y. Wang, C.W. Nan, L. Chen, and J. Yu, Appl. Phys. Lett. 90, 172507 (2007).
D. Lee, M.G. Kim, S. Ryu, H.M. Jang, and S.G. Lee, Appl. Phys. Lett. 86, 222903 (2005).
G.L. Yuan and S.W. Or, J. Appl. Phys. 100, 024109 (2006).
D.H. Wang, W.C. Goh, M. Ning, and C.K. Ong, Appl. Phys. Lett. 88, 212907 (2006).
W.N. Su, D.H. Wang, Q.Q. Cao, Z.D. Han, J. Yin, J.R. Zhang, and Y.W. Du, Appl. Phys. Lett. 91, 092905 (2007).
V.R. Palkar, D.C. Kundaliya, and S.K. Malik, J. Appl. Phys. 93, 4337 (2003).
V.A. Khomchenko, D.A. Kiselev, J.M. Vieira, A.L. Kholkin, M.A. Sa, and Y.G. Pogorelov, Appl. Phys. Lett. 90, 242901 (2007).
V.A. Khomchenko, D.A. Kiselev, J.M. Vieira, L. Jian, A.L. Kholkin, A.M.L. Lopes, Y.G. Pogorelov, J.P. Araujo, and M. Maglione, J. Appl. Phys. 103, 024105 (2008).
V.A. Khomchenko, M. Kopcewicz, A.M.L. Lopes, Y.G. Pogorelov, J.P. Araujo, J.M. Vieira, and A.L. Kholkin, J. Phys. D: Appl. Phys. 41, 102003 (2008).
D. Kothari, V.R. Reddy, A. Gupta, V. Sathe, A. Banerjee, S.M. Gupta, and A.M. Awaasthi, Appl. Phys. Lett. 91, 202505 (2007).
B. Yu, M. Li, J. Liu, D. Guo, L. Pei, and X. Zhao, J. Phys. D: Appl. Phys. 41, 065003 (2008).
J. Li, Y. Duan, H. He, and D. Song, J. Alloys and Compounds 315, 259 (2001).
K.S. Rao, D.M. Prasad, P. Murali Krishna, B.H. Bindu, and K. Suneetha, J. Mater. Sci. 42, 7363 (2007).
A.R. Makhdoom, M.J. Akhtar, M.A. Rafiq, and M.M. Hassan, Ceram. Int. 38, 3829 (2012).
F.S. Jesus, C.A. Escobedo, and A.M.B. Miro, Ceram. Int. 44, 15298 (2018).
B. Song, J. Shen, H. Zhao, A. Kumar, Q. Xu, Y. Zhai, and Q. Li, Ceram. Int. 47, 10873 (2021).
N.R.M. Reddy, M.V. Ramana, K. Krishnaveni, K.V.S. Kumar, and V.R.K. Murthy, Bull. Mater. Sci. 30, 357 (2007).
Z. Yu, C. Ang, R. Guo, A.S. Bhalla, and L.E. Cross, Appl. Phys. Lett. 80, 1034 (2002).
N. Uchida and T. Ikeda, Jpn. J. Appl. Phys. 4, 867 (1965).
H. Bouaamlat, N. Hadi, N. Belghiti, H. Sadki, M.N. Bennani, F. Abdi, T. Lamcharfi, M. Bouachrine, and M. Abarkan, Adv. Mater. Sci. Eng. Vol. 8689150, 8 (2020).
N.F. Mott, Philos. Mag. 19, 835 (1969).
M.B. Bellakki and V. Manivannan, J. Mater. Sci. 45, 1137 (2010).
J. Nogues and I.K. Schuller, J. Magn. Magn. Mater. 192, 203 (1999).
R. Mazumder, P.S. Devi, D. Bhattacharya, P. Choudhury, A. Sen, and M. Raja, Appl. Phys. Lett. 91, 062510 (2007).
Acknowledgment
The authors are thankful to the honorable vice-chancellor and dean of Manav Rachna University, Faridabad, for constant encouragement and support for conducting research in the physics department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehta, K., Sharma, A. & Jaiparkash Ca Doping Concentration Induced Enhancement in Dielectric and Magnetic Properties of Bi1−xCaxFeO3 (x = 0.2 and 0.4) Ceramics. J. Electron. Mater. 51, 4145–4151 (2022). https://doi.org/10.1007/s11664-022-09723-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09723-3