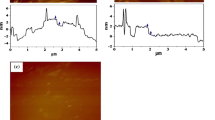
Abstract
In this work, we performed an integrated study on the physicochemical changes of graphene oxide (GO) during the drying process in terms of their biological effects on red blood cells (hemolysis) and interactions with human plasma (protein corona formation). GO in aqueous dispersion (GO-Disp) was dried exploring two procedures: using a vacuum system at room temperature (GO-VD) and lyophilization (GO-LP). The nanomaterials were well characterized by microscopic (TEM, SEM, and AFM), spectroscopic (FTIR, UV–Vis, Raman, and 13C NMR), and XRD techniques. The lyophilization process produced a nanomaterial with a three-dimensional porous macrostructure and the lowest oxidation degree. In contrast, the vacuum-drying process at room temperature provided a nanomaterial with a film-like macrostructure, presenting a higher oxidation degree as well as physicochemical properties more similar to those of GO-Disp. All of the nanomaterials adsorbed human plasma proteins; however, the protein adsorption was more selective for GO-Disp. GO-VD induced hemolysis of red blood cells in a lower concentration than GO-Disp and GO-LP, but the protein corona formation suppressed the hemolytic effect for all nanomaterials. Finally, our results indicate that the method applied to dry GO nanomaterials has a critical influence on their nanobiointeractions with cells and proteins, suggesting that more attention should be paid to biomedical applications and toxicological evaluations associated with these promising nanomaterials.
Graphical abstract







Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339. https://doi.org/10.1021/ja01539a017
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240. https://doi.org/10.1039/B917103G
Lerf A, He H, Forster M, Klinowski J (1998) Structure of graphite oxide revisited. J Phys Chem B 102:4477–4482. https://doi.org/10.1021/jp9731821
Georgakilas V, Otyepka M, Bourlinos AB et al (2012) Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev 112:6156–6214. https://doi.org/10.1021/cr3000412
Li X, Zhang G, Bai X et al (2008) Highly conducting graphene sheets and Langmuir-Blodgett films. Nat Nanotechnol 3:538–542. https://doi.org/10.1038/nnano.2008.210
Kim J, Cote LJ, Kim F et al (2010) Graphene oxide sheets at interfaces. J Am Chem Soc 132:8180–8186. https://doi.org/10.1021/ja102777p
Taheriazam A, Abad GGY, Hajimazdarany S et al (2023) Graphene oxide nanoarchitectures in cancer biology: nano-modulators of autophagy and apoptosis. J Control Release 354:503–522. https://doi.org/10.1016/j.jconrel.2023.01.028
Han S, Teng F, Wang Y et al (2020) Drug-loaded dual targeting graphene oxide- based molecularly imprinted composite and recognition of carcino-embryonic antigen. RSC Adv 10:10980–10988. https://doi.org/10.1039/D0RA00574F
Sontakke AD, Tiwari S, Purkait MK (2023) A comprehensive review on graphene oxide-based nanocarriers: synthesis, functionalization and biomedical applications. FlatChem 38:100484–100515. https://doi.org/10.1016/j.flatc.2023.100484
Bałaban J, Wierzbicki M, Zielińska-Górska M et al (2023) Graphene oxide decreases pro-inflammatory proteins production in skeletal muscle cells exposed to SARS-CoV-2 spike protein. Nanotechnol, Sci Appl 16:1–8. https://doi.org/10.2147/NSA.S391761
Caputo D, Coppola A, Quagliarini E et al (2022) Multiplexed detection of pancreatic cancer by combining a nanoparticle-enabled blood test and plasma levels of acute-phase proteins. Cancers 14(19):4658–4670. https://doi.org/10.3390/cancers14194658
Caputo D, Quagliarini E, Coppola A et al (2023) Inflammatory biomarkers and nanotechnology: new insights in pancreatic cancer early detection. Int J Surg 109(10):2934–2940
Bhatt S, Pathak R, Punetha VD, Punetha M (2023) Recent advances and mechanism of antimicrobial efficacy of graphene-based materials: a review. J Mater Sci 58:7839–7867. https://doi.org/10.1007/s10853-023-08534-z
Braccia C, Castagnola V, Vazquez E et al (2021) The lipid composition of few layers graphene and graphene oxide biomolecular corona. Carbon 185:591–598. https://doi.org/10.1016/j.carbon.2021.09.052
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Tarcan R, Todor-Boer O, Petrovai I et al (2020) Reduced graphene oxide today. J Mater Chem C 8:1198–1224. https://doi.org/10.1039/C9TC04916A
Vryonis O, Andritsch T, Vaughan AS, Lewin PL (2019) An alternative synthesis route to graphene oxide: influence of surface chemistry on charge transport in epoxy-based composites. J Mater Sci 54:8302–8318. https://doi.org/10.1007/s10853-019-03477-w
Tan CP, Zawawi RM, ZA NH. (2021) Effect of sonication time and heat treatment on the structural and physical properties of chitosan/graphene oxide nanocomposite films. Food Packag Shelf Life 28:100663–100674. https://doi.org/10.1016/j.fpsl.2021.100663
Kumar S, Garg A, Chowdhuri A (2019) Sonication effect on graphene oxide (GO) membranes for water purification applications. Mater Res Express 6:85620–85629. https://doi.org/10.1088/2053-1591/ab1ffd
Ghulam AN, Dos Santos OA, Hazeem L et al (2022) Graphene oxide (GO) materials—applications and toxicity on living organisms and environment. J Funct Biomater 13(2):77–108. https://doi.org/10.3390/jfb13020077
Mazánek V, Luxa J, Matějková S et al (2019) Ultrapure graphene is a poor electrocatalyst: definitive proof of the key role of metallic impurities in graphene-based electrocatalysis. ACS Nano 13:1574–1582. https://doi.org/10.1021/acsnano.8b07534
Zhang Z, Ma M, Chen C et al (2016) The morphology, structure and electrocatalytic ability of graphene prepared with different drying methods. RSC Adv 6:28005–28014. https://doi.org/10.1039/C5RA23123J
Platero E, Fernandez ME, Bonelli PR, Cukierman AL (2017) Graphene oxide/alginate beads as adsorbents: influence of the load and the drying method on their physicochemical-mechanical properties and adsorptive performance. J Colloid Interface Sci 491:1–12. https://doi.org/10.1016/j.jcis.2016.12.014
Gies V, Zou S (2018) Systematic toxicity investigation of graphene oxide: evaluation of assay selection, cell type, exposure period and flake size. Toxicol Res (Camb) 7:93–101. https://doi.org/10.1039/C7TX00278E
Pinto AM, Gonçalves IC, Magalhães FD (2013) Graphene-based materials biocompatibility: a review. Colloids Surf B Biointerfaces 111:188–202. https://doi.org/10.1016/j.colsurfb.2013.05.022
Seabra AB, Paula AJ, de Lima R et al (2014) Nanotoxicity of graphene and graphene oxide. Chem Res Toxicol 27:159–168. https://doi.org/10.1021/tx400385x
Sanchez VC, Jachak A, Hurt RH, Kane AB (2012) Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol 25:15–34. https://doi.org/10.1021/tx200339h
Fadeel B, Bussy C, Merino S et al (2018) Safety assessment of graphene-based materials: focus on human health and the environment. ACS Nano 12:10582–10620. https://doi.org/10.1021/acsnano.8b04758
Liao K-H, Lin Y-S, Macosko CW, Haynes CL (2011) Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl Mater Interfaces 3:2607–2615. https://doi.org/10.1021/am200428v
Palmieri V, Perini G, De Spirito M, Papi M (2019) Graphene oxide touches blood: in vivo interactions of bio-coronated 2D materials. Nanoscale Horizons 4:273–290. https://doi.org/10.1039/C8NH00318A
Sasidharan A, Panchakarla LS, Sadanandan AR et al (2012) Hemocompatibility and macrophage response of pristine and functionalized graphene. Small 8(2012):1251–1263. https://doi.org/10.1002/smll.201102393
Monopoli MP, Åberg C, Salvati A, Dawson KA (2012) B Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol 7:779–786. https://doi.org/10.1038/nnano.2012.207
Franqui LS, De Farias MA, Portugal RV et al (2019) Interaction of graphene oxide with cell culture medium: evaluating the fetal bovine serum protein corona formation towards in vitro nanotoxicity assessment and nanobiointeractions. Mater Sci Eng C 100:363–377. https://doi.org/10.1016/j.msec.2019.02.066
de Faria AF, Martinez DST, Meira SMM et al (2014) Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf B Biointerfaces 113:115–124. https://doi.org/10.1016/j.colsurfb.2013.08.006
de Moraes ACM, Andrade PF, de Faria AF et al (2015) Fabrication of transparent and ultraviolet shielding composite films based on graphene oxide and cellulose acetate. Carbohydr Polym 123:217–227. https://doi.org/10.1016/j.carbpol.2015.01.034
Fonseca LC, de Araújo MM, de Moraes ACM et al (2018) Nanocomposites based on graphene oxide and mesoporous silica nanoparticles: preparation, characterization and nanobiointeractions with red blood cells and human plasma proteins. Appl Surf Sci 437:110–121. https://doi.org/10.1016/j.apsusc.2017.12.082
de Sousa M, Martins CHZ, Franqui LS et al (2018) Covalent functionalization of graphene oxide with d-mannose: evaluating the hemolytic effect and protein corona formation. J Mater Chem B 6:2803–2812. https://doi.org/10.1039/C7TB02997G
Reina G, González-Domínguez JM, Criado A et al (2017) Promises, facts and challenges for graphene in biomedical applications. Chem Soc Rev 46:4400–4416. https://doi.org/10.1039/C7CS00363C
Kang Y, Wang C, Shi X et al (2018) Crystallization, rheology behavior, and antibacterial application of graphene oxide-graft-poly (l-lactide)/poly (l-lactide) nanocomposites. Appl Surf Sci 451:315–324. https://doi.org/10.1016/j.apsusc.2018.04.271
Li W, Wang J, Ren J, Qu X (2013) 3D graphene oxide-polymer hydrogel: near-infrared light-triggered active scaffold for reversible cell capture and on-demand release. Adv Mater 25:6737–6743. https://doi.org/10.1002/adma.201302810
Chetwynd AJ, Wheeler KE, Lynch I (2019) Best practice in reporting corona studies: minimum information about nanomaterial biocorona experiments (MINBE). Nano Today 28:100758–100770. https://doi.org/10.1016/j.nantod.2019.06.004
Chen K, Liu F, Song S, Xue D (2014) Water crystallization to create ice spacers between graphene oxide sheets for highly electroactive graphene paper. CrystEngComm 16:7771–7776. https://doi.org/10.1039/C4CE01030B
Pan Y, Wu T, Bao H, Li L (2011) Green fabrication of chitosan films reinforced with parallel aligned graphene oxide. Carbohydr Polym 83:1908–1915. https://doi.org/10.1016/j.carbpol.2010.10.054
Stankovich S, Dikin DA, Dommett GHB et al (2006) Graphene-based composite materials. Nature 442:282–286. https://doi.org/10.1038/nature04969
Schniepp HC, Li J-L, McAllister MJ et al (2006) Functionalized single graphene sheets derived from splitting graphite oxide. J Phys Chem B 110:8535–8539. https://doi.org/10.1021/jp060936f
Cheng Q, Wu M, Li M, Jiang L, Tang Z (2013) Ultratough artificial nacre based on conjugated cross-linked graphene oxide. Angew Chemie Int Ed 52:3750–3755. https://doi.org/10.1002/anie.201210166
Seehra MS, Narang V, Geddam UK, Stefaniak AB (2017) Correlation between X-ray diffraction and Raman spectra of 16 commercial graphene-based materials and their resulting classification. Carbon NY 111:380–384. https://doi.org/10.1016/j.carbon.2016.10.010
Krishnamoorthy K, Veerapandian M, Yun K, Kim S-J (2013) The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon NY 53:38–49. https://doi.org/10.1016/j.carbon.2012.10.013
Huh SH (2014) X-ray diffraction of multi-layer graphenes: instant measurement and determination of the number of layers. Carbon NY 78:617–621. https://doi.org/10.1016/j.carbon.2014.07.034
Ju H-M, Huh SH, Choi S-H, Lee H-L (2010) Structures of thermally and chemically reduced graphene. Mater Lett 64:357–360. https://doi.org/10.1016/j.matlet.2009.11.016
Liaros N, Iliopoulos K, Stylianakis MM et al (2013) Optical limiting action of few layered graphene oxide dispersed in different solvents. Opt Mater (Amst) 36:112–117. https://doi.org/10.1016/j.optmat.2013.04.036
Scherrer P (1918) Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen. Math Phys 2:98–100
Dikin DA, Stankovich S, Zimney EJ et al (2007) Preparation and characterization of graphene oxide paper. Nature 448:457–460. https://doi.org/10.1038/nature06016
Akbari A, Sheath P, Martin ST et al (2016) Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide. Nat Commun 7:10891–10903. https://doi.org/10.1038/ncomms10891
Moon IK, Lee J, Ruoff RS, Lee H (2010) Reduced graphene oxide by chemical graphitization. Nat Commun 1:73–79. https://doi.org/10.1038/ncomms1067
Eigler S, Dimiev AM (2016) Characterization techniques. Graphene Oxide 85–120. https://doi.org/10.1002/9781119069447.ch3
Konkena B, Vasudevan S (2012) Understanding aqueous dispersibility of graphene oxide and reduced graphene oxide through pKa measurements. J Phys Chem Lett 3:867–872. https://doi.org/10.1021/jz300236w
Acik M, Lee G, Mattevi C et al (2011) The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J Phys Chem C 115:19761–19781. https://doi.org/10.1021/jp2052618
Yuan R, Yuan J, Wu Y et al (2017) Efficient synthesis of graphene oxide and the mechanisms of oxidation and exfoliation. Appl Surf Sci 416:868–877. https://doi.org/10.1016/j.apsusc.2017.04.181
Rabchinskii MK, Dideikin AT, Kirilenko DA et al (2018) Facile reduction of graphene oxide suspensions and films using glass wafers. Sci Rep 8:14154–14165. https://doi.org/10.1038/s41598-018-32488-x
Guex LG, Sacchi B, Peuvot KF et al (2017) Experimental review: chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 9:9562–9571. https://doi.org/10.1039/C7NR02943H
Wu J-B, Lin M-L, Cong X et al (2018) Raman spectroscopy of graphene-based materials and its applications in related devices. Chem Soc Rev 47:1822–1873. https://doi.org/10.1039/C6CS00915H
Ferrari AC, Meyer JC, Scardaci V et al (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97:187401–87405. https://doi.org/10.1103/PhysRevLett.97.187401
Toh SY, Loh KS, Kamarudin SK, Daud WRW (2014) Graphene production via electrochemical reduction of graphene oxide: synthesis and characterisation. Chem Eng J 251:422–434. https://doi.org/10.1016/j.cej.2014.04.004
Bussy C, Ali-Boucetta H, Kostarelos K (2013) Safety considerations for graphene: lessons learnt from carbon nanotubes. Acc Chem Res 46:692–701. https://doi.org/10.1021/ar300199e
Gao Y, Chen K, Ren X et al (2018) Exploring the aggregation mechanism of graphene oxide in the presence of radioactive elements: experimental and theoretical studies. Environ Sci Technol 52:12208–12215. https://doi.org/10.1021/acs.est.8b02234
Yang K, Chen B, Zhu X, Xing B (2016) Aggregation, adsorption, and morphological transformation of graphene oxide in aqueous solutions containing different metal cations. Environ Sci Technol 50:11066–11075. https://doi.org/10.1021/acs.est.6b04235
Derjaguin B, Landau L (1993) Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog Surf Sci 43:30–59. https://doi.org/10.1016/0079-6816(93)90013-L
Hong BJ, Compton OC, An Z et al (2012) Successful stabilization of graphene oxide in electrolyte solutions: enhancement of biofunctionalization and cellular uptake. ACS Nano 6:63–73. https://doi.org/10.1021/nn202355p
Wei X-Q, Hao L-Y, Shao X-R et al (2015) Insight into the interaction of graphene oxide with serum proteins and the impact of the degree of reduction and concentration. ACS Appl Mater Interfaces 7:13367–13374. https://doi.org/10.1021/acsami.5b01874
Chen J, Yao B, Li C, Shi G (2013) An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon NY 64:225–229. https://doi.org/10.1016/j.carbon.2013.07.055
Cai B, Hu K, Li C et al (2015) Bovine serum albumin bioconjugated graphene oxide: red blood cell adhesion and hemolysis studied by QCM-D. Appl Surf Sci 356:844–851. https://doi.org/10.1016/j.apsusc.2015.08.178
Acknowledgements
This work is “in memoriam” of Professor Oswaldo Luiz Alves and Dr Douglas Soares da Silva. The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Instituto Nacional de Ciência e Tecnologia em Materiais Complexos Funcionais (INCT-Inomat) and NanoBioss/SisNANO/MCTI for financial support. The authors acknowledge the Open facilities: Nanotoxicology and Nanosafety (NANOTOX) and Biophysics of Macromolecules (BFM) at CNPEM. The authors further acknowledge the Hemocenter of the School of Medicine at the UNICAMP for providing the plasma and red cells from human blood for the experiments.
Funding
Financial support was received by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) with a research grant (process number 88882.329207/2019-01).
Author information
Authors and Affiliations
Contributions
DLSM did conceptualization, methodology, experiments, investigation, data curation, writing—original draft. FC did experiments, data curation and analysis, writing—original draft, review and editing. LSF, LCF, DSS performed data analysis, conduction of experiments, writing—initial draft. KBS and CHZM contributed to conceptualization, review of data, writing—initial draft. FSD was involved in methodology, conduction of experiments and data curation. DSTM and OLA done project administration, supervision, validation, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This work was approved by the Research Ethical Committee (No. 5.719.081) from the University of Campinas linked to the Plataforma Brasil system at the Ministry of Health/Brazil.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 494 KB)
The online version contains supplementary material available. Table S1 presented the chemical composition results of GO samples analyzed by energy-dispersive spectroscopy (EDS). Table S2 presented the total protein quantification in the human plasma measured by Braford assay. And the total protein profile of human plasma applied in this study was demonstrated by SDS-PAGE in supplementary material (Figure S1).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Sousa Maia, D.L., Côa, F., da Silva, K.B. et al. Drying of graphene oxide: effects on red blood cells and protein corona formation. J Mater Sci 59, 577–592 (2024). https://doi.org/10.1007/s10853-023-09163-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-09163-2