Abstract
Key message
Different environmental conditions affect tree senescence by different patterns of carbohydrate concentrations and have specific impact on the dissection of the photosynthetic apparatus.
Abstract
A proactive cultivation of Mediterranean broadleaf species, including oaks, has been suggested to fill possible temporal and spatial gaps in forestry created by Climate Change in Central Europe in the future. Climate can affect trees in several different ways, e.g., by modulating the course of leaf senescence. Senescence-associated processes, like regulation of carbohydrates and changes in chlorophyll fluorescence under drought stress conditions were studied with leaf tissue of drought-tolerant downy oak (Quercus pubescens). Two months of consistent drought stress in a frost-free greenhouse led to significantly earlier senescence and significant increased amounts of soluble sugars in the leaves of the drought-stressed group in comparison to a well-watered control group. Similar sugar accumulation was observed in trees outdoors, after exposure to frost. In contrast to monocarpic plants the accumulation of free sugars is neither triggering leaf senescence, nor is it a side effect of age-depending changes in Q. pubescens. Instead, sugar accumulation is induced by abiotical factors, like drought and frost. Furthermore, we suggest that the senescence process in the absence of drought stress or frost depends on the source status of the leaf, which, in term, is a function of light (through photosynthesis) and night temperature (through respiration). Contents of the storage metabolite starch decreased during late summer in all three groups. Drought-stressed plants showed a decline of the connectivity of photosystem II antenna, reflected as the L-band in the chlorophyll fluorescence induction curves, and stronger correlations between the declines in the capacity of photosynthetic dark reactions and electron transport-associated chlorophyll fluorescence parameters. We conclude that the disassembly of single parts of the photosynthetic apparatus during leaf senescence is a uniform process, but the onset of this process depends on abiotical environmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forestry always has to deal with climate, but the climatic changes have never been as fast as currently (Folland et al. 2001). An average temperature increase, less precipitation and frequent heat waves in the forthcoming 100 years (Schär et al. 2004; Jacob et al. 2014) will put nowadays already dry forestry areas to the test. Under Climate Change, drought stress (DS) will become one of the major problems of agri- and silviculture in Central Europe with increasing importance also in regions where it has been negligible until now (Chaves and Oliveira 2004). Distribution models of European broadleaf trees like oak and beech reveal that there could be a shift of these species’ distribution ranges towards northeast in the forthcoming 90 years (Hickler et al. 2012). Around 34 % of European forest land may only be suitable for Mediterranean oak type forest instead of more economically valuable beech, conifers or Central European oak species (Hanewinkel et al. 2012). In this context, proactive cultivation of Mediterranean broadleaf species, including oaks, has been suggested to fill possible gaps created by Climate Change in Central Europe on difficult stands in the future (Schmiedinger et al. 2009; Bolte et al. 2009, Pflug and Brüggemann 2012). Climate Change may affect trees in several different ways. Whereas warming has positive effects on the biomass production via earlier bud burst and earlier intra-annual shoot growth (Kuster et al. 2013), drought stress leads to earlier senescence behaviour and shortens the length of the growth period. This process offers a possibility to maintain a favourable water balance in the whole plant, in case water supply becomes limited through reduction of the transpiring surface (Larcher 1995; Munné-Bosch and Alegre 2004). This regulation of leaf senescence allows monocarpic plants to complete their life cycle and ensures the survival for perennial plants until the next favourable growth period, even under stressful conditions (Gan and Amasino 1997; Ono et al. 2001). Drought-adapted perennials can revert to accelerated cellular senescence and their nutrients during a drought period (Günthardt-Goerg and Vollenweider 2007) already before autumn starts. In Mediterranean ecosystems, the onset of drought stress is correlated with earlier leaf senescence in deciduous as well as in evergreen trees (Escudero and Del Arco 1987). Strong drought periods can cause higher annual litter fall in evergreen conifers (Pedersen and Bille-Hansen 1999). Field observations on the drought-tolerant deciduous tree species Q. pubescens under severe drought also indicated the induction of the leaf senescence processes (Damesin and Rambal 1995; Haldimann et al. 2008). The aim of this well-balanced process is the storage of nutrients from the leaves in other plant organs. In deciduous trees, these nutrients support the development of new leaves in the following spring (Clausen and Apel 1991; Larcher 2003; Matyssek et al. 2010). The dissection of the photosynthetic apparatus during senescence, monitored by chlorophyll a fluorescence, gives information about the stage of senescence (Holland et al. 2014). Senescence processes are often accompanied by changing levels of carbohydrates, especially of free sugars (Wingler and Roitsch 2008), which are increasing in several plant species during senescence (reviewed by Nooden et al. 1997). It is still under debate, whether the accumulation of free sugars is a trigger for senescence or if it is a consequence of age-depending changes in the metabolism (Dai et al. 1999; Wingler and Roitsch 2008). Accumulation of soluble sugars was not only observed during monocarpic senescence, but also as stress response in several plant species (Wingler and Roitsch 2008). In this context, the accumulation of free sugars, especially hexoses, could be a protection mechanism for the cell tissue. In our study, we focused on the physiological impact of environmental factors such as drought and frost on senescence behaviour and sugar accumulation. Q. pubescens, as a species with mainly Mediterranean distribution is extending its area of distribution northwards to the warmest regions of Germany. The species is traditionally ranked among the deciduous oaks, but has the ability to keep a fraction of leaves green if the climate conditions stay mild during autumn and winter. The present study investigates senescence in a frost-free control group (C) and a drought-stressed group (DS) of Q. pubescens plants and compares the developmental processes with the behaviour under natural (frost) conditions. The main questions of the study were:
-
1.
Is the senescence process accompanied with an accumulation of carbohydrates in the leaves?
-
2.
Are there differences in the dissection of the photosynthetic apparatus during senescence under control, DS and frost conditions?
Materials and methods
Plant material, drought stress induction and sampling dates
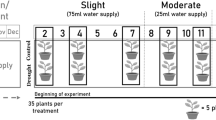
Ten specimens of Q. pubescens WILLD. trees, approximately 2 m high and 5 years old originating from Languedoc, France (F-QPU701, supplied by Darmstädter Forstbaumschule, Darmstadt, GER), were grown during the vegetation period 2011 (mid-August until the end of October) in a (frost-free) foliar greenhouse (Nitsch & Sohn GmbH & Co, Kreuztal-Eichen, GER) in plastic pots of 45 L volume, respectively. Trees were planted in loamy soil (sand + silt 86–89 %, clay 11–14 %), and watered thoroughly every second day until the beginning of the DS experiment to ensure controlled water supply. Plants were randomly selected and split in two groups after the control measurements. Volumetric soil water content was measured with a Theta probe ML2x (Delta-T Devices, Cambridge, UK). Pre-dawn leaf water potentials (Ψ PD) were recorded with a Scholander pressure bomb (SKPM 1400 pressure chamber (SKYE Instruments, Powys, Wales, UK) on one branchlet per tree. The maximum midday light intensities in the greenhouse were 1430 µmol m−2 s−1 photosynthetic active radiation (PAR) and we estimated the average (PAR) light sum in the greenhouse to be approximately 70–75 % of the outdoors insolation. Light intensity and temperature within the greenhouse were monitored continuously with an iMetos SMT280 climate station (Pessl Instruments, Weiz, AT). While the 5 plants of the control group were continuously watered, water was withheld from the stress group until Ψ PD was below −1 MPa. These pots were weighed daily and transpirational water loss was individually replaced at the end of the day. The experiment started on August 11th (day of year (DOY) 223) and the DS induction started 1 week later on August 18th (DOY 230). After the control measurements, the main sampling dates followed on September 29th (DOY 272) and October 27th (DOY 301). South-exposed sun leaves were used for all measurements.
Comparison with a field study: To obtain a more comprehensive picture of senescence processes, we compared the results to an outdoor Common Garden study, which was carried out in the same year under natural conditions with eight different (deciduous: Q. robur, Q. cerris, Q. frainetto, Q. pubescens; semi-evergreen: Q. x hispanica, Q. x turneri; evergreen: Q. ilex, Q. suber) well-watered oak taxa, including Q. pubescens from the same source and age as in the greenhouse experiment (experimental design and data shown in Holland et al. 2014). Precipitation and temperature data were collected from German Meteorological Survey (Deutscher Wetterdienst, Offenbach, Germany; www.dwd.de).
Chlorophyll contents and characterization of tree senescence stages
From the 10 trees, three trees per treatment group were chosen randomly for the characterization of the senescence level: all leaves per tree were counted and visually grouped into five senescence stages: green, leaves visually healthy without any damages; green-yellow, leaves with 10–90 % yellow area; yellow, no green colour is visible; brown, leaves are dry and without pigments; shed, leaves are no longer connected to the tree (Table 1). Averaged relative dimensionless chlorophyll contents were assessed by so-called SPAD-readings (SPAD-502, Konica-Minolta, Osaka, JP). The SPAD 502 is a portable chlorophyll metre that assesses leaf absorbance at 650 nm (red) and at 940 nm (near-infrared) and displays a value, which is calculated using the 940 nm absorbance as a reference for structural (i.e., non-pigment associated) absorption. The device, thus, measures the relative chlorophyll content of the sample non-destructively. To ensure comparability of the measurement itself and the CF methods on the patchy senescing leaves, the SPAD-readings as well as the CF measurements were taken from the same position on the leaf.
Chlorophyll fluorescence measurements
To assess the overall plant vitality and to dissect different partial processes of the photosynthetic apparatus, we used the fast CF induction of Photosystem (PS) II described by Strasser et al. (2000, 2004). PS II functionality was investigated by the JIP-test with a portable fluorimeter (Pocket-PEA, Hansatech Instruments Ltd, King´s Lynn, UK) on 3*x leaves per tree and measuring date measured at the same leaf and leaf position throughout the experiment according to Strasser et al. (2000, 2004), using the 50 μs fluorescence level as F 0. In situ measurements were carried out pre-dawn. The JIP-test parameters derived from the original induction curves were calculated using the PEA Plus Software (PEA Plus 1.0.0.1, Hansatech Instruments Ltd, King´s Lynn, UK) and using Excel 2010 formulae (Microsoft corporation, Albuquerque, USA) according to Strasser et al. (2000) as explained in Table 2. The individual induction curves (n = 8–21) were averaged for each taxon and sampling date. The averaged CF induction curves of the measurements taken in August were used as references for comparison with the averaged curves of the following dates to calculate double normalised (between F 0 and F m) differential induction curves (e.g., Jiang et al. 2008; Yordanov et al. 2008).
\(\Delta {\text{V}}\left( {\text{t}} \right) = \left[ {\left( {F_{\text{t}} {-}F_{0} } \right)_{\text{md}} /\left( {F_{\text{m}} {-}F_{0} } \right)_{\text{md}} } \right]{-}\left[ {\left( {F_{\text{t}} {-}F_{0} } \right)_{\text{c}}/\left({F_{\text{m}} {-}F_{0} } \right)_{\text{c}} } \right]\) with md = measuring date and c = control date.
Photosynthetic activity of leaves with different senescence stages
Gas-exchange rates of south-exposed leaves of the different senescence stages were measured with a Clark oxygen electrode in an LD 2/3 setup (Hansatech, King´s Lynn, UK). For the calculation of the net assimilation rate (A) at saturated CO2 concentration, leaf discs (13–32 mm in diameter, depending on leaf size) were excised with a cork borer. O2 was measured after 10 min of dark adaptation for 10 min, at 25 °C, 900 μmol quanta m−2 s−1 PAR (emitted by the LH36/2R light source). In order to avoid the effect of drought-induced closing of the stomata on the assessment of the capacity of the photosynthetic apparatus, the photosynthetic capacity was measured under low O2 and high CO2 conditions (2 % O2, 4.5 % CO2 in N2) to ensure CO2 saturation.
Carbohydrate concentration of leaves with different senescence stages
Soluble sugars were determined enzymatically in extracts obtained according to Stitt et al. (1983) with some modifications: two leaves of each plant and each senescence stage were harvested around 12 a.m., pooled, frozen in liquid nitrogen and ground at 30 Hz in the frozen state for 1 min in a mixing mill (MM 400, Retsch GmbH & Co., Haan, GER). 500 µl of 20 % HClO4 were added to 300 mg frozen leaf powder and ground. After thawing 1000 µl H2O were added and the samples were placed on ice for 10 min and then centrifuged (20 min, RT, 10,000g). To the supernatant 165 mg Polyclar (SERVA, Heidelberg, GER) was added to remove phenolic compounds. The samples were centrifuged again (10 min, RT, 10,000g) and the pH of the supernatant was adjusted to pH 8 with 5 M KOH and 133 mM tri-ethanol amine (TRA). After 10 min on ice, KClO4 was removed by centrifugation (10 min, 4 °C, 10,000g). In the supernatant, soluble sugars were determined enzymatically (Bergmeyer 1962) and presented in hexose units as the sum of glucose, fructose and 2* sucrose.
Starch was measured after washing 0.5 g of the frozen leaf powder from the mixing mill for 30 min at 80 °C in 6 ml 80 % EtOH to remove soluble sugars. After centrifugation, the pellet was suspended in 5 ml of 3 % HC1 and starch was hydrolyzed for 1 h at 98 °C. 100 µl of the hydrolysate was neutralised with ca. 30 µl 0.5 M KOH and 133 mM TRA and used for glucose determination. Then glucose was measured according to Bergmeyer (1962).
Statistical analysis
For comparison of data from different dates within one treatment group, we used an unpaired t test with Welch’s correction. The statistical correlation analyses of different parameters were performed using a Spearman correlation analysis. All statistical analyses were calculated using GraphPad PRISM 5.04 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
During the vegetation period 2011, spring was relatively dry (55 mm precipitation, March–May), but summer was quite wet (223 mm precipitation, June–August). Normal precipitation in autumn prevented any possible drought periods for the outdoor growing plants. An additional irrigation system secured an efficient water supply through out the whole year. C and DS group installations displayed average soil water contents of 23 vol% at the beginning of the experiment on DOY 223. Whereas the control (C) group was well watered during the whole experiment and its soil water content stayed stable, water was withheld from the plants of the DS group and soil water content decreased to 5–10 vol% on DOY 242. While Ψ PD of the C plants never fell below −0.38 MPa during the experiment, Ψ PD of the DS plants decreased significantly from DOY 244 on (p value < 0.0001) and remained always below −1 MPa with an average value of −1.79 MPa, due to the controlled daily replacement of water loss by transpiration until the end of the experiment on DOY 301 (ESM 1).
Figure 1 depicts the senescence development of the C and DS group over time. At the beginning of the measurements on DOY 223 all trees bore only green leaves. Seven weeks later, on DOY 272, both groups showed clear senescence behaviour. While around 70 % of the leaves in the control group were still green, the amount of green leaves in the DS group had decreased to <5 %. One-third of the leaves had turned brown with no chlorophyll content measurable using the SPAD-metre and the rest had turned yellow at this time point. After 4 weeks, on DOY 301, the C group still showed only 10–15 % green leaves, with nearly all leaves were still on the trees, whereas the DS group had shed more than half of the leaves and no green leaves were retained.
The soluble sugars and the starch concentration in the C and DS groups were nearly identical at the beginning of the experiment (DOY 223). The starch content was about 280–315 µmol g −1FW and thus twice as high as the content of the soluble sugars (150 µmol g −1FW ; Fig. 2a, b). Seven weeks later (DOY 272) the soluble sugars had increased significantly (p value = 0.0013) in the green leaves of the DS group (Fig. 2d) due to increases in the monosaccharide glucose and fructose (individual data not shown). At the same time, a significant decrease in starch content was observed (p value = 0.0035), while the contents of soluble sugars and starch in the control leaves had not changed significantly (Fig. 2c). The leaves of the DS group with green-yellow or yellow senescence level showed a further (not significant) decrease trend in the starch contents, whereas a significant decrease (p value = 0.016) of the soluble sugar contents from the green leaves to the yellow leaves was observed (Fig. 2d). Another 4 weeks later (DOY 301), at the end of the experiment (Fig. 2e, f), the starch contents of the C group had dropped significantly (p value < 0.0001) to one-third of the initial value. The concentration of the soluble sugars in the C group showed a noticeable decline from DOY 272 to DOY 301, with very similar values in all senescence stages. In the last residual green-yellow and yellow leaves (DOY 301) of the DS group, the starch concentration remained very low, and the concentrations of the soluble sugar decreased below the initial values on the control date DOY 223.
For a better understanding of the senescence processes under DS, we compared our data to observations on plants growing outdoors under natural temperature and light conditions (Fig. 3). In these plants first signs of senescence (chlorophyll degradation) were only visible from DOY 290 on, i.e., much later than in C or DS plants in the greenhouse drought experiment. From DOY 286 on (first frost nights), starch concentrations remained constant at a low level (±26 µmol hexose g −1FW ) until the end of the vegetation period. During a warmer period after the frost, the concentration of soluble carbohydrates increased significantly, exhibiting near equimolar increases of glucose and fructose (data not shown). A second significant increase of soluble sugars was observed after the temperature dropped below 0 °C again. Similar behaviour of leaf starch and soluble sugar contents as in Q. pubescens were observed in five other (evergreen and semi-evergreen) Quercus taxa grown under identical conditions. At the same time, fully deciduous taxa, shedding their leaves earlier than Q. pubescens, revealed constantly low starch and soluble sugar contents until abscission (ESM 2).
Senescence behaviour of Quercus pubescens under natural conditions: a concentration of soluble carbohydrates (black lines) and starch (black dotted line) and the relative chlorophyll values (SPAD, deposited in grey) in µmol hexose g −1FW (DOY 242-329; n = 3, of pooled leaf samples, mean ± SD); b minimum temperature 2 m above ground
To analyse the developmental changes in the photosynthetic apparatus in the course of senescence, we compared C leaves at DOY 301 and DS leaves at DOY 272. At this respective date, all trees contained green and green-yellow senescing (Fig. 4). While the F v/F m values (Fig. 4a) as well as the rate of photosynthesis (Fig. 4c) did not show significant differences between the green leaves from C and DS plants, a significant decline of PI(abs) of the green leaves was found in DS plants (Fig. 4b). The green-yellow leaves differed only slightly between the C and the DS group in their assimilation rates (A) and PI(abs), but showed only a significant decline of F v/F m in the leaves from the DS.
Effects of drought treatment on photosynthesis parameters in leaves of different senescence stages. a Maximum quantum yield of PS II (F v/F m). b Performance Index on absorption basis [PI(abs)] and c Photosynthetic capacity (A [µmol m−2 s−1]) of C plants (DOY 301, shaded bars) and of DS plants (DOY 272,. white bars) (mean ± SD, n = 10–40). Statistical significance: p value = ★ < 0.05, ★★ < 0,01
The averaged differential CF induction curves in Fig. 5 revealed similar shapes in the C and the DS groups in the course of senescence. The induction curves of both DS and C group were nearly identical in the beginning of the experiment (DOY 223) and were pooled and considered as reference curves for both groups. Relative fluorescence intensity changed considerably during the course of senescence in both groups in leaves of different senescence stages. The differences are presented in Fig. 5 as differential (ΔV) curves between green-yellow and green leaves.
The leaves of the DS group as well as the leaves of the C group showed positive values in the differential curves (Fig. 5a). Both groups showed an increase of fluorescence around 0.5–1 ms in the green-yellow leaves compared to the non-senescing references in August (DOY 223). Furthermore in both groups, a strong peak occurred in the ΔVOJ-curves (Fig. 5b), i.e., a so-called K-band (Fig. 5b). In the ΔVOK-curves of the DS group, additionally, a so-called L-band was visible in the green-yellow leaves (Fig. 5c).
To assess whether the well-known decrease of photosynthetic capacity (assimilation rate A under near-saturating conditions) during senescence (cf. Holland et al. 2014 and references therein) occurs independently of or in a well-coordinated manner with the disassembly of the chloroplast electron transport chain, correlation analysis were carried out. The quantum yields of different processes associated with electron transport of individual leaves derived from the CF induction curves (i.e. φD0, φP0, φE0, φR0), were plotted against their respective photosynthesis rate under saturating CO2 (A [µmol m−2 s−1]; Fig. 6). The statistical correlation analysis revealed that A of the green and green-yellow leaves of the C and the DS group correlated significantly especially with φE0 (electron transport). In the DS group the alterations of the values in the green-yellow leaves in general were stronger and yielded higher Spearman rank correlation coefficients (r S) than in the control group, best visible in φD0 and φP0.
Spearman correlation analysis of the net assimilation rate (A [µmol m−2 s−1]) and the quantum yields φD0, φP0, φE0, φR0 of pooled green and green-yellow leaves of the C (n = 40) and the DS group (n = 47) depict as r S (Spearman rank correlation coefficient). p values of correlation analysis of both leaf stages depicted as asterisk
Discussion
Different accumulation of soluble sugars under different senescence scenarios
In accordance to field observations on Q. pubescens under severe drought (Damesin and Rambal 1995), the DS treatment of our Q. pubescens in the greenhouse experiment inducted an accelerated senescence processes. The onset of senescence in the DS group happened about 1 month earlier than in the C group and impaired a larger number of leaves (Fig. 1).
Decreasing concentrations of amino acids and starch, but increasing concentrations of free sugars have been observed in several plant species during senescence (reviewed by Nooden et al. 1997; Wingler and Roitsch 2008). Between August and October, starch contents decreased in all three experimental groups, both under outdoor conditions (Fig. 3) and in the greenhouse (Fig. 2). In oaks, late summer is the main period of starch storage in the trunk (Maunoury et al. 2007). However, the concentration of soluble sugars increased in the DS plants. It is known that DS leads to an increase of soluble carbohydrates as osmotic adjustment even without inducing senescence. This accumulation promotes the influx of water, thus maintaining cell volume and counteracting the wilting process (Larcher 1995). Patakas and Noitsakis (2001) e.g. showed a doubling of monosaccharide concentrations in immature leaves of DS grapevines (Vitis vinifera) in comparison to C leaves, similar to our findings. The contents of soluble sugars increased also in the outdoor growing Q. pubescens after the first frost (Fig. 3). Glucose, fructose and sucrose accumulate together with other osmolytes during cold treatments, possibly as a result of a feedback effect from limited sink strength, provided the ambient conditions still allow photosynthesis (Brüggemann et al. 1992). This accumulation also decreases the freezing point of the cell sap and has been interpreted as a proactive physiological process during winter acclimation (Cook et al. 2004; Kaplan et al. 2007). In field conditions, the sugar accumulation was also observed after frost events in Q. pubescens as well as in the semi-deciduous hybrids and the evergreen species in the course of autumn, but not in Q. robur, Q. frainetto or Q. cerris during their earlier senescence process before the occurrence of frosts (EMS 2). In contrast to the DS greenhouse plants and the outdoor-grown plants, the C group in the frost-free greenhouse showed no significant changes in the soluble sugar contents (Fig. 2a, c, e). As demonstrated by the chlorophyll contents (Fig. 3), the senescence process of the Q. pubescens plants in the Common Garden experiment started after DOY 286, 2 weeks later than in the C plants (DOY 272) and 5 weeks later than the DS plants in the frost-free greenhouse (DOY 251). A possible reason for the earlier start of senescence of the C plants in the greenhouse as compared to the outdoor plants could be a change in its source strength, indicated by the low starch contents (Fig. 2e, green leaves). While outdoor growing plants also contained low starch contents already at the same point of time (Fig. 3; ESM 2), greenhouse plants only received 70–75 % of the outdoor insolation. In combination with the warmer night temperatures in the greenhouse (and thus higher respiration rates), a decreased 24-h net assimilation rate as compared to the outdoor growing plants can thus be reasonably assumed. This would lead to a faster change of the leaf status from source to sink and could be a reason for the earlier occurrence of senescence. However, to elucidate the way by which the light sum in combination with temperature triggers senescence, further experimental investigations by monitoring the plants under controlled light and temperature regimes would be required.
The DS plants showed the same patchy pattern of chlorophyll degradation, as C plants and the course of senescence did not differ from the C plants in discoloration or length of the senescence period in single leaves. Many molecular processes of DS-induced and developmental leaf senescence have been reported to occur in the same way during the re-organisation and terminal phases of senescence, whereas differences have been observed during the initial phase (reviewed by Munné-Bosch and Alegre 2004). Several genes have been identified to play a role in the initiation of developmental senescence (Quirino et al. 2000 and citations therein), but only some of them are regulated in DS-induced senescence. Hensel et al. (1993) assumed that a decline in photosynthetic rate acts as one of the basic triggers of developmental leaf senescence in monocarpic species. Pic et al. (2002) pointed out that in Pisum sativum under DS senescence is already initiated, while the leaves of the plants are still able to perform high rates of photosynthesis. Once senescence is initiated, the decline of the photosynthetic capacity is accompanied by the breakdown of thylakoid membranes and stromal proteins (Nooden 1988) and the decrease of the expression of several photosynthesis genes (Woo et al. 2002). The accumulation of free sugars could be a part of the occurring signal transduction chain in drought-induced senescence by the inhibition of the expression of photosynthesis genes (Krapp et al. 1993; Jang and Sheen 1994; Jones et al. 1996).
Differential disassembly of the photosynthetic apparatus under different senescence scenarios
In the green leaves of the DS group (Fig. 2b, d), an intermittent sugar accumulation in comparison to the C group had been observed (Fig. 2a, c), together with a decrease of the O2 evolution rates (23 %, n.s., Figure 4c) and significant changes in the functionality of the photosynthetic electron transport chain (Fig. 4a, b). Figure 4b shows a clear decline in PIabs, a factor combining three components associated with light capturing and PS II electron transport. Similarly, in several deciduous oak taxa under natural senescence, first strong changes occurred in PIabs prior to measurable effects of senescence on F v/F m (φP0; Holland et al. 2014).
To investigate whether there are differences between DS-induced and natural senescence in the disassembly of the photosynthetic apparatus we compared the differential CF induction curves of the plants (Fig. 5). At the end of September (DOY 272), the green-yellow leaves of the DS group (Fig. 5a), like those of the C group four weeks later (DOY 301) showed impaired electron transport at 0.5–1 ms (i.e., higher CF = positive values in the differential ΔVOP curves) as compared to green leaves at DOY 223. These data match with the photosynthesis measurements. We observed the development of K-bands (Fig. 5b) in the CF induction curves (ΔVOJ) in the green-yellow leaves of both groups, indicative of constraints in electron donation to PS II, e.g. by a damage to the water-splitting apparatus (Srivastava et al. 1997), but only in the green-yellow leaves of the DS plants the occurrence of a well-developed L-band could be observed (Fig. 5c). L-bands are considered indicators of decreasing connectivity of PS II antennae and have been described under DS in barley (Oukarroum et al. 2007) as a stress symptom. The connection between the CF parameters and the photosynthetic capacity (A) is shown in Fig. 6. The correlation plots of A with the quantum efficiency fluxes, φD0, φP0, φE0, φR0, representing different processes of the photosynthetic electron transport (Fig. 6), showed the same patterns during senescence in both tested groups. Generally, the significance of the (positive or negative) correlations was stronger in the DS plants as evidenced by higher Spearman rank correlation coefficients. In detail, φD0, the maximum quantum efficiency of energy dissipation increased with decreasing A, while the quantum efficiencies of light trapping (φP0), electron transport (φE0) and PSI end acceptor side reduction (φR0) decreased (Fig. 6). The higher Spearman rank correlation coefficients during DS-induced senescence may indicate that the decrease of the capacity of the dark reactions is better coordinated with the disassembly of the electron transport chain (e.g., visualised in the parameter φE0) than under developmental senescence. The frost exposed plants from the field study exhibited practically unchanged F v/F m, strongly decreasing PIabs and the occurrence of the 300 µs K-band in the O, J double normalised plot of the CF induction curves after the first frost. An L-band was not observed in those studies (Holland et al. 2014).
Conclusions
In Table 3, compiling the data from Holland et al. (2014) and this study, we summarise the different processes associated with leaf senescence under the different environmental conditions. We conclude that the time course of the disassembly of the photosynthetic apparatus during senescence is identical, but shows stress-related additional side effects and the onset of the process depends on the abiotic factors, which act on the plants during senescence. In the case of the Mediterranean, potentially wintergreen Q. pubescens, DS shortens the leaf life span significantly. DS-induced senescence comes along with a significant increase in free sugars. We propose that the senescence in the greenhouse C group must have been triggered by the source status of the leaves, since these plants did not experience any frost or other external factors. Accumulation of free sugars also takes place under outdoor conditions, when frost events occur before a possible day length trigger starts the senescence process. Neither the accumulation of soluble sugars in the leaves is likely to be the crucial senescence trigger, nor occasional mild frost events seem to be an effective senescence trigger in Q. pubescens, as it was the case in Populus tremula (Keskitalo et al. 2005). We further conclude that the disassembly of single parts of the photosynthetic apparatus follows a unified scheme under all senescence scenarios. DS plants showed an additional decline of the connectivity of PS II antennae, reflected as the L-band in the CF induction curve, and a higher correlation coefficient between the declines in photosynthetic dark reactions and electron transport-associated parameters, indicative of a more coordinated disassembly of the electron transport chain components together with the proteins involved in the dark reactions.
In our opinion the drought-adapted Q. pubescens could be a suitable cultivation alternative for Central Europe. The trees would benefit from the warming climate in the summer and from the predicted wet and mild winter conditions. Additionally, they have the ability to avoid the more frequently occurring severe drought periods or the from time to time still appearing strong frost conditions of future climate scenarios through environmentally controlled accelerated senescence.
Author contribution statement
The first author VH and SK designed the experiment according to prior agreement with WB. VH and SK performed the experiments. SL undertook laboratory measurements of the carbohydrates. VH analysed the data and wrote the manuscript with the contribution of WB. SK and SL commented on the manuscript.
Abbreviations
- A:
-
Net assimilation rate (under saturating CO2 and high light)
- C:
-
Control(s)
- CF:
-
Chlorophyll fluorescence
- DOY:
-
Day of year
- DS:
-
Drought stress(ed)
- FW:
-
Fresh weight
- PAR:
-
Photosynthetic active radiation
- PS:
-
Photosystem
References
Bergmeyer HU (1962) Methoden der enzymatischen analyse. Verlag Chemie, Weinheim, FRG
Bolte A, Eisenhauer D, Ehrhart HP, Groß J, Hanewinkel M, Kölling C, Profft I, Rohde M, Amereller K (2009) Climate change and forest management-accordances and differences between the German states regarding assessments for needs and strategies towards forest adaptation. Agric For Res 59:269–278
Brüggemann W, van der Kooij TA, van Hasselt PR (1992) Long-term chilling of young tomato plants under low light and subsequent recovery. Planta 186(2):179–187
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55(407):2365–2384
Clausen S, Apel K (1991) Seasonal changes in the concentration of the major storage protein and its mRNA in xylem ray cells of poplar trees. Plant Mol Biol 17(4):669–678
Cook D, Fowler S, Fiehn O, Thomashow MF (2004) A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc Natl Acad Sci USA 101(42):15243–15248
Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, Ratner K, Granot D (1999) Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell Online 11(7):1253–1266
Damesin C, Rambal S (1995) Field study of leaf photosynthetic performance by a Mediterranean deciduous oak tree (Quercus pubescens) during a severe summer drought. N Phytol 131:159–167
Escudero A, Del Arco JM (1987). Ecological significance of the phenology of leaf abscission. Oikos 11–14
Folland CK, Rayner NA, Brown SJ, Smith TM, Shen SSP, Parker DE, Macadam I, Jones PD, Jones RN, Sexton DMH (2001) Global temperature change and its uncertainties since 1861. Geophys Res Lett 28(13):2621–2624
Gan S, Amasino RM (1997) Making sense of senescence (molecular genetic regulation and manipulation of leaf senescence). Plant Physiol 113(2):313
Günthardt-Goerg MS, Vollenweider P (2007) Linking stress with macroscopic and microscopic leaf response in trees: new diagnostic perspectives. Environ Pollut 147(3):467–488
Haldimann P, Gallé A, Feller U (2008) Impact of an exceptionally hot dry summer on photosynthetic traits in oak (Quercus pubescens) leaves. Tree Physiol 28(5):785–795
Hanewinkel M, Cullmann DA, Schelhaas M-J, Nabuurs G-J, Zimmermann NE (2012) Climate change may cause severe loss in the economic value of European forest land. Nat Clim Chang 3:203–207
Hensel LL, Grbic V, Baumgarten DA, Bleecker AB (1993) Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissue in Arabidopsis. Plant Cell 5:553–564
Hickler T, Vohland K, Feehan J, Miller PA, Smith B, Costa L, Giesecke T, Fronzek S, Carter TR, Cramer W, Kühn I, Sykes MT (2012) Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation model. Glob Ecol Biogeogr 21(1):50–63
Holland V, Koller S, Brüggemann W (2014) Insight into the photosynthetic apparatus in evergreen and deciduous European oaks during autumn senescence using OJIP fluorescence transient analysis. Plant Biology 16(4):801–808
Jacob D et al (2014) EURO-CORDEX: new high-resolution climate change projections for European impact research. Reg Environ Chang 14(2):563–578
Jang JC, Sheen J (1994) Sugar sensing in higher plants. Plant Cell 6:1665–1679
Jiang HX, Chen LS, Zheng JG, Han S, Tang N, Smith BR (2008) Aluminum-induced effects on Photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol 28:1863–1871
Jones PG, Lloyd JC, Raines CA (1996) Glucose feeding of intact wheat plants represses the expression of a number of Calvin cycle genes. Plant Cell Environ 19(2):231–236
Kaplan F, Kopka J, Sung DY, Zhao W, Popp M, Porat R, Guy CL (2007) Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J 50(6):967–981
Keskitalo J, Bergquist G, Gardeström P, Jansson S (2005) A cellular timetable of autumn senescence. Plant Physiol 139(4):1635–1648
Krapp A, Hofmann B, Schäfer C, Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the ‘sink regulation’ of photosynthesis? Plant J 3(6):817–828
Kuster TM, Arend M, Bleuler P, Günthardt-Goerg MS, Schulin R (2013) Water regime and growth of young oak stands subjected to air-warming and drought on two different forest soils in a model ecosystem experiment. Plant Biol 15(s1):138–147
Larcher W (1995) Physiological plant ecology. Springer, Heidelberg. (ISBN 038798260)
Larcher W (2003) Physiological plant ecology. Springer, Berlin, DE, pp 237–240
Matyssek R, Fromm J, Rennenberg H, Roloff A (2010) Biologie der Bäume. Eugen Ulmer, Stuttgart, DE, pp 83–140
Maunoury F, Berveiller D, Lelarge C, Pontailler J-Y, Vanbostal L, Damesin C (2007) Seasonal, daily and diurnal variations in the stable carbon isotope composition of carbon dioxide respired by tree trunks in a deciduous oak forest. Oecologia 151:268–279
Munné-Bosch S, Alegre L (2004) Die and let live: leaf senescence contributes to plant survival under drought stress. Funct Plant Biol 31(3):203–216
Nooden LD (1988) The phenomena of senescence and aging. In: Nooden LD, Leopold AC (eds) Senescence and aging in plants. Academic Press, San Diego, CA, pp 1–50
Nooden LD, Giuamét JJ, John I (1997) Senescence mechanism. Physiol Plant 101:746–753
Ono K, Nishi Y, Watanabe A, Terashima I (2001) Possible mechanisms of adaptive leaf senescence. Plant Biol 3(3):234–243
Oukarroum A, El Madidi S, Schansker G, Strasser RJ (2007) Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and rewatering. Environ Exp Bot 60:438–446
Patakas A, Noitsakis B (2001) Leaf age effects on solute accumulation in water-stressed grapevines. J Plant Physiol 158:63–69
Pedersen LB, Bille-Hansen J (1999) A comparison of litterfall and element fluxes in even aged Norway spruce, sitka spruce and beech stands in Denmark. For Ecol Manag 114(1):55–70
Pflug E, Brüggemann W (2012) Frost-acclimation of photosynthesis in overwintering Mediterranean holm oak, grown in Central Europe. Int J Plant Biol 3(1):1–8
Pic E, de La Serve BT, Tardieu F, Turc O (2002) Leaf senescence induced by mild water deficit follows the same sequence of macroscopic, biochemical, and molecular events as monocarpic senescence in pea. Plant Physiol 128(1):236–246
Quirino BF, Noh YS, Himelblau E, Amasino RM (2000) Molecular aspects of leaf senescence. Trends Plant Sci 5(7):278–282
Schär C, Vidale PL, Lüthi D, Frei C, Häberli C, Liniger MA, Appenzeller C (2004) The role of increasing temperature variability in European summer heatwaves. Nature 427:332–336
Schmiedinger A, Bachmann M, Kölling C, Schirmer R (2009) How to select tree species for 381 trials against the background of climate change. Forstarchiv 80:15–22
Srivastava A, Guissé B, Greppin H, Strasser RJ (1997) Regulation of antenna structure and electron transport in Photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim Biophys Acta 1320(1):95106
Stitt M, Gerhardt R, Kürzel B, Heldt HW (1983) A role for fructose-2,6-bisphosphate in the regulation of sucrose synthesis in spinach leaves. Plant Physiol 72:1139–1141
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms, regulation and adaptation. Taylor and Francis, London, pp 445–483
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorphyll a fluorescence transient. In: Papageorgiou C, Govindjee (eds), Chlorophyll a fluorescence: a signature of Photosynthesis. Advances in Photosynthesis and Respiration. vol. 19 (Govindjee, Series editor.). Kluwer Academic, Dordrecht, NL, pp 321–362
Wingler A, Roitsch T (2008) Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biol 10:50–62
Woo HR, Goh CH, Park JH, Teyssendier de la Serve B, Kim JH, Park Y-I, Nam HG (2002) Extended leaf longevity in the ore4-1 mutant of Arabidopsis with a reduced expression of a plastid ribosomal protein gene. Plant J 31:331–340
Yordanov I, Goltsev V, Stefano D, Chernev P, Zaharieva I, Kirova M, Gecheva V, Strasser RJ (2008) Preservation of photosynthetic electron transport from senescence-induced inactivation in primary leaves after decapitation and defoliation of bean plants. J Plant Physiol 165:1954–1963
Acknowledgments
The present study was financially supported by the research funding programme “LOEWE – Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz” of Hesse’s Ministry of Higher Education, Research and the Arts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by U. Lüttge.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM. 1
Soil water content and pre-dawn water potential of control and stress plants in the course of the drought stress experiment (mean ± SEM; n = 5). (TIFF 18582 kb)
ESM. 2
Senescence behaviour of 8 different oak taxa under natural conditions: concentration of soluble carbohydrates (black lines) and starch (black dotted line) in µmol hexose/g FW, the relative chl values (SPAD, deposited in grey) (DOY 242-329; n = 3, of pooled leaf samples, mean ± SD) and the minimum temperature 2 m above ground [°C]. (TIFF 109872 kb)
Rights and permissions
About this article
Cite this article
Holland, V., Koller, S., Lukas, S. et al. Drought- and frost-induced accumulation of soluble carbohydrates during accelerated senescence in Quercus pubescens . Trees 30, 215–226 (2016). https://doi.org/10.1007/s00468-015-1290-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1290-4