Abstract
Background
The two middle contacts of directional leads (d-leads) for deep brain stimulation are split into three segments, allowing current steering toward desired axial directions. To facilitate programming, their final orientation needs to be reliably determined. However, it is currently unclear whether d-leads rotate after implantation. Our objective was to assess the degree of d-lead rotation after implantation.
Methods
We retrospectively analyzed d-lead orientation on intraoperative X-rays, postoperative CT scans (latencies to surgery: 108–189 min postoperatively), and rotational fluoroscopies (4–9 days postoperatively) for a consecutive series of 32 implanted d-leads. For five d-leads, a CT scan with a mean follow-up of 57 days (range 28–182) was available. All d-leads were implanted with the marker facing anterior and the intention to hit an “iron sight” (ISi) on the X-ray, indicating anterior orientation (i.e., 0° ± 6°).
Results
In nine d-leads, an ISi was visible on the final X-ray; median orientation was 1.5° (range 0.5–6.0°) at the first follow-up CT, confirming anterior orientation. In d-leads without ISi or where ISi was not evaluable, the median rotation was 15.5° (9.5–35.0°) and 26.5° (5.5-62.0°), respectively. The orientation of the initial CT was comparable with the orientation determined by the postoperative rotational fluoroscopy and second CT in all d-lead groups.
Conclusion
D-lead orientation does not change within the first week after implantation. We provide first indications that d-lead orientation remains stable for several weeks after surgery. Determination of lead orientation using marker-based X-ray alone seems too imprecise; adding the ISi method can increase determination of intraoperative orientation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Directional leads (d-leads) are a recent advance in deep brain stimulation (DBS). The two middle contact rows of d-leads are split into three segments, which allow steering of the electrical current into desired axial directions [6]. Recent studies indicate favorable therapeutic effects along with reduced delivery of electrical power to the tissue and lower side-effect thresholds compared with standard ring electrodes [1, 2, 9, 10], but long-term clinical benefit of directional leads will need to be investigated in future studies. The additional steering options available, however, are associated with increased programming complexity and thus higher expenditure of time spent in clinical routine [11]. To facilitate and improve programming, knowledge of the final orientation of the d-leads is essential, especially when steering options are used in patients with a narrow therapeutic window or when image-guided programming software that integrates d-lead orientation is used.
To determine d-lead orientation, several intra- and postoperative methods have been described, including stereotactic X-ray, flat-panel computed tomography (CT), standard CT, or rotational fluoroscopy (RF) [4, 5, 7, 8]. However, it is currently unclear whether d-leads rotate after implantation. The first clinical study to address this issue showed large deviations from the time point of the intraoperatively intended anterior lead orientation to the first postoperative CT scan. More than one-third of their d-leads had rotated by > 30°, with maximal rotation angles seen up to 90° [3]. Ongoing lead rotation due to internal torsion and difficulties in manually adjusting d-lead orientation were considered as possible explanations for their observations [3].
It is of paramount importance to establish if and for how long d-leads continue to rotate. This information supports clinicians in the decision when to determine the d-lead orientation and when it is feasible to establish steering programs with long-term sustainability. If d-leads were found to rotate within the first hours or days after surgery, an additional imaging-based assessment of the final lead orientation would be required at a later time point. If there was no further rotation, images at any time point after implantation could be used to determine d-lead orientation. To date, evidence-based recommendations in this regard are lacking.
The aim of our study was to ascertain whether there is ongoing rotation of d-leads within the first week after implantation by comparing images at different time points.
Materials and methods
Study cohort and study design
This was a retrospective data analysis. All patients who underwent DBS surgery with d-leads between September 2019 and February 2020 at our institution with available radiological data were included. All available intraoperative X-rays, postoperative CT scans, and postoperative RFs were analyzed.
The d-lead orientation was determined at four different time points: intraoperatively by the means of X-ray, at the first postoperative CT scan (latencies after X-ray, 108–189 min) and at the RF (median latency after surgery, 4 days (range 4–9)). In a subgroup of d-leads (n = 5), a CT scan was acquired several weeks after d-lead implantation (median latency after surgery, 57 days (range 28–182)), and their orientation values were also included in the analysis. D-lead orientation results were compared as described in the statistical methods section.
Surgical procedure
Pre-planning was performed on the day before surgery using Brainlab® Elements (Brainlab). On the day of surgery, a stereotactic CT scan was performed (0.7 mm, 2 × 192 slice detector, Somatom Force, Siemens) after placing a Leksell frame (Leksell G-frame, Elekta) and fusing to the preoperatively acquired MRI scan (3 Tesla Magnetom Skyra, Siemens).
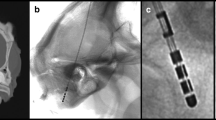
Lead placement was carried out under local anesthesia while patients were awake (13 patients) or under general anesthesia (two patients). A 14-mm burr hole was drilled, and the ring of the burr hole cover (Boston Scientific) was fixated with screws. A mobile flat detector C-arm (Philips Zenition 70, Philips) was used for intraoperative lateral X-rays, aligned perpendicular to the frame to determine the depth of the d-lead (Vercise Cartesia™ Model DB-2202-30 or DB-2202-45; Boston Scientific) as well as its orientation. The d-lead was inserted into the brain with the marker facing anterior. Single X-rays were performed while adjusting the depth. The lead was then rotated, trying to visualize an “iron sight” (ISi). This is a bright line that appears between the gaps of the split contacts on an X-ray, indicating an anterior orientation (Fig. 1). If the ISi was not visible (e.g., due to overlap with the contralateral lead), only the marker was used to determine the orientation. When the desired depth was reached, the lead was locked (even when the ISi was not visible) using the inner part of the burr hole cover (Boston Scientific) (Supplementary Fig. 1A), the stylet was removed, and an X-ray was performed (pre-final image). Finally, the end of the lead was fixated at the ring of the burr hole cover, the lid was placed on top (Supplementary Fig. 1D), and another X-ray was performed (final X-ray). The same surgeon (MTK) performed all surgeries using the same methodology. All patients received a postoperative CT scan directly after surgery for postoperative fusion (0.7 mm, 2 × 192 slice detector, Somatom Force, Siemens) and to rule out any surgical complications.
Explanation of “iron sight” in X-ray in reference to the frame and ACPC line. A Schematic drawing of a directional lead in reference to the ACPC line (green) and X-ray beam (yellow), which is perpendicular to the frame anterior-posterior axis (red). B Appearance of an “iron sight” every 60° on a schematic drawing of a directional lead. C “Iron sight” on intraoperative X-ray when the radiation beam traverses the frame laterally at a 90° angle. Abbreviations: ACPC, anterior commissure–posterior commissure. Figure was created using Procreate® on an iPad Pro (Apple®) and power point (Microsoft®)
Determination of lead orientation
Visualization of the ISi (frame coordinate system)
The two last X-rays, one after locking the lead (i.e., “pre-final X-ray”; Supplementary Fig. 1A) and one after fixating the lead (i.e., “final X-ray”; Supplementary Fig. 1D), were analyzed. D-lead orientation was determined by the ISi method as previously described [7]. In brief, when a radiation beam traverses a d-lead from the side at a 90° angle, an ISi becomes visible every 60° (360°/6) (Fig. 1). When the marker is facing strictly anterior and the ISi is perfectly visible, the rotation of the lead can be regarded as 0°. To be precise, due to the d-lead dimensions, oblique lead angles, and conventional X-ray resolution, we observed the gap to be visible over a range of approximately 12° (i.e., − 6 to + 6°) (Fig. 2).
Schematic drawing to explain groups A–E. Upper row shows a directional lead with orientations from − 12° to + 12°. A An ISi is visible on both the pre-final and final X-rays. B An ISi is not visible on the pre-final but is in the final X-ray. C An ISi is visible on the pre-final but not the final X-ray. D An ISi is not visible on either the pre-final or final X-rays. E An ISi is not evaluable, only the marker is used for evaluation. Abbreviations: ISi, iron sight
Determination of lead orientation on CT scans (ACPC coordinate system)
Images of the postoperative CT scan were loaded in Brainlab Elements and fused to the preoperative MRI images. The tip and axis of the implanted leads were detected in Elements Lead Localization using the automatic DBS lead position detection tool and visually verified. After assigning the Boston Scientific Vercise Cartesia Directional Lead model in the software, the orientation of each d-lead was automatically determined by Elements Lead Localization, based on the same characteristic CT artifact patterns described by Hellerbach and colleagues in 2018 [4] and using the intended implantation orientation (in this case anterior) as input. The same approach was used to determine lead orientation in those d-leads that were analyzed several weeks after implantation. The d-lead orientation relative to the patient anterior could be determined from postoperative CT in all cases.
Determination of lead orientation on postoperative CT (frame coordinate system)
In a DBS setup, the X-ray is aligned perpendicular to the frame when the target rods are perfectly aligned (Fig. 1C). However, in postoperative images (RF and CT), the orientation of the lead is defined in relation to the patients’ midline (ACPC line) [7]. Therefore, if the frame is not fixed perfectly straight on the patient’s head, and since the leads are not inserted perpendicular to the x- and y-axis, the difference of these angles (frame to ACPC) has to be adjusted accordingly. Therefore, the preoperatively performed stereotactic CT scan with the frame was uploaded into Brainlab Elements planning software. Images were fused to the preoperative MRI scan and postoperative CT scan with detected d-leads. The ACPC line was changed to the frame line in all three axes to determine the lead rotation in reference to the frame (Supplementary Fig. 2). Because the X-ray is acquired perpendicular to the frame, this approach was necessary to compare the rotation determined on the postoperative scan with the results from the ISi visualization.
Rotational fluoroscopy
As part of our clinical routine, all patients received an RF after surgery using a flat-panel detector C-arm system (Artis Q, Siemens Healthcare). Lead rotation in RF was determined using the ISi method in Philips DICOM Viewer (Philips DICOM Viewer, Version R3.0 SP3; Philips Healthcare) and evaluated by an experienced neurosurgeon (MTK) and neuroradiologist (JW) [7].
Statistical analysis
D-lead orientation was analyzed in a stepwise approach (Fig. 3). In analysis step 1, we evaluated whether the ISi was visible on the pre-final and final intraoperative X-ray and allocated d-leads to five groups according to their ISi visibility: group A, ISi visible on the pre-final and final X-rays (Fig. 2A); group B, ISi not visible on the pre-final but in the final X-ray (Fig. 2B); group C, ISi visible on the pre-final but not final X-ray (Fig. 2C); group D, ISi not visible on either the pre-final or final X-rays (Fig. 2D); and group E, ISi not evaluable, determination of orientation only by marker (Fig. 2E). For leads with a visible ISi in the final X-ray (group A/B), the intraoperative lead orientation (0° to frame anterior) was compared with the orientation determined on the first postoperative CT which was adjusted to the frame coordinates as described above. In analysis step 2, we determined the orientation of the d-leads in each group on both the first postoperative CT and RF in reference to the ACPC line and compared the orientation values at the two different time points when the images were acquired. In analysis step 3, d-lead orientation determined on the first postoperative CT scan was compared with that determined on the CT scan obtained several weeks after implantation (Fig. 3).
Study design. Comparison of X-rays with ISi to the postoperative CT in reference to the frame (step 1). Comparison of postoperative CT to RF (step 2) and to the second postoperative CT in reference to the ACPC (step 3). Abbreviations: ACPC, anterior commissure–posterior commissure; CT, computed tomography; ISi, iron sight; RF, rotational fluoroscopy
Methods of descriptive statistics were used. The data are presented as median values (range). The Mann-Whitney U test and Kruskal-Wallis test were used for group comparisons. Significance level was set to p < 0.05. Statistical analysis was performed using SPSS 20 (IBM).
Results
Patients
Seventeen eligible patients with a total of 32 d-leads were identified and included into the study (eight males, nine females; mean age 62 years (range 23–82)). Two patients were implanted unilaterally and 15 bilaterally. Ten patients suffered from Parkinson’s disease, five from essential or dystonic tremor, one from generalized idiopathic dystonia, and one from spasmodic dysphonia. The targets were the subthalamic nucleus in 22 leads (69%), the ventral intermediate nucleus in eight leads (25%), and the internal globus pallidus in two leads (6%) (Supplementary Table).
Lead orientation on intraoperative X-rays vs. first postoperative CT (analysis step 1)
The rotation values of each group are shown in Table 1. The rotation angles were comparable in both groups in which the ISi was seen on the final X-ray (i.e., groups A and B; p = 0.41). Likewise, the rotation angles in both groups in which no ISi was seen on the final X-ray (i.e., groups C and D) were comparable (p = 0.09) (Table 1). Therefore, groups A and B as well as C and D were merged for further analysis. The median deviation of those leads in which an ISi was visible on the final X-ray (group A/B) was 1.5° (range 0.5–6.0°) on the first postoperative CT scan with the frame as reference. The median deviation of those leads in which an ISi was not visible (group C/D) on the final X-ray was 15.5° (range 9.5–35.0°). Leads in group E, where only the marker orientation could be used, showed a median deviation of 26.5° (5.5–62.0°). The deviation angles were significantly smaller in group A/B than in groups C/D and E (p < 0.001), while the values were comparable in the groups C/D and E (p = 0.52) (Table 1 and Supplementary Table).
Lead orientation on postoperative CT vs. RF (analysis step 2)
Median time latencies between lead implantation (X-ray) and the postoperative CT were widely comparable across the three groups (p = 0.074) (Table 1). Latencies between postoperative CT and RF were also comparable in all three groups (p = 0.69). The orientation did not change (from left to right) in relation to the ACPC line in any lead in the time period between the postoperative CT and RF. The median deviation between the postoperative CT and RF was 1.5° (0–8.0°) in the whole cohort. Stratified according to the ISi visibility, the median deviation was 2.5° (0.5–5.0°) in group A/B, 2.0° (0–4.0°) in group C/D, and 1.0° (0–8.0°) in group E. The degree of rotation was comparable in all three groups (p = 0.71) (Table 1).
Lead orientation on first postoperative CT vs. second postoperative CT (analysis step 3)
In those d-leads with a mean longer follow-up time of 57 days (range 28–182), there was no change in lead orientation (median deviation 0° (range 0–1°)), when compared with the first postoperative CT.
Discussion
We did not find any deviation in the d-lead orientation after their implantation. First, there was no further rotation within the first 2-3 h after surgery in d-leads with a visible ISi on the final intraoperative X-ray. A visible ISi reliably indicates an anterior orientation of the d-leads (0 ± 6°) in reference to the frame at the time of their implantation [7]. A comparable degree of rotation was seen on the first postoperative CT scan in these patients when adjusting the reference to the frame. On the other hand, the rotation angles were significantly larger in d-leads in which no ISi was seen on the final X-ray, and none of those leads were within the ISi window range of ± 6°. This observation indicates that there is a larger deviation from anterior direction in d-leads without a visible ISi from the time point when the electrodes are intraoperatively fixated. Second, when we compared the orientation of the d-leads on the first postoperative CT scan with that on the RF 4–9 days later, we found a median deviation of 1.5° in all d-leads regardless of the intraoperative visibility of the ISi. More importantly, the deviation did not exceed 8.0° in any lead. Third, in patients with available CT scans acquired more than 4 weeks after surgery, we still found a stable d-lead orientation compared with the first postoperative CT scan.
Our observations are partially contrary to the findings by Dembek and colleagues. They demonstrated that d-leads implanted with an intended anterior orientation showed large deviations of up to 90° from the intraoperative orientation detection to the first postoperative CT scan, whereas we only found a median deviation of 1.5° within a comparable time period. There are two reasons for the incongruence of observations. In their study, the orientation of the d-leads was determined using the marker alone. However, we observed that d-leads for which only the marker could be used to determine orientation showed the largest deviation from 0.0°, with a median deviation of 26.9°. Our observation suggests that the marker alone cannot be used to reliably determine the orientation of the d-leads on intraoperative X-ray. Furthermore, Dembek and colleagues used bone cement and a microplate to fix the leads, whereas we used a burr hole cover. It should be noted, however, that in a subgroup of d-leads, we observed some rotation between the time when the lead was locked (i.e., at the pre-final X-ray) and after the lead was fixated (i.e., at the final X-ray). This rotation was indicated by the disappearance or appearance of the ISi between the two X-rays and depended on the amount of twist given to the lead during the process of locking. We therefore suggest that different surgical techniques and locking mechanisms need to be considered in future studies when the question of perioperative lead rotation is addressed.
Our study results have several implications for the care of DBS patients. We provide evidence that there is only minimal rotation of d-leads upon implantation. Since we found a median rotation of only 1.5° in our cohort, we suggest that one scan within the first week may be sufficient to reliably determine the d-lead orientation. For those centers that perform a postoperative CT scan, this would be the imaging modality of choice. If an ISi is visible on the final intraoperative X-ray, theoretically this information can be used to sufficiently determine the d-lead orientation and can be particularly valuable for centers that routinely perform a postoperative MRI scan, which cannot determine the orientation. On the other hand, we propose that use of marker-based techniques alone to determine the d-lead orientation is not precise enough. Knowledge regarding the orientation of the d-lead is important for clinicians who program the DBS system, since stable d-lead orientation is an essential prerequisite for sustained efficacy of DBS settings when steering options are used. Furthermore, patients may be prevented from repeating scans to determine d-lead orientation, e.g., when therapeutic effects are lost.
There are some limitations to our study. First, we included only five d-leads with a follow-up time of more than 4 weeks. To the best of our knowledge, there is no long-term stability data on d-lead orientation published to date. Thus, studies with a larger number of patients and followed up for a longer period of time are warranted to confirm our results. Second, we determined the orientation of the d-leads using imaging techniques, but did not validate our results by including clinical data on thresholds for therapeutic effects and side effects for each electrode segment. It should be noted that there is no published consensus defining a threshold above which d-lead rotation can be regarded as clinically significant. A clinical assessment would have provided information regarding the degree of d-lead rotation that may be regarded as clinically relevant and may need to be addressed in future studies. Third, our results refer to leads that were only moderately turned (usually not more than 120°), and we cannot exclude that greater intraoperative torsion may result in delayed rotation.
Conclusion
We have demonstrated that d-lead orientation does not deviate within the first week after implantation. Our study also provides first indications that d-lead orientation remains stable for more than 4 weeks after surgery. Postoperative images performed within this time frame can be used to reliably determine d-lead orientation. The first postoperative CT scan should be reliable enough to determine d-lead orientation and, since it is often routinely performed to rule out surgical complications, would be the imaging modality of choice. Marker-based determination of lead orientation alone may be too imprecise; adding the ISi method can improve determination of intraoperative orientation. These findings have clinical implications for clinical routine because stable d-lead orientation is an essential prerequisite for a sustained clinical benefit when steering programs are established.
References
Asahi T, Ikeda K, Yamamoto J, Tsubono H, Sato S (2019) Pilot study for considering subthalamic nucleus anatomy during stimulation using directional leads. J Mov Disord 12(2):97–102
Dembek TA, Reker P, Visser-Vandewalle V, Wirths J, Treuer H, Klehr M, Roediger J, Dafsari HS, Barbe MT, Timmermann L (2017) Directional DBS increases side-effect thresholds—a prospective, double-blind trial. Mov Disord 32(10):1380–1388
Dembek TA, Hoevels M, Hellerbach A et al (2019) Directional DBS leads show large deviations from their intended implantation orientation. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2019.08.017
Hellerbach A, Dembek TA, Hoevels M, Holz JA, Gierich A, Luyken K, Barbe MT, Wirths J, Visser-Vandewalle V, Treuer H (2018) DiODe: Directional orientation detection of segmented deep brain stimulation leads: a sequential algorithm based on CT imaging. Stereotact Funct Neurosurg 96(5):335–341
Hunsche S, Neudorfer C, Majdoub FE, Maarouf M, Sauner D Determining the rotational orientation of directional deep brain stimulation leads employing flat-panel computed tomography. Oper Neurosurg. https://doi.org/10.1093/ons/opy163
Pollo C, Kaelin-Lang A, Oertel MF, Stieglitz L, Taub E, Fuhr P, Lozano AM, Raabe A, Schüpbach M (2014) Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain 137(7):2015–2026
Reinacher PC, Krüger MT, Coenen VA, Shah M, Roelz R, Jenkner C, Egger K (2017) Determining the orientation of directional deep brain stimulation electrodes using 3D rotational fluoroscopy. Am J Neuroradiol. https://doi.org/10.3174/ajnr.A5153
Sitz A, Hoevels M, Hellerbach A, Gierich A, Luyken K, Dembek TA, Klehr M, Wirths J, Visser-Vandewalle V, Treuer H (2017) Determining the orientation angle of directional leads for deep brain stimulation using computed tomography and digital x-ray imaging: a phantom study. Med Phys 44(9):4463–4473
Steigerwald F, Müller L, Johannes S, Matthies C, Volkmann J (2016) Directional deep brain stimulation of the subthalamic nucleus: a pilot study using a novel neurostimulation device. Mov Disord 31(8):1240–1243
Steigerwald F, Matthies C, Volkmann J (2018) Directional deep brain stimulation. Neurotherapeutics. https://doi.org/10.1007/s13311-018-0667-7
Ten Brinke TR, Odekerken VJJ, Dijk JM, van den Munckhof P, Schuurman PR, de Bie RMA (2018) Directional deep brain stimulation: first experiences in centers across the globe. Brain Stimulat. https://doi.org/10.1016/j.brs.2018.04.008
Acknowledgments
We thank Rebecca Kurtev-Rittstieg (Brainlab) for her support regarding technical and software aspects. Editorial assistance was provided by Deborah Nock (Medical WriteAway, Norwich, UK).
Author information
Authors and Affiliations
Contributions
Research project: A. conception, MTK, and PCR; B. organization, MTK, YN, and FB; C. execution, MTK, FC, JW, and YN
Statistical analysis: A. design, FB and MTK; execution, FB, GK, and SHL; B. review and critique, all authors
Manuscript: A. writing of first draft, MTK and FB; B. review and critique, all authors
Images: A. conception and drawings, MTK; B. review and critique, all authors
Corresponding author
Ethics declarations
Conflict of interest
Related to this work: MTK has received travel funding from Boston Scientific (manufacturer of the electrodes used in this study). She is a consultant for Brainlab (manufacturer of the planning software used in this study). PCR has received occasional honoraria and travel support from Brainlab AG; he is a consultant for Boston Scientific. The companies had no involvements in the collection, analysis, and interpretation of the data nor in writing, editing, or submitting the manuscript.
Unrelated to this work: MTK has received travel funding from Medtronic. FC holds shares in varying amounts of different companies in medically related fields as a private investor (Amyra Biotech AG, Fresenius SE & Co. KGaA). JW has a partnership with Siemens Healthineers and Artis Q. SHL has received travel funding from AbbVie, Switzerland. FB has received a scholarship by the Baasch-Medicus Foundation, Zurich, and a research grant by the KSSG Forschungskommission.
Statement of ethics
The clinical characteristics and scan-related outcome parameters were obtained as part of the patients’ routine care. All data was stored on an in-house database that was approved by the local ethics committee. All patients gave their written informed consent to be included in the database.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Functional Neurosurgery–Other
Electronic supplementary material
ESM 1
(DOCX 709 kb)
Rights and permissions
About this article
Cite this article
Krüger, M.T., Naseri, Y., Cavalloni, F. et al. Do directional deep brain stimulation leads rotate after implantation?. Acta Neurochir 163, 197–203 (2021). https://doi.org/10.1007/s00701-020-04568-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04568-3