Abstract
The field of microelectronic devices and 5G communication has an increasing demand for polymer composites with low dielectric constant, low dielectric loss, and good hydrophobicity. However, traditional polymer composites cannot simultaneously satisfy them, which severely hinders their application. In this work, a liquid crystal epoxy resin (LCE4) consisted of a flexible chain and rigid mesogenic unit was prepared and cured with methylhexahydrophthalic anhydride (MHHPA). And the mechanism of the curing reaction and the phase structure of the liquid crystal epoxy resins were investigated. In addition, dielectric hydrophobic liquid crystal epoxy nanometer composite materials were successfully prepared with functionalized mesoporous silica (SBA-15) as a filler. The results showed that pure LCE4 exhibited excellent dielectric properties and thermal stability. Compared with pure LCE4, the composite material for SBA-15 modified with KH560 displayed lower dielectric constant, lower dielectric loss, higher glass transition temperature, and better hydrophobicity. For example, with a 0.5 wt% SBA-15, the dielectric constant and dielectric loss of the composite material were as low as (2.35, 0.025) compared with pure LCE4 (3.25, 0.036) that was reduced by 24.7% and 31%. In addition, the glass transition temperature and water contact angle are increased by 16 °C and 14°, respectively. Composites also showed good thermal stability and mechanical properties. The reasons may mainly be derived from the internal structure of LCE4, the effective modification of mesoporous SBA-15 by KH560, and the excellent dispersion of organic and inorganic phases.
Graphical abstract

Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of 5G communication technology and the continuous miniaturization and integration of electronic equipment, higher and stricter requirements are put forward for the performance, reliability, and processing technology of advanced electronic packaging materials [1,2,3,4,5,6]. Microelectronic device packaging materials required low dielectric constant, dielectric loss, easy processing, and excellent hydrophobicity [7,8,9,10]. However, most polymers and composites can achieve some of the properties mentioned above [11] but still have high dielectric constant and poor hydrophobicity. Therefore, research on materials with excellent dielectric properties and hydrophobicity is imminent and has become a research hotspot of functional polymer composites [12,13,14,15,16]. As we all know, epoxy resin has a wide range of applications for the fields of coatings, composites, construction, electronics, and electrical due to its excellent comprehensive properties and broad applicability [17,18,19,20]. However, due to the relatively high dielectric constant and dielectric loss, some applications are limited.
Previous studies reported that by introducing structurally ordered mesogenic units into the polymer matrix (the mesogenic unit is the rigid rod part of the liquid crystal epoxy molecule) and then induced by the curing agent to form a tight chemical cross-linked network [21]. Compared with ordinary epoxy resins, liquid crystal epoxy resin (LCE) has more evident advantages in electronic packaging materials, electronic and electrical applications. It is characterized by better thermal stability, mechanical properties, dielectric properties, and hydrophobicity [22,23,24,25]. In recent years, the application of liquid crystal epoxy resin in electronic packaging and electronic and electrical materials has gradually attracted people's attention [26,27,28,29]. Owing to its orderly structure and highly cross-linked network characteristics, it hinders the movement of molecules in an external alternating electric field. At the same time, it also contains many low-polarity groups (C–C, C–H, C–O), which is highly conducive to the preparation of low-dielectric materials.
In addition, the most effective way to prepare low dielectric constant materials is to introduce pore structure into the material to increase its porosity. Owing to the existence of the pore structure, part of the air can be introduced into the pores (the dielectric constant of air is 1), thereby significantly reducing the dielectric constant of the material [30,31,32]. In general, introducing the pore structure into the material is achieved through a foaming agent. The pore size obtained by this method is not uniform, and the thermal and mechanical properties of the prepared composites are easily deteriorated [33, 34]. Many studies have shown that the pore size of inorganic nanoparticles with microporous and mesoporous structures is uniform, and the introduction to an ordered network structure can improve the thermal and mechanical properties of the material [35,36,37,38]. Among them, mesoporous silica (SBA-15) has a wide range of applications because of its uniform pore size, good thermal stability, and biocompatibility, and its surface is easily chemically modified [39, 40]. According to reports, mesoporous SBA-15 has a low dielectric constant, and the texture is chemically modifiable [41, 42]; its hydrophilic surface groups can be changed into hydrophobic groups of modification. The mesoporous SBA-15, after surface modification, is expected to be suitable for preparing polymer composites with excellent dielectric properties and good hydrophobicity.
This work reports a method for preparing organic–inorganic nanocomposites by mixing liquid crystal epoxy resin (LCE4) with surface-functionalized mesoporous silica (SBA-15). First, LCE4 with the flexible chain was synthesized by a two-step synthesis method, and its structure and properties were characterized. Secondly, the surface functionalization of mesoporous SBA-15 was carried out synchronously byγ-glycidoxypropyltrimethoxysilane (KH560) and then uniformly dispersed in the liquid crystal epoxy resin as a low dielectric hydrophobic filler. Finally, corresponding SBA-15/LCE4 composites were prepared by in situ polymerization. The effects of the LCE4 system and different mass fractions of surface-functionalized mesoporous SBA-15 on the dielectric properties, thermal properties, mechanical properties, and hydrophobicity of SBA-15/LCE4 composites were systematically discussed.
Materials and methods
Materials
4,4′-Dihydroxydiphenyl (97%), 6-bromo-1-hexene (95%), potassium carbonate (99%), potassium iodide (99%), 3-chloroperoxybenzoic acid (MCPBA, 85%), methylhexahydrophthalic anhydride (MHHPA, 95%), N,N-dimethylbenzylamine (BDMA, 99%) were purchased from Aladdin; mesoporous silica (SBA-15,95%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd; γ-glycidoxypropyltrimethoxysilane (KH560, 98%) was from Nanjing Shuguang Chemical Group Co., Ltd; N, N-dimethylformamide (99.5%), dichloromethane (99.5%), ethanol anhydrous (99.7%), and acetone were provided by Guangzhou reagent factory; all chemicals were used as they were, and no further purification was required.
Synthesis of liquid crystal epoxy (LCE)
The liquid crystal epoxy resin was prepared by a two-step synthesis method as shown in Scheme 1
Preparation of 4, 4′- bis (5-propenyloxy) biphenyl (L4)
The 4,4′-dihydroxydiphenyl (6 g, 0.032 mol) was dissolved in 150 mL N, N-dimethylformamide, 6-bromo-1-hexene (11 mL, 0.082 mol) was added, potassium carbonate (8.83 g, 0.064 mol) and potassium iodide (0.5 g, 0.003 mol) were mixed well and refluxed at 80 °C under nitrogen atmosphere for 24 h. The mixed solution was evaporated under reduced pressure, filtered, washed, and dried to obtain white powdery crystals, and the yield was 88% (9.8 g), named L4.
1H NMR (400 MHz, CDCl3, ppm): 7.48 (d, 2H, Ar–H), 6.96 (d, 2H, Ar–H), 5.81 (m, 1H, CH=CH2), 5.11–4.87 (d, 2H, CH=CH2), 4.01 (t, 2H, OCH2), 2.12 (m, 2H, CH2–CH=CH2), 1.76 (dd, 2H, OCH2 CH2), 1.56 (m, 2H, CH2).
13C NMR (400 MHz, CDCl3, ppm):158.22, 138.56, 133.42, 127.68, 115.59, 114.78, 67.89, 33.44, 28.78, 25.37.
Preparation of 4,4′-bis(2,3-epoxyhexyloxy) biphenyl (LCE4)
The prepared L4 (7.0 g, 0.02 mol) was dissolved in 180 mL of dichloromethane, added MCPBA (10.38 g, 0.06 mol) in batches, and reacted at 0 °C for 1 h. The reaction mixture was slowly heated to 40 °C and refluxed for 24 h. After the reaction, the crude product was filtered and then washed with 10% Na2SO3 solution, 5% NaHCO3 solution, and deionized water successively. The organic phase was dried with anhydrous MgSO4, and dichloromethane was evaporated under reduced pressure to obtain light yellow solid. Crude product was repeatedly washed by anhydrous ethanol several times to get a white solid, and the yield was 84% (6.4 g) named LCE4. The epoxy value was determined by the hydrochloric acid-acetone method to be 0.52.
1H NMR (400 MHz, CDCl3, ppm): 7.48 (d, 2H, Ar–H), 6.96 (d, 2H, Ar–H), 4.01 (t, 2H, OCH2), 2.95 (s, 1H, CH2 in epoxy), 2.78 (t, 1H, CH2 in epoxy), 2.51 (dd, 1H, CH in epoxy), 1.85 (dd, 2H, OCH2CH2), 1.73–1.55 (m, 4H, CH2CH2).
13C NMR (400 MHz, CDCl3, ppm):158.14, 133.45, 127.69, 114.76, 67.74, 52.17, 47.02, 32.21, 29.10, 22.68.
The curing process of LCE4/MHHPA
LCE4 (5 g, 0.013 mol) was dissolved in 30 mL of acetone, and MHHPA (4.3 g, 0.026 mol) was added for sufficient mechanical stirring, then 1% (relative to the total mass of LCE4 and MHHPA) BDMA as accelerator was added to the mixture, after mixing uniformly, evaporated under reduced pressure, and removed most of the solvent, then cast the mixture into the mold, and removed the solvent again under vacuum. Then, the temperature was raised, and the curing was carried out in stages at 110 °C for 3 h, 140 °C for 5 h, and 170 °C for 2 h.
Surface modification of SBA-15 and preparation of SBA-15/LCE4 composite
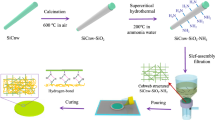
Before surface modification, the mesoporous silica was placed in a vacuum drying oven at 200 °C for 24 h; then, 2 g of activated SBA-15 was dispersed in 30 mL of toluene at room temperature ultrasonically and 1 g of silane coupling agent dropwise and continue ultrasonic dispersion for 1 h. Subsequently, the mixed solution was heated to 80 °C and refluxed for 6 h. After the reaction, the hybrid solution was suction-filtered and washed with toluene several times; finally, silane coupling agent-modified SBA-15 nanoparticles were obtained by drying in a vacuum drying oven. Figure 1 shows a schematic diagram of the modification process of mesoporous SBA-15.
The SBA-15/LCE4 composite was prepared by in situ polymerization. First, under the cutting-edge ultrasonic treatment, the stoichiometric ratio of LCE4, KH560-SBA-15, MHHPA, and BDMA (Table 1) was ultrasonically dispersed in toluene for 1.5 h. Then, the mixture was mixed with a Thinky Mixer (ARE250. Thinky) at 5000r/min stir at a rate of 1 h; after removing most of the solvent, we cast the mixture into a mold and removed the solvent again under vacuum. Then, the temperature was raised, and the curing was carried out in stages at 110 °C for 3 h, 140 °C for 5 h, and 170 °C for 2 h. Figure 1 shows the schematic diagram of the preparation process of the composites.
Characterization
Fourier transform infrared (FT-IR) spectroscopy was recorded on a German Bruker TENSOR 27 Fourier transform infrared spectrometer. The sample was mixed with KBr powder and pressed into a thin sheet. The measured spectrum range was 4000 to 400 cm−1.
1H NMR and 13C NMR spectra were recorded on a BRUKER AVANCE 400 spectrometer at room temperature, and the samples use CDCl3 as a solvent.
A polarized light optical microscopy (Orthoglan, LEITZ, Germany with hot stage and temperature controller) was used to investigate the liquid crystal phase structure of the LCE4. A small amount of the mixture of LCE4, MHHPA, and BDMA was placed on a glass slide, and then, the glass slide was placed on a hot stage to increase the temperature for curing, and the formation of the liquid crystal phase was studied.
The crystal structure of the cured resins was studied with wide-angle X-ray diffraction (WAXD). The diffraction patterns were recorded with a Rigaku diffractometer (D/MAX-1200), using monochromatic CuKα radiation (40 kV, 30 mA), and the scanning speed was 4°/min.
Fully automatic specific surface and porosity analyzer (BET, Mike 2460) were used to measure the nitrogen adsorption–desorption isotherm under a nitrogen atmosphere to obtain the nanopore size and specific surface area of the SBA-15 before and after modification were obtained. Before the test, the SBA-15 particles were preheated at 200 °C for 24 h.
Scanning electron microscope was used to observe the dispersibility of mesoporous SBA-15 in the composites.
A broadband dielectric spectrometer (Agilent 4294 A) was used to study the dielectric properties of the composites. The temperature was 25 °C, the frequency was 1 Hz to 1 MHz, and the sample size was 15 mm × 15 mm × 1 mm.
Differential scanning calorimetry (DSC) was examined via a TA Instrument NETZSCH DSC 200F3 to acquire glass transition (pure LCE4 and SBA-15/LCE4 composites). The measurements were heated from 25 to 250 °C with heating and cooling rates of 10 °C/min.
In the tensile mode, a dynamic thermomechanical analyzer (DMA, TA-Q800) was used to measure the dynamic thermomechanical properties. A sample with a size of 30 mm × 5 mm × 2 mm was scanned from 25 to 300 °C, the heating rate was 5 °C/min, the amplitude was 25 μm, and the frequency was 1 Hz.
The thermal stability of the composites was analyzed by a thermogravimetric analyzer (PE TGA8000, USA), and the test was conducted under nitrogen at a heating rate of 10 °C /min from 30 to 800 °C.
A universal testing machine (SHT5000 Shenzhen SANS testing machine) was used to conduct a tensile test on the composites, and the strain rate was tested at a rate of 3 mm/min.
A contact angle measuring instrument (Shanghai Zhongchen Digital Technology Equipment Co., Ltd. JC2000C type) was used to test the water contact angle of the composites.
The water absorption test was measured by the gravimetric method. A 15 mm × 15 mm × 1 mm sample was polished and dried in a vacuum oven at a constant temperature of 100 °C for one week. Then, it was soaked in deionized water. During the period, it was regularly eliminated from the water and wiped dry with a paper towel, then weighed it on an electronic balance of 1/10000, repeated the test for each sample three times, and calculated the average value.
Results and discussion
Synthesis and characterization of liquid crystal epoxy resin
Using 4,4′-dihydroxy biphenyl and 6-bromo-1-hexene as basic raw materials, a liquid crystal epoxy resin matrix with flexible chains was prepared by a two-step synthesis method. Previous studies had reported that introducing a flexible spacer between the mesogenic nucleus and the reactive group could effectively reduce the melting point of LCE4 and improve the stability of the mesophase [43,44,45,46,47,48]. It was worth mentioning that LCE4 was prepared by epoxidation of olefins. Usually, the reaction took about a week at room temperature. In this study, two methods of ice-water bath and heating reflux were used in the preparation process. It only took a day to complete. FT-IR and NMR confirmed the successful preparation of a high-purity LCE4 matrix. It could be obtained from Fig. 2a that the characteristic absorption peak of phenol –OH at 3362 cm−1 disappeared before and after the 4,4′-dihydroxy biphenyl reaction, and new ones appeared 2939 cm−1 and 2873 cm−1. The absorption peak of –CH2– and the appearance of a new absorption peak of –C=C– near 1642 cm−1 could prove the formation of a further monomer L4. In addition, the absorption peak of –C=C– to 1642 cm−1 disappeared after the L4 monomer was oxidized, which can be seen from Fig. 2b. Similarly, the chemical shift of H and the chemical change of C at each position in 1H NMR and 13C NMR could be a one-to-one correspondence. The above results fully proved the successful preparation of the liquid crystal epoxy resin matrix (LCE4).
The liquid crystal phase structure and curing mechanism of LCE4/MHHPA
In this study, biphenyl is invoked as the liquid crystal rigid unit, combined with the appropriate flexible segment main chain, the perfect combination of rigidity and flexibility, giving LCE4 unique liquid crystal properties [27]. POM and XRD analyzed the liquid crystal phase structure of LCE4. The polarizing microscope images of cured LCE4/MHHPA are shown in Fig. 3, and birefringence could be observed in all POM images. With the extension of curing time, the density of liquid crystal microdomains could be observed to increase gradually, indicating the existence of the liquid crystal phase, which was mainly due to the interaction between polarized light and the highly ordered structure of liquid crystal molecules. Figure 3f shows that the liquid crystal microdomain density is highest when the curing time was 180 min. At the same time, the “schlieren-like” structure could be observed in all POM images.
The room-temperature wide-angle X-ray diffraction (WAXD) pattern of LCE4 cured with MHHPA at 140 °C is shown in Fig. 4b. It offered a robust and broad peak at 2θ = 19.8°. The interplanar spacing d = 4.49 Å was calculated by the Bragg equation. The diffraction peak at this position represented the nematic structure in LCE4, corresponding to the lateral stacking of liquid crystal molecules [49]. The curing mechanism of LCE4 is shown in Fig. 4a, including early linear chain extension, then branching, and finally cross-linking, which may have a significant impact on the mesogenic orientation and structure of the liquid crystal epoxy resin [21]. Figure 4c shows the uniaxial orientation and nematic structure model of LCE4, which was more helpful in understanding mesogenic units’ direction. Based on the orderly and highly cross-linked characteristics of LCE4, its application prospects will be more and more extensive.
Surface modification and characterization of mesoporous silica SBA-15
In this study, silane coupling agent KH560 was used to modify the surface of mesoporous SBA-15. The whole modification process was mainly divided into three steps: firstly, KH560 was hydrolyzed to produce Si–OH. Secondly, the Si–OH obtained by the hydrolysis and the -OH on the surface of the mesoporous SBA-15 was dehydrated to form an oligosiloxane. Finally, in the process of heating and dehydration, KH560 was combined with the character of mesoporous SBA-15 in the form of covalent bonds to complete the modification of mesoporous SBA-15. Through chemical modification, the inorganic phase and the organic phase could be closely combined, which significantly enhanced the interfacial adhesion and improved the material’s performance [50, 51]. Figure 5a shows the FTIR image of SBA-15 before and after modification. As could be seen from the figure, the characteristic absorption peak of mesoporous SBA-15 at 3425 cm−1 was the stretching vibration of Si–OH. After being modified by KH560, it widened, indicating that the Si–OH of SBA-15 nanoparticles reacts chemically with the alkoxy group in KH560. In addition, a new absorption peak appeared at 2912 cm−1 after modification of mesoporous SBA-15, which could be attributed to the symmetric stretching vibration of –CH2–. Surprisingly, however, no characteristic absorption peak was observed near 910 cm−1 for the epoxy group, possibly because the peak was covered by the strong absorption peak of the Si–O–Si antisymmetric stretching vibration at 1094 cm−1. Figure 5b shows the thermal weight loss analysis of the SBA-15 nanoparticles before and after modification. It could be seen from the figure that the KH560-modified SBA-15 nanoparticles lose weight with the increased of temperature, and the weight loss rate was about 10%. In contrast, for the unmodified SBA-15 nanoparticles the mesoporous SBA-15 has not changed.
Figure 5c and d shows the BJH pore size distribution diagram and N2 adsorption–desorption isotherm of mesoporous SBA-15 measured by the automatic specific surface area and porosity analyzer at 77 K. It could be seen from the figure that according to the classification of IUPAC mesoporous SBA-15 was behaved as a typical Langmuir IV isotherm with a clear H1 hysteresis loop, indicating that it had the distinctive characteristics of a porous material. At the same time, it showed that the modification did not destroy its pore structure [52,53,54]. The changes in specific surface area, pore volume, and pore diameter of mesoporous SBA-15 before and after modification are shown in Table 2.
Through characterization methods such as FT-IR, TGA, BET, and BJH pore size distribution, it could be seen that the characteristic absorption peak, thermal weight loss, specific surface area, and pore size of Si–OH before and after the modification had changed significantly, indicating that KH560 affects mesoporous SBA-15 the modification of was triumphant.
Scanning electron microscope analysis of SBA-15/LCE4 composite
The SEM images of LCE4 and SBA-15/LCE4 composites are illustrated in Fig. 6; the dispersibility of mesoporous SBA-15 in LCE4 varies with the amount of addition. When the addition amount of mesoporous SBA-15 was 0.5 wt%, the dispersibility of mesoporous SBA-15 and LCE4 was good, and the result is shown in Fig. 6b. To further verify the excellent dispersibility of mesoporous SBA-15 and LCE4 when the additional amount is 0.5 wt%, we had done EDS element analysis to illustrate it. The results are shown in Fig. 6d, e, and f; C, N, O, Si, and other elements all showed excellent dispersibility, which confirmed that the mesoporous SBA-15 and LCE4 could be dispersed well. However, when the amount of mesoporous SBA-15 added was 2.0 wt%, there was some agglomeration of mesoporous SBA-15 in the composite, and the result is shown in Fig. 6c.
Dielectric properties and potential applications of SBA-15/LCE4 composite
Low dielectric constant (Dk) and low dielectric loss (Df) epoxy resins and their composites are expected to play an increasingly important role in high-performance microelectronic devices and electronic packaging materials [21]. Therefore, new epoxy resins and composites with low dielectric constant and low dielectric loss were desirable. In this study, LCE4 with a liquid crystal phase structure was prepared and used as a resin matrix to mix with modified mesoporous SBA-15 nanoparticles to prepare SBA-15/LCE4 composites. The effect of the addition of mesoporous SBA-15 on the broadband dielectric properties of the composites is shown in Fig. 7.
Figure 7a and b shows the dielectric constant and dielectric loss of pure LCE4 and the composites at 25 °C and frequencies from 1 Hz to 1 MHz. It can be seen from Fig. 7a and b that the dielectric constant and dielectric loss of pure LCE4 at high frequency were 3.12 and 0.036. However, what is exciting is that the dielectric constant and dielectric loss of the composites gradually decrease with the increase in mesoporous SBA-15 content. When the addition amount of mesoporous SBA-15 was 0.5 wt%, the dielectric constant and dielectric loss of the composites were reduced to the lowest of about 2.35 and 0.025, which were decreased, respectively, compared with pure LCE4 24.7% and 31%. The reason for the significant decrease in the dielectric constant and dielectric loss was mainly due to the internal structure of LCE4 and mesoporous SBA-15, as well as the dispersibility of mesoporous SBA-15 in composites.
The order of the orientation structure and the gradual increase in the packing density of the mesogenic unit in LCE4 can effectively hinder the movement of molecular chains in the applied alternating electric field and reduce the dielectric constant and dielectric loss of the composite. In addition, since air has a low dielectric constant (DK≈1), air voids could be used to effectively reduce the dielectric constant of composites [55, 56]. In this work, the mesoporous SBA-15 has a large pore size. A part of the air could be introduced into the mesopores during the preparation of the composites, thereby significantly reducing the dielectric constant. On the other hand, the dispersibility of the KH560-modified mesoporous SBA-15 in the composites was improved so that the inorganic phase and the organic phase were tightly combined, which enhanced the interfacial adhesion and further improved the dielectric performance. However, with increasing content of mesopore SBA-15, the dielectric constant and dielectric loss of the composite showed a growing trend when the additional amount was 1.5 wt% and 2.0 wt%, compared with that of pure LCE4. The reason could be that with the increase of mesoporous SBA-15 content, the dispersity of mesoporous SBA-15 in the composites gradually becomes worse and finally leaded to the agglomeration of mesoporous SBA-15 nanoparticles. Previous studies had shown that adding too much mesoporous SBA-15 will cause aggregation and poor dispersion, reducing the air volume [37]. Thus, the dielectric constant and dielectric loss are increased. Therefore, when the additional amount of mesoporous SBA-15 was 0.5 wt%, the effect of reducing the dielectric constant and the dielectric loss of the composites was significant.
Hydrophobicity of SBA-15/LCE4 composite
The water contact angle of the prepared samples was measured to study the wettability of LCE4 and SBA-15/LCE4 composites. As shown in Fig. 8a, the contact angle of pure LCE4 before adding SBA-15 was 82°. Meanwhile, compared with LCE4, the composites were more hydrophobic. When the addition amount of SBA-15 was 0.1 wt%, 0.5 wt%, 1.0 wt%, 1.5 wt%, and 2.0 wt%, the water contact angles of the composite were 90.2°, 96°, 94.6°, 90.8°, 85.4°. This was primarily attributed to the hydrophilic group (–OH) on the surface of SBA-15 changed into the hydrophobic group after KH560 modification and the dispersion difference of SBA-15. As shown in Fig. 8a, with the increased of SBA-15 content, the water contact angle increased and then decreased. When the content of SBA-15 increased gradually, more SBA-15 aggregates would occur, leading to uneven dispersion of the composites, resulting in poor compatibility and increased porosity between SBA-15 and LCE4. Therefore, when the additional amount of SBA-15 exceeded 0.5 wt%, the water contact angle showed a decreasing trend.
Figure 8b shows the water absorption capacity of LCE4 and the composites. In this study, the water absorption of the prepared sample was evaluated according to the weight difference between LCE4 and the composites before and after soaking in deionized water. When different samples were soaked at room temperature for 24 h, the water absorption rate of pure LCE4 was 0.54%, and the addition amount was 0.1 wt %, 0.5 wt %, 1.0 wt %, 1.5 wt %, and 2.0 wt %. The water absorption rates of the composites were 0.34%, 0.28%, 0.38%, 0.46%, and 0.51%, respectively. Under the condition of the low additional amount, SBA-15 and LCE4 were uniformly distributed in the resin matrix to form a compact inorganic–organic hybrid composites, which hindered the penetration of oxygen and water to a certain extent water absorption. However, with the increase of SBA-15 addition, SBA-15 may aggregate and lead to more porosity. Therefore, low addition of SBA-15 could effectively reduce the water absorption of the composites.
Thermomechanical properties of SBA-15/LCE4 composite
Glass transition temperature (Tg) is an important performance index used to measure thermoset materials. In this study, DSC and DMA were used to characterize the Tg of SBA-15/LCE4 composites, as shown in Fig. 9 and Fig. 10. The datum is summarized in Table 3. Obviously, with the addition of mesoporous SBA-15, the Tg of the composites changed. It was worth noting that the Tg of the composite increased by 16 °C compared with that of pure LCE4 when the addition amount was 0.5 wt%, reaching a maximum of 101 °C. However, with the increase of SBA-15, the Tg of the composites decreased. The change of Tg of the composite was related to the pore structure of SBA-15 and the cross-link density of the composite. With the increase of mesoporous SBA-15 content, the cross-linking density of the composites increased slightly. (The cross-linking density is calculated by Eq. 1.) At the same time, the pore structure of SBA-15 restricted the movement of molecular chains, increasing Tg. In addition, the Tg of the composites was closely related to the dispersion between SBA-15 and LCE4. Under the condition of low addition, the homogeneous dispersion of SBA-15 and LCE4 enhanced the interaction force between inorganic and organic phases, which further improved the Tg. Unfortunately, when the additional amount of SBA-15 exceeded 1.0 wt%, a slight agglomeration phenomenon occurred between SBA-15 and LCE4, weakening the interaction force between the inorganic and organic phases, which results in a decrease in Tg.
where \({E}^{^{\prime}}r\) represents the rubber platform modulus at Tg + 30 °C, R represents the gas mole constant, Tr represents the Kelvin temperature at Tg + 30 °C.
The thermal decomposition behavior of LCE4 and SBA-15/LCE4 composites was compared by TGA in the temperature range of 50–800 °C. The specific data are given in Fig. 11 and Table 3. It could be seen from the figure that LCE4 and the composites degraded through one step, indicating that there was a good phase connection between mesoporous SBA-15 and LCE4 matrix, and KH560 was successfully grafted to the surface of mesoporous SBA-15 and that water and other volatile substances were not present in the composite [57, 58]. It can be seen from Table 3 that with the gradual increase in the addition of mesoporous SBA-15, the thermal decomposition temperatures of the composites in T10% and T50% were significantly increased compared with that of pure LCE4, which proved that the addition of mesoporous SBA-15 into LCE4 could improve the thermal stability of composites. The improvement of thermal stability could be attributed to the good thermal stability of LCE4 and mesoporous SBA-15. In addition, the most critical factor was that LCE4 and the modified mesoporous SBA-15 form a close cross-linking network under the action of a curing agent, which enhanced the interaction.
Figure 12 shows the mechanical properties of the prepared LCE4 and SBA-15/LCE4 composites, including tensile strength (Fig. 12a), tensile modulus (Fig. 12b), elongation at break (Fig. 12c), and stress–strain curves (Fig. 12d); the specific values are shown in Table 4. Interestingly, it can be seen from Fig. 12a that as the mass fraction of mesoporous SBA-15 increased from 0 wt% (49.35 MPa) to 0.5 wt% (55.44 MPa), the tensile strength of the SBA-15/LCE4 composite increased. However, with the gradual increase of mesoporous SBA-15 content of 1.0 wt% (51.62 MPa), 1.5 wt% (50.63 MPa), 2.0 wt% (49.17 MPa), the tensile strength of SBA-15/LCE4 composites decreased. This phenomenon originated from the strong interaction between mesoporous SBA-15 and LCE4 evenly dispersed on the one hand and with the appropriate affinity and chemical interaction between SBA-15 and LCE4 on the other hand. Compared with the 0.5-SBA-15/LCE4 composites, the tensile strength decreased when the addition amount was 1.0 wt%, 1.5 wt%, and 2.0 wt%, which could be attributed to the slight agglomeration phenomenon in the mesopore SBA-15 of LCE4 matrix with the increase of the addition amount of mesopore SBA-15. Most importantly, the elongation at break of the 0.5- SBA-15/LCE4 composite was 5.07%, increasing 33.1% compared with pure LCE4.
Conclusions
In this work, a LCE4 consisting of flexible chain and rigid mesogenic units was prepared and thermally cured by MHHPA. The mechanism of the curing reaction and the liquid crystal phase structure was studied. The results showed that with the progress of the cross-linking and curing reaction, a nematic liquid crystal with a "schlieren-like" structure was observed, and the microdomain density of the liquid crystal gradually increased. Due to the introduction of mesogenic elements, the dielectric constant and dielectric loss of the prepared LCE4/MHHPA samples were as low as 3.25 and 0.036, which were significantly better than ordinary epoxy resins. Even compared with the liquid crystal epoxy resin systems studied by Zhang et al. [24], Liu et al. [25], Guo et al. [59], they all have different degrees of reduction. Secondly, the mesoporous SBA-15 was surface-functionalized with KH560, and then, it was used as a low-dielectric hydrophobic filler uniformly dispersed in the liquid crystal epoxy resin to prepare the corresponding SBA-15/LCE4 composites. The results showed that KH560 successfully modified the surface of mesoporous SBA-15, and the composites exhibited better dielectric, thermal, mechanical, and hydrophobic properties than pure LCE4. Specifically, when the additional amount of mesopore SBA-15 was 0.5 wt%, the dielectric constant and dielectric loss of the composites were 2.35 and 0.025, which decreased 24.7% and 31%, respectively, compared with pure LCE4. The Tg of the composite was 101 °C, which was 16 °C higher of pure LCE4. In addition, the introduction of mesoporous SBA-15 made the hydrophobicity, thermal stability, and mechanical properties of the composites significantly improved compared with pure LCE4, which makes it have a broad application prospect in electronic packaging, electrical, and electronic fields.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
References
Feng CP, Chen L, Wei F, Ni HY, Chen J, Yang W (2016) Highly thermally conductive UHMWPE/graphite composites with segregated structures. RSC Adv 6(70):65709–65713
Kim K, Kim M, Kim J (2014) Thermal and mechanical properties of epoxy composites with a binary particle filler system consisting of aggregated and whisker type boron nitride particles. Compos Sci Technol 103:72–77
Luo T, Lloyd JR (2012) Enhancement of thermal energy transport across graphene/graphite and polymer interfaces: a molecular dynamics study. Adv Func Mater 22(12):2495–2502
Hopkins PE, Baraket M, Barnat EV, Beechem TE, Kearney SP, Duda JC, Robinson JT, Walton SG (2012) Manipulating thermal conductance at metal-graphene contacts via chemical functionalization. Nano Lett 12(2):590–595
Taha-Tijerina J, Narayanan TN, Gao G, Rohde M, Tsentalovich DA, Pasquali M, Ajayan PM (2012) Electrically insulating thermal nano-oils using 2D fillers. ACS Nano 6(2):1214–1220
Liu Y, Qian C, Qu L, Wu Y, Zhang Y, Wu X, Zou B, Chen W, Chen Z, Chi Z, Liu S, Chen X, Xu J (2015) A bulk dielectric polymer film with intrinsic ultralow dielectric constant and outstanding comprehensive properties. Chem Mater 27(19):6543–6549
Huang X, Iizuka T, Jiang P, Ohki Y, Tanaka T (2012) Role of interface on the thermal conductivity of highly filled dielectric epoxy/AlN composites. J Phys Chem C 116(25):13629–13639
Jiang Q, Zhang W, Hao J, Wei Y, Mu J, Jiang Z (2015) A unique “cage-cage’’’ shaped hydrophobic fluoropolymer film derived from a novel double-decker structural POSS with a low dielectric constant.” J Mater Chem C 3(44):11729–11734
Tousignant MN, Rice NA, Niskanen J, Richard CM, Ritaine D, Adronov A, Lessard BH (2021) High performance organic electronic devices based on a green hybrid dielectric. Adv Electron Mater. https://doi.org/10.1002/aelm.202100700
Wu B, Liu H, Fu R, Song X, Su X, Liu X (2021) Epoxy-matrix composite with low dielectric constant and high thermal conductivity fabricated by HGMs/Al2O3 co-continuous skeleton. J Alloys Compd 869:159332. https://doi.org/10.1016/j.jallcom.2021.159332
Xu X, Hu R, Chen M, Dong J, Xiao B, Wang Q, Wang H (2020) 3D boron nitride foam filled epoxy composites with significantly enhanced thermal conductivity by a facial and scalable approach. Chem Eng J 397:125447. https://doi.org/10.1016/j.cej.2020.125447
Zhang P, Zhao J, Zhang K, Bai R, Wang Y, Hua C, Wu Y, Liu X, Xu H, Li Y (2016) Fluorographene/polyimide composite films: Mechanical, electrical, hydrophobic, thermal and low dielectric properties. Compos Part a-Appl Sci Manuf 84:428–434
Wahab MA, Chaobin H, Altalhi T, Albaqami MD, Alothman ZA, Haque R (2021) Nanopore engineered tortuosity towards thermo-mechanically enhanced low-k polymer-mesoporous organosilica composite membranes. Compos Sci Technol 211:108854. https://doi.org/10.1016/j.compscitech.2021.108854
Wang L, Bai Z, Liu C, Wei R, Liu X (2021) Porous fluorinated polyarylene ether nitrile as ultralow permittivity dielectrics used under humid environment. J Mater Chem C 9(3):860–868
Liu X, Yue D, Yang C, Li N, Gao S, Liu Y, Mo G, Wu Z, Yin J, Su B, Li L (2019) Fluorinated carbon nanofiber/polyimide composites: Electrical, mechanical, and hydrophobic properties. Surf Coat Technol 361:206–211
Yu Z, Wu S, Li C, Xiao Y, Zheng L, Liu J, Zhang B (2021) Ultra-low dielectric constant fluorinated graphene/polybenzoxazole composite films with excellent thermal stabilities and mechanical properties. Compos Part a-Appl Sci Manuf 145:106387. https://doi.org/10.1016/j.compositesa.2021.106387
Chrusciel JJ, Lesniak E (2015) Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog Polym Sci 41:67–121
Pan Y-T, Zhang L, Zhao X, Wang D-Y (2017) Interfacial engineering of renewable metal organic framework derived honeycomb-like nanoporous aluminum hydroxide with tunable porosity. Chem Sci 8(5):3399–3409
Jiang S-D, Tang G, Chen J, Huang Z-Q, Hu Y (2018) Biobased polyelectrolyte multilayer-coated hollow mesoporous silica as a green flame retardant for epoxy resin. J Hazard Mater 342:689–697
Xu Y-J, Wang J, Tan Y, Qi M, Chen L, Wang Y-Z (2018) A novel and feasible approach for one-pack flame-retardant epoxy resin with long pot life and fast curing. Chem Eng J 337:30–39
Guo H, Lu M, Liang L, Wu K, Ma D, Xue W (2017) Liquid crystalline epoxies with lateral substituents showing a low dielectric constant and high thermal conductivity. J Electron Mater 46(2):982–991. https://doi.org/10.1007/s11664-016-5003-6
Carfagna C, Amendola E, Giamberini M (1994) Rigid-rod networks - liquid-crystalline epoxy-resins. Compos Struct 27(1–2):37–43
Li Y, Badrinarayanan P, Kessler MR (2013) Liquid crystalline epoxy resin based on biphenyl mesogen: Thermal characterization. Polymer 54(12):3017–3025
Zhang X, Gu A, Liang G, Zhuo D, Yuan L (2011) Liquid crystalline epoxy resin modified cyanate ester for high performance electronic packaging. J Polym Res 18(6):1441–1450
Liu Z, Yuan L, Liang G, Gu A (2015) Tough epoxy/cyanate ester resins with improved thermal stability, lower dielectric constant and loss based on unique hyperbranched polysiloxane liquid crystalline. Polym Adv Technol 26(12):1608–1618
Y Li, V Ambrogi, P Cerruti, M Goswami, Z Yang, MR Kessler, O Rios (2021) Functional liquid crystalline epoxy networks and composites: from materials design to applications, Int Mater Rev
Yang X, Zhong X, Zhang J, Gu J (2021) Intrinsic high thermal conductive liquid crystal epoxy film simultaneously combining with excellent intrinsic self-healing performance. J Mater Sci Technol 68:209–215
Hong Y, Goh M (2021) Advances in liquid crystalline epoxy resins for high thermal conductivity. Polymers 13(8):1302. https://doi.org/10.3390/polym13081302
Tian K, Yang S, Niu J, Wang H (2021) Enhanced thermal conductivity and mechanical toughness of the epoxy resin by incorporation of mesogens without nanofillers. Ieee Access 9:31575–31580
Kim Y, Yoo S, Lee HG, Won Y, Choi J, Kang K (2021) Structural analysis of silica aerogels for the interlayer dielectric in semiconductor devices. Ceram Int 47(21):29722–29729
Nam J-Y, Kim H-K, Song Y-S (2021) Fabrication and analysis of sepiolite/glass microcapsules/liquid crystal polymer composites. Molecules 26(9):2522. https://doi.org/10.3390/molecules26092522.
Shwaykani H, El-Hajj A, Costantine J, Al-Husseini M (2021) A calibration-free method for the dielectric constant calculation of low-loss materials. Ieee Trans Instrum Meas 70:1–10. https://doi.org/10.1109/TIM.2020.3011765
Zhang J, Xu R, Yu D (2007) A novel poly-benzoxazinyl functionalized polyhedral oligomeric silsesquioxane and its nanocomposite with polybenzoxazine. Eur Polymer J 43(3):743–752
Du W, Shan J, Wu Y, Xu R, Yu D (2010) Preparation and characterization of polybenzoxazine/trisilanol polyhedral oligomeric silsesquioxanes composites. Mater Des 31(4):1720–1725
Yang PD, Zhao DY, Margolese DI, Chmelka BF, Stucky GD (1998) Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 396(6707):152–155
Park I, Peng HG, Gidley DW, Xue SQ, Pinnavaia TJ (2006) Epoxy-silica mesocomposites with enhanced tensile properties and oxygen permeability. Chem Mater 18(3):650–656
Lin J, Wang X (2008) Preparation, microstructure, and properties of novel low-kappa brominated epoxy/mesoporous silica composites. Eur Polymer J 44(5):1414–1427
Purushothaman R (2015) Amine functionalized SBA-15/terpolyimide composites with low dielectric constant. J Porous Mater 22(3):585–594
Hukkamaki J, Suvanto S, Suvanto M, Pakkanen TT (2004) Influence of the pore structure of MCM-41 and SBA-15 silica fibers on atomic layer chemical vapor deposition of Cobalt carbonyl. Langmuir 20(23):10288–10295
Yao J, Sheng M, Bai S, Su H, Shang H, Deng H, Sun J (2021) Ionic Liquids grafted mesoporous silica for chemical fixation of CO2 to cyclic carbonate: morphology effect. Catal Lett. https://doi.org/10.1007/s10562-021-03667-9
Lu Y, Lin Q, Ren W, Zhang Y (2015) Investigation on the preparation and properties of low-dielectric ethylene-vinyl acetate rubber/mesoporous silica composites. J Polym Res 22(4). https://doi.org/10.1007/s10965-015-0694-6
Lasmi S, Zoukrami F, Antonio Marcos-Fernandez A, Guerba H (2020) Influence of modified mesoporous silica SBA-15 and compatibilizer on the properties and structure of ethylene-vinyl acetate copolymer-based nanocomposites. Polym-Plast Technol Mater 59(18):2003–2017
Jahromi S, Kuipers WAG, Norder B, Mijs WJ (1995) Liquid-crystalline epoxide thermosets - dynamic-mechanical and thermal-properties. Macromolecules 28(7):2201–2211
Mormann W, Broche M, Schwarz P (1997) Mesogenic azomethine based diepoxides - monomers for the synthesis of “liquid crystal” thermoset networks. Macromol Chem Phys 198(11):3615–3626
Lee JY, Jang JS (1998) Effect of substituents on the curing of liquid crystalline epoxy resin. J Polym Sci Part a-Polym Chem 36(6):911–917
Ortiz C, Wagner M, Bhargava N, Ober CK, Kramer EJ (1998) Deformation of a polydomain, smectic liquid crystalline elastomer. Macromolecules 31(24):8531–8539
Shiota A, Ober CK (1998) Smectic networks obtained from twin LC epoxy monomers - Mechanical deformation of the smectic networks. J Polym Sci Part B-Polym Phys 36(1):31–38
Ribera D, Mantecon A, Serra A (2001) Synthesis and crosslinking of a series of dimeric liquid crystalline epoxy resins containing imine mesogens. Macromol Chem Phys 202(9):1658–1671
Zhang Q, Chen G, Wu K, Shi J, Liang L, Lu M (2020) Biphenyl liquid crystal epoxy containing flexible chain: Synthesis and thermal properties. J Appl Polym Sci 137(38):49143. https://doi.org/10.1002/app.49143
Luo T, Wei X, Luo S, Li H, Li W (2013) Study of Surface Modification of Al2O3 Nanoparticles with KH-560. Asian J Chem 25(12):6777–6779
Zheng W, Tang C, Xie J, Gui Y (2019) Micro-scale effects of nano-SiO2 modification with silane coupling agents on the cellulose/nano-SiO2 interface. Nanotechnology 30(44):445701. https://doi.org/10.1088/1361-6528/ab3546
Kruk M, Jaroniec M (2001) Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem Mater 13(10):3169–3183
Ma J, Liu Q, Chen D, Wen S, Wang T (2015) Synthesis and characterisation of pore-expanded mesoporous silica materials. Micro Nano Letters 10(2):140–144
Luo P, Xu M, Wang S, Xu Y (2017) Structural Dynamic Mechanical and Dielectric Properties of Mesoporous Silica/Epoxy Resin Nanocomposites. Ieee Trans Dielectr Electr Insul 24(3):1685–1697
Jin C, Lin S, Wetzel JT (2001) Evaluation of ultra-low-k dielectric materials for advanced interconnects. J Electron Mater 30(4):284–289
Zhao X-Y, Liu H-J (2010) Review of polymer materials with low dielectric constant. Polym Int 59(5):597–606
Lin J, Wang X (2008) New type of low-dielectric composites based on o-cresol novolac epoxy resin and mesoporous silicas: fabrication and performances. J Mater Sci 43(13):4455–4465. https://doi.org/10.1177/0954008313511347
Prabunathan P, Sethuraman K, Alagar M (2014) Development of bio-based F-SBA-15 reinforced epoxy nanocomposites for low-k dielectric applications. High Perform Polym 26(3):283–289
Guo H, Zheng J, Gan J, Liang L, Wu K, Lu M (2015) Relationship between crosslinking structure and low dielectric constant of hydrophobic epoxies based on substituted biphenyl mesogenic units. RSC Adv 5(107):88014–88020
Acknowledgements
The study was supported by the Guangzhou Science and Technology Program Key Projects (No. 201904010244). The authors would like to thank Huang Zhenyun from shiyanjia laboratory for support of DSC and BET analysis (www.Shiyanjia.com).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Maude Jimenez.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, Z., Liu, X., Zhang, W. et al. Functionalized mesoporous silica liquid crystal epoxy resin composite: an ideal low-dielectric hydrophobic material. J Mater Sci 57, 1156–1173 (2022). https://doi.org/10.1007/s10853-021-06672-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06672-w