Abstract
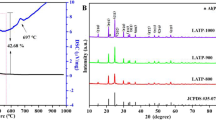
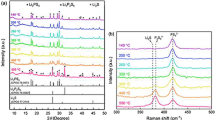
Li1.3Al0.3Ti1.7(PO4)3 (LATP) has become a promising solid electrolyte material for energy storage due to its high ionic conductivity, low cost, and good air stability. However, its application development is limited by the problems of high grain boundary resistance and low density. In this work, the boron-based B2O3 glass are added into LATP by solid method at external concentrations of 1 wt%, 2 wt%, 3 wt% and 4 wt%. And the effect of B2O3 on the structure and properties of LATP solid electrolyte was studied. As the results, the main diffraction peaks of all prepared samples sintered at 850 °C for 7 h maintain the NASICON-type with the R-3c space group. Meanwhile, the symmestric stretching vibrations of P–O bond is redshifted with the addition of boron-based glass, which plays a positive role in bulk conductivity. Additionally, high density can be obtained by controlling the amount of flux, while controlling the production of the second phase LiTiPO5 and reducing the influence of the second relative ionic conductivity. When the amount of B2O3 is 2 wt%, the relative density, total ionic conductivity and conductance activation energy are the best: σtot = 0.307 mS/cm, ρ = 95.1%, Ea = 0.26 eV.












Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Data availability
Data are available upon request.
References
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414(6861):359–367. https://doi.org/10.1038/35104644
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603. https://doi.org/10.1021/cm901452z
Jiang P, Du G, Cao J, Zhang X, Zou C (2023) Solid-state Li ion batteries with oxide solid electrolytes: progress and perspective. Energy Technol-Ger 11(3):2201288. https://doi.org/10.1002/ente.202201288
Il’ina E (2023) Recent strategies for lithium-ion conductivity improvement in Li7La3Zr2O12 solid electrolytes. Int J M Sci 24(16):12905. https://doi.org/10.3390/ijms241612905
Benabed Y, Rioux M, Rousselot S, Hautier G, Dolle M (2021) Assessing the electrochemical stability window of NASICON-type solid electrolytes. Front Energy Res 9:682008. https://doi.org/10.3389/fenrg.2021.682008
Arbi K, Jimenez R, Šalkus T, Orliukas AF, Sanz J (2015) On the influence of the cation vacancy on lithium conductivity of Li1+xRxTi2−x(PO4)3Nasicon type materials. Solid State Ionics 271:28–33. https://doi.org/10.1016/j.ssi.2014.10.016
Pinus IY, Arkhangel’skii IV, Zhuravlev NA, Yaroslavtsev AB (2009) Cation mobility in modified Li1+xTi2−xGax(PO4)3 lithium titanium NASICON phosphates. Russ J Inorg Chem+ 54:1173–1176. https://doi.org/10.1134/S0020168511120181
Svitan’Ko AI, Novikova SA, Safronov DV, Yaroslavtsev AB (2011) Cation mobility in Li1+xTi2−xCrx(PO4)3 NASICON-type phosphates. Inorg Mater+ 47:1391–1395. https://doi.org/10.1134/S0020168511120181
Capsoni D, Bini M, Ferrari S, Massarotti V, Mozzati MC (2012) Role of lithium excess and doping in Li1+xTi2–xMnx(PO4)3 (0.00 ≤ x ≤ 0.10). J Phys Chem C 116(1):1244–1250
Venkateswara Rao A, Veeraiah V, Prasada Rao AV, Prasada R, Kishore Babu B (2015) Effect of Fe3+ doping on the structure and conductivity of LiTi2(PO4)3. Res Chem Intermediat 41:2307–2315. https://doi.org/10.1007/s11164-013-1348-0
Pfalzgraf D, Mutter D, Urban DF (2021) Atomistic analysis of Li migration in Li1+xAlxTi2−x(PO4)3(LATP) solid electrolytes. Solid State Ionics 359:115521. https://doi.org/10.1016/j.ssi.2020.115521
Zhang B, Lin Z, Dong H, Wang L, Pan H (2020) Revealing cooperative Li-ion migration in Li1+xAlxTi2−x(PO4)3 solid state electrolytes with high Al doping. J Mater Chem A 8(1):342–348. https://doi.org/10.1039/c9ta09770h
Zhu Y, Wu T, Sun J, Kotobuki M (2020) Highly conductive lithium aluminum germanium phosphate solid electrolyte prepared by sol-gel method and hot-pressing. Solid State Ionics 350:115320. https://doi.org/10.1016/j.ssi.2020.115320
Chang CM, Lee YI, Hong SH, Park HM (2005) Spark plasma sintering of LiTi2(PO4)3-based solid electrolytes. J Am Ceram Soc 88(7):1803–1807. https://doi.org/10.1111/j.1551-2916.2005.00246.x
Chen S, Nie L, Hu X, Zhang Y (2022) Ultrafast sintering for ceramic-based all-solid-state lithium-metal batteries. Adv Mater 34(33):2200430. https://doi.org/10.1002/adma.202200430
Lachal M, El Khal H, Bouvard D, Chaix J, Bouchet R (2021) Flash sintering of cationic conductive ceramics: A way to build multilayer systems. J Am Ceram Soc 104(8):3845–3854. https://doi.org/10.1111/jace.17787
Kwatek K, Ślubowska W, Nowiński JL et al (2021) Electrical and structural properties of Li1. 3Al0.3Ti1.7(PO4)3-based ceramics prepared with the addition of Li4SiO4. Materials 14(19):5729. https://doi.org/10.3390/ma14195729
Xiao Z, Shang C, Guo M (2011) Influence of Li3PO4 addition on properties of lithium ion-conductive electrolyte Li1.3Al0.3Ti1.7(PO4)3. T Nonferr Metal Soc 21(11):2454–2458. https://doi.org/10.1016/s1003-6326(11)61036-4
Kwatek K, Ślubowska W, Trébosc J, Lafon O, Nowiński JL (2020) Impact of Li2.9B0.9S0.1O3.1 glass additive on the structure and electrical properties of the LATP-based ceramics. J Alloy Compd 820:153072. https://doi.org/10.1016/j.jallcom.2019.153072
Kothari DH, Kanchan DK (2021) Effect of adding Li2O and MgO on Li-conductivity in NASICON material. Physica B 600:412489. https://doi.org/10.1016/j.physb.2020.412489
Zhao X, Luo Y, Zhao X (2022) Effect of TeO2 sintering aid on the microstructure and electrical properties of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. J Alloys Compd 927:167019. https://doi.org/10.1016/j.jallcom.2022.167019
Pershina SV, Vovkotrub EG, Antonov BD (2022) Effects of B2O3 on crystallization kinetics, microstructure and properties of Li1.5Al0.5Ge1.5(PO4)3-based glass-ceramics. Solid State Ionics 383:115990. https://doi.org/10.1016/j.ssi.2022.115990
Rai K, Kundu S (2023) B2O3 as an effective sintering aid in enhancing the lithium-ion conductivity of LiTa2PO8 solid electrolyte. J Power Sources 576:233229. https://doi.org/10.1016/j.jpowsour.2023.233229
Kang J, Guo X, Gu R, Tang Y, Hao H (2023) Effect of boron-based glass additives on the ionic conductivity of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. J Alloys Compd 941:168857. https://doi.org/10.1016/j.jallcom.2023.168857
Hupfer T, Bucharsky EC, Schell KG, Hoffmann MJ (2017) Influence of the secondary phase LiTiOPO4 on the properties of Li1+xAlxTi2−x(PO4)3(x= 0; 0.3). Solid State Ionics 302:49–53. https://doi.org/10.1016/j.ssi.2016.10.008
Mariappan CR, Galven C, Crosnier-Lopez MP, Le Berre F, Bohnke O (2006) Synthesis of nanostructured LiTi2(PO4)3 powder by a Pechini-type polymerizable complex method. J Solid State Chem 179(2):450–456. https://doi.org/10.1016/j.jssc.2005.11.005
Luo Z, Qin C, Xu W, Liang H, Lei W (2020) Crystal structure refinement, microstructure and ionic conductivity of ATi2(PO4)3 (A= Li, Na, K) solid electrolytes. Ceram Int 46(10):15613–15620. https://doi.org/10.1016/j.ceramint.2020.03.108
Hupfer T, Bucharsky EC, Schell KG, Hoffmann MJ (2017) Influence of the secondary phase LiTiOPO4 on the properties of Li1+xAlxTi2−x(PO4)3 (x= 0; 0.3). Solid State Ionics 302:49–53. https://doi.org/10.1016/j.ssi.2016.10.008
Lang B, Ziebarth B, Elsässer C (2015) Lithium ion conduction in LiTi2(PO4)3 and related compounds based on the NASICON structure: a first-principles study. Chem Mater 27(14):5040–5048. https://doi.org/10.1021/acs.chemmater.5b01582
Pfalzgraf D, Mutter D, Urban DF (2021) Atomistic analysis of Li migration in Li1+ xAlxTi2−x(PO4)3(LATP) solid electrolytes. Solid State Ionics 359:115521. https://doi.org/10.1016/j.ssi.2020.115521
Tarte P, Cahay R, Garcia A (1979) Infrared spectrum and structural role of titanium in synthetic Ti-garnets. Phys Chem Miner 4:55–63. https://doi.org/10.1007/BF00308359
Xu W, Qin C, Zhang S, Liang H, Lei W (2021) Thermal, structural and electrical properties of fluorine-doped Li3.6Al0.8Ti4.0P7.6O30-x/2Fx(x=0, 0.5, 1,2) glass ceramic electrolytes. J Alloy Compd 853:157191. https://doi.org/10.1016/j.jallcom.2020.157191
Golosov OA, Khvostov SS, Glushkova NV, Evseev MV, Staritsyn SV (2021) Corrosive and mechanical resistance of MgO ceramics under metallizing and mild chlorination of spent nuclear fuel in molten salts. Ceram Int 47(3):3306–3311. https://doi.org/10.1016/j.ceramint.2020.09.171
Yang B, Li X, Guo H, Wang Z, Xiao W (2015) Preparation and properties of Li1.3Al0.3Ti1.7(PO4)3 by spray-drying and post-calcining method. J Alloy Compd 643:181–185. https://doi.org/10.1016/j.jallcom.2015.04.019
Xu Q, Tsai CL, Song D, Basak S, Kungl H (2021) Insights into the reactive sintering and separated specific grain/grain boundary conductivities of Li1.3Al0.3Ti1.7(PO4)3. J Power Sources 492:229631. https://doi.org/10.1016/j.jpowsour.2021.229631
Wu P, Zhou W, Su X, Li J, Su M (2023) Recent Advances in Conduction Mechanisms, Synthesis Methods, and Improvement Strategies for Li1+xAlxTi2−x(PO4)3 Solid Electrolyte for All-Solid-State Lithium Batteries. Adv Energy Mater 13(4):2203440. https://doi.org/10.1002/aenm.202203440
Miao C, Kou Z, Li J, Liu C, Chen Q (2022) LiF-doped Li1.3Al0.3Ti1.7(PO4)3 superionic conductors with enhanced ionic conductivity for all-solid-state lithium-ion batteries. Ionics 2022:1–11. https://doi.org/10.1007/s11581-021-04324-2
Bai H, Hu J, Li X, Duan Y, Shao F (2018) Influence of LiBO2 addition on the microstructure and lithium-ion conductivity of Li1+xAlxTi2−x(PO4)3 (x = 0.3) ceramic electrolyte. Ceram Int 44(6):6558–6563. https://doi.org/10.1016/j.ceramint.2018.01.058
Dai L, Wang J, Shi Z, Yu L, Shi J (2021) Influence of LiBF4 sintering aid on the microstructure and conductivity of LATP solid electrolyte. Ceram Int 47(8):11662–11667. https://doi.org/10.1016/j.ceramint.2021.01.004
Kang J, Guo X, Gu R, Tang Y, Hao He (2023) Effect of boron-based glass additives on the ionic conductivity of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. J Alloy Compd 941:168857. https://doi.org/10.1016/j.jallcom.2023.168857
Acknowledgements
This work was financially supported by The Jiangxi Provincial Department of Education's Graduate Innovation Fund (YC2023-B235).
Funding
This study was supported by Education Department of Jiangxi Province, YC2023-B235, Binxuan Jiang
Author information
Authors and Affiliations
Contributions
Jiale Yuan and Binxuan Jiang helped in project administration, conceptualization, methodology, writing, contributed equally to the manuscript. Yueming Li participated in conceptualization, formalism of methodology, resources, validation, supervision and writing-review and editing. Xu Guo, Yadzo Emmanuel Kwame and Mengzhen He assisted in data handling.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: David Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, J., Jiang, B., Li, Y. et al. Effect of flux B2O3 on structure and properties of LATP solid electrolyte. J Mater Sci 59, 16629–16643 (2024). https://doi.org/10.1007/s10853-024-10186-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-10186-6