Abstract
Purpose
To evaluate the relationship between non-tumor liver (NTL) dose and adverse events (AE) in patients with hepatocellular carcinoma (HCC) treated with glass-based Yttrium-90 radioembolization (Y90-RE).
Materials and Methods
A retrospective analysis of patients with HCC treated with Y90-RE between 2013 and 2018 was performed. Baseline characteristics including demographics and Y90-RE treatment approach were captured. Common Terminology Criteria for Adverse Events v5 was assessed at months 3 and 6 post-treatment. Using voxel-based dosimetry with MIM Software V. 6.9, dose-volume histograms of treated area of liver were created. Receiver operator characteristic curve was used to determine NTL dose threshold predicting AEs. Multivariate analysis was used to determine independent clinical factors of predicting severe AEs. Chi-square analysis was used to compare proportions.
Results
Two hundred and twenty-nine consecutive patients (115(50.2%) lobar and 114(49.8%) segmental) were included. At 3 months, there was a lower rate of any grade AE (55(46%) segmental and 36(31%) lobar, p = 0.009) and increased rate of severe AEs for lobar compared to segmental (2(2%) segmental and 9(8%) lobar, p = 0.029). At 6 months, severe AEs were greater for lobar than segmental (1(1%) segmental vs 10(9%) lobar, p = 0.005). For lobar Y90-RE, mean NTL dose of 112 Gy predicted severe AE (89% sensitivity and 91% specificity (AUC = 0.95, p = < 0.0001) at 3 and 6 months. For the segmental group, no significant association was found between NTL dose and severe treatment-related AE at 3 and 6 months.
Conclusion
In patients with HCC undergoing glass-based lobar Y90-RE, NTL dose of > 112 Gy is associated with severe treatment-related AEs at 3–6 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Personalized dosimetry improves patient survival and tumor progression outcomes in patients with liver cancer undergoing Y90 radioembolization (Y90-RE) [1]. Additionally, much research has focused on the clinical toxicities patients face when undergoing Y90-RE [2,3,4,5,6,7]. Clinical follow-up duration after Y90-RE has been mixed, with adverse events recorded at 1 week to 3 months [2,3,4,5,6,7]. This study builds upon the research focused upon Y90-RE clinical toxicities and seeks to determine a threshold dose at which clinical toxicity occurs after Y90-RE. Individualized Y90 dosimetry has become a promising strategy to improve patient survival, as a recent, prospective, and randomized trial demonstrated a survival benefit for tumors treated with over 205 Gy of glass Y90 [1]. Another recent study correlating tumor dose to pathological necrosis in explanted HCC tumors demonstrated that tumor dose of 400 Gy was required to achieve complete pathological necrosis in patients with HCC treated with glass-based Y90 microspheres [8]. Emerging data suggest that higher doses of radiation to tumors confer both increased tumor response and increased patient survival [1, 8]. However, there remains a paucity of data on both the clinical adverse events and the exact non-tumoral liver (NTL) dose that can predict treatment-related toxicity in patients with HCC undergoing glass-based Y90-RE.
The aim of this study is to evaluate whether a NTL dose threshold can predict significant clinical toxicity for both segmental and lobar glass-based Y90-RE of HCC.
Materials and Methods
Study Design and Population
This single-institution, multi-hospital, and retrospective study was approved by the Institutional Review Board and enrolled 229 consecutive patients with HCC who underwent Y90-RE either with segmental or lobar treatment from March 2013 until December 2018. Clinical and imaging data were attained from the electronic medical record. Baseline albumin–bilirubin (ALBI) grade, Child–Pugh (CP) score, Model for End Stage Liver Disease (MELD) Scores, Barcelona Clinic Liver Classification (BCLC), and demographics were collected via chart review.
Yttrium-90 Selective Internal Radiation Therapy Technique
Using previously described methods [9, 10], all patients were treated with glass-based Y90 microspheres (TheraSphere, Boston Scientific, Marlborough, Massachusetts, USA). Approximately 2–4 weeks prior to Y90-RE, all patients underwent a mapping procedure (shunt study) using Technetium-99 m macroaggregated albumin (Tc-99 m MAA). The Y90 activity to be administered was calculated via the MIRD model as recommended by the TheraSphere package insert with the goal of 120 Gy to the perfused lobe of the liver [11]. The type of Y90-RE to be performed, in a segmental or lobar fashion, was determined by the treating interventional radiologist. Tumor perfusion from the microcatheter location position where the Y90 was to be delivered was ensured with intraprocedural cone beam-computed tomography (CBCT).
Dosimetry Analysis
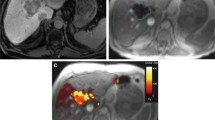
Y90 bremsstrahlung SPECT/CT was obtained immediately following radioembolization for each patient. MIM SurePlan software v6.9 (MIM Software, Cleveland, OH, USA) was utilized retrospectively to create a volumetric region of interest around the treated tumor and perfused portion of the liver using the semi-automated dosimetry module of the software. Contours around the targeted tumor and NTL were created on the pre-Y90-RE contrast-enhanced MRI or CT [12]. Contoured images were then fused to Y90 bremsstrahlung SPECT/CT images using the MIM software and voxel-based dose to the targeted tumor, perfused non-tumoral liver, and lungs were calculated using software-generated dose-volume histograms [12,13,14,15,16,17,18,19,20] Fig. 1.
Post-Y90 dosimetry workflow involves first inputting an anatomic CT/MRI, then attenuation correction CT, and lastly a bremsstrahlung CT. The MIMsureplan software guides the creation of liver and tumor contours with manual correction by the user. After the administered activity is entered, the workflow creates body and non-tumoral liver contours automatically and creates a heat map of the activity delivered. Dosimetry is calculated via the partition model. Dose contours are created automatically using the semi-automated tumor and liver contours, and the data are output as a dose-volume histograms
Treatment-Related Toxicity Analysis
Clinical and laboratory toxicities were evaluated using Common Terminology Criteria for Adverse Events (CTCAE) version 5 at 3 and 6 months post-Y90-RE [21]. Adverse events (AE) were classified as “any grade”, i.e., grade 1–5, or “severe”, i.e., grade ≥ 3. Clinical toxicities assessed were encephalopathy, ascites, fatigue, abdominal pain, nausea, vomiting, anorexia, constipation, or fever. Laboratory toxicities assessed were international normalized ratio (INR), white blood cell count (WBC), alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (Alk Phos), total bilirubin (tBili), creatinine, albumin, and sodium. Performance status was determined using Eastern Cooperative Oncology Group (ECOG) toxicity [22].
Statistical Analysis
Receiver operating characteristic (ROC) curve was used to calculate the area under the curve (AUC), which allowed the evaluation of the reliability of NTL dose to predict any grade or severe clinical/laboratory toxicities at 3 and 6 months post-Y90-RE. Multivariate analysis was employed to determine whether gender, NTLD > 110 Gy, albumin–bilirubin grade 2 or 3, Child–Pugh B or C, or age over 65 independently predicted severe, ≥ grade 3 clinical toxicities at 3 or 6 months. Chi-square analysis was used to compare proportions, and Student’s t-test was used to compare means.
Results
Baseline Demographics
Two hundred and twenty-nine consecutive patients were included with 115 (50.2%) treated with segmental and 114 (49.8%) treated with lobar Y90-RE. The mean age of the entire cohort was 64 years, 119 (78%) male, 150 (66%) ECOG 0, 150 (65.5%) ALBI grade 1, and 178 (78%) with Child–Pugh class A pretherapy. For patients treated with segmental Y90-RE, they were more likely to be ECOG 0 (n = 81, 70.4% segmental vs n = 69, 60.5% lobar, p = 0.01) and be classified as Child–Pugh class A (n = 97, 84% segmental vs n = 81, 72% lobar p = 0.03) (Table 1). Mean cumulative tumor size was significantly larger for the lobar group compared to the segmentectomy group (lobar: mean 86 mm SD 51 mm vs. segmental: mean 54 mm SD 30 mm p < 0.001). The segmental group had a significantly higher mean TD and mean NTLD compared to the lobar group (TD: 227 Gy segmental vs 188 Gy lobar p = 0.04, NTLD: 234 Gy segmental vs 82 Gy lobar, p = < 0.01) (Table 1). There was no statistical difference for the other demographic characteristics such as age, gender, BCLC stage or ALBI grade between the lobar and segmental cohorts (p’s > 0.05) (Table 1). Mean Y90 activity was not significantly different between the segmental and lobar groups (segmental 2.52 GBq vs lobar 2.44 GBq p = 0.57). For further tumor dosimetry details, please see Table 1.
Over the course of the study, ALBI score significantly decreased for those in the lobar group (pre-treatment score − 2.15 vs 3 month score − 1.89 p < 0.01, and 6 month score − 1.91 p < 0.01). For the segmental group, there was no significant change between pre-treatment and follow-up ALBI scores (pre-treatment − 2.26 vs 3 months − 2.12 p = 0.08, vs 6 months − 2.25 p = 0.96).
Clinical and Laboratory Toxicities
The incidence of total number of clinical and laboratory adverse events (AE) was significantly different between the two groups when assessed at 3 and 6 months. Patients with progression of disease were excluded from severe toxicity analysis to reduce confounding effect of disease progression on potential liver toxicity. At 3 months, there was significantly more AEs of any grade in the cohort treated with segmental versus that of lobar therapy (48% segmental vs 31% lobar, p < 0.01) and fewer severe AE (2% segmental vs 8% lobar, p = 0.03) (Table 2). Multivariate analysis of the entire cohort revealed that NTLD > 110 Gy (HR 16.2 (14.0–18.3) p = 0.01) and Child–Pugh B or B (HR 32.6 (30.8–34.4) p = 0.001) were associated with grade 3 or greater clinical toxicities at 3 months (Table 3). For the lobar group, the same variables were associated with grade 3 toxicities at three months, NTLD > 110 Gy (HR 93.5, (91.0–96.1) p = 0.001) and Child–Pugh B or C (HR 19.6 (16.9–22.3) p = 0.03). No characteristics predicted grade 3 or greater clinical toxicities at 3 months for the segmental group (Table 3).
Individual event rates of clinical and laboratory adverse events at 3 months were not significantly different among the segmental or lobar groups (p > 0.05) (Table 4). Grade 3 AE at 3 months included the development of ascites requiring paracentesis (1 event (1%) in the segmental group and 3 events (2.6%) in the lobar group p = 0.83) (Table 4). At the 3-month follow-up, 6 patients in the segmental Y90-RE group were deceased and 4 patients in the lobar Y90-RE group were deceased (5% segmental vs 4% lobar, p = 0.32).
At 6 months, the total number of any grade AE observed was not statistically different between the groups (p > 0.05) and only severe AEs were significantly less prevalent in the segmental Y90-RE group (1% segmental vs 9% lobar, p < 0.01) (Table 2). For the entire cohort, NTLD > 110 Gy (HR 15.9 (13.7–18.1) p = 0.01) and Child–Pugh class B or C (HR 12.4 (8.0–16.8) p = 0.03) predicted grade 3 or greater clinical toxicities at 6 months (Table 3). For the lobar group, only Child–Pugh class B or C predicted serious clinical toxicity at 6 months (HR 44.1 (37.9–50.3) p = 0.01). For the segmental group, no factors predicted serious clinical toxicity (Table 3).
There were no significant differences between the incidences of individual severe clinical or laboratory toxicities at 6 months between the lobar and segmental groups (p > 0.05) (Table 4). At the 6-month follow-up, 9 patients in the segmental Y90-RE group were deceased and 17 patients in the lobar Y90-RE group were deceased (8% segmental vs 15% lobar p = 0.031).
NTL Dose Predicting Clinical Toxicities
For patients in the lobar Y90-RE group, a mean NTL dose of 111 Gy predicted severe AE with 89% sensitivity and 92% specificity (AUC = 0.95, p ≤ 0.0001) at 3 months (Fig. 2, Table 5), and a mean NTL dose of 112 Gy predicted severe AE with 80% sensitivity and 92% specificity (AUC = 0.958, p ≤ 0.0001) at 6 months (Fig. 2, Table 5). No NTL dose threshold was identified for any grade AE in the lobar Y90-RE group at 3 or 6 months (p > 0.05). For patients treated with segmental Y90-RE, no significant association was found between a NTL dose threshold and any grade or severe AE at 3 and 6 months (p’s > 0.05).
Receiver Operant Curves (ROC) for patients treated with Y90 RE predicting clinical toxicities at 3–6 months. a ROC demonstrating the ability to predict severe toxicity (CTCAE toxicity ≥ 3) at 3 months in the patients treated in a lobar fashion (black line, significant predictive value) and segmental fashion (blue line, insignificant predictive value). Curves to the right of or crossing the red line depicts an insignificant predictive value. b ROC demonstrating the ability to predict severe toxicity (CTCAE toxicity ≥ 3) at 6 months in the patients treated in a lobar fashion (black line, significant predictive value) and segmental fashion (blue line, insignificant predictive value). Curves to the right of or crossing the red line depicts an insignificant predictive value. CTCAE common terminology for Clinical Adverse Events, ROC receiver operating characteristic
Discussion
This study compared the treatment-related toxicity profiles of glass-based Y90-RE as it is used to treat HCC in a segmental or lobar fashion. Treatment-related clinical and laboratory toxicities over 3–6 months were evaluated with a particular focus on whether the Y90 dose delivered to the non-tumoral portion of the liver predicted clinically significant AE. Importantly, these two cohorts of patients (i.e. segmental treatment vs lobar treatment) demonstrated significant differences, with the lobar cohort showing a higher proportion of patients with worse ECOG performance status and higher Child–Pugh class. Nevertheless, the objective of this study was mainly to determine the NTL dose as an independent predictor of serious adverse events post-glass Y90. The study showed that a non-tumoral liver dose of greater than 112 Gy predicted severe AE at 3–6 months for patients who underwent lobar treatments with glass-based Y90. Conversely, no dose threshold was observed for predicting any grade AE in either lobar or segmental Y90-RE group. Additionally, no dose toxicity threshold was identified for severe AEs in patients who underwent segmental Y90-RE therapy. Smaller, clinical studies have reported similar dose thresholds. For example, a 2013 retrospective study by Garin et al. reported a surrogate of NTL dose, healthy-injected liver dose (HILD), > 100 Gy of glass Y90 in patients with low hepatic reserve was associated with grade 3 clinical AE (p = 0.03) [23]. In a retrospective 2015 study, values as high as 100 Gy to the whole liver were tolerated without treatment-related liver disease (hyperbilirubinemia and other laboratory changes) being observed when treated with glass Y90 [24]. In a 2017 study by Garin et al., a dose threshold of 120 Gy with a hepatic reserve < 30% was associated with post-Y90-RE decompensation (defined as any adverse laboratory changes) in an 85 patient case series treated with glass Y90 [25]. Lastly, in 2018, Chan et al. showed that a dose threshold of 100 Gy was associated with grade 2/3 AE in a prospective 35 patient single-arm study of primary and metastatic liver cancers primarily treated with glass Y90 [26]. This study reports a slightly higher NTL dose ceiling of 112 Gy to predict adverse effects. The authors hypothesize that this may be because Y90 SPET/CT was used for dosimetry calculations instead of the Y90 PET/CT in the other studies.
Clinical toxicities observed in this study were concordant with those reported in the literature. In a study by Moreno-Luna et al. that evaluated AE after lobar Y90-RE, fatigue and abdominal pain were reported in 46–30% of the participants post-Y90-RE, respectively [3]. The current study saw lower rates of both AE, potentially due to the increased use of proton pump inhibitors and steroids as gastrointestinal prophylaxis after Y90 [27, 28]. Laboratory toxicities have also been reported as common after glass-based lobar Y90-RE, with rates of transaminitis quoted at 11% and increased bilirubin in 20% of patients [6]. These reported rates are lower than those observed in our study, where transaminitis appeared in 27% of the lobar group and 15% in the segmental group, and hyperbilirubinemia in 24% of the lobar group and 30% of the segmental group. The recently published TARGET study demonstrated no associated NTL dose with hyperbilirubinemia [29]. The retrospective, multinational, single-arm study enrolled 209 participants and evaluated the NTL dose association with grade 3 AE hyperbilirubinemia. Due to low rates of hyperbilirubinemia (4.8%), there was no ability to establish a correlation between hyperbilirubinemia with non-tumor absorbed dose [29].
Death after Y90-RE is a rare occurrence within 30 days after Y90-RE [30]. The current study did not observe any deaths in the first month after Y90-RE. However, several deaths were observed within 6 months after Y90-RE for both patients treated in a segmental (n = 9) and lobar fashion (n = 17) (8% segmental vs 15% lobar p = 0.031). This increased death rate in the lobar group may explain why more clinical AEs were observed in the segmental group—as those patients who were facing hepatic decompensation in the lobar group may have been hospitalized outside the system or unable to come in for their follow-up appointments.
A strength of the current study is its detailed six-month follow-up and assessment of clinical and laboratory parameters for patients who underwent glass-based Y90-RE for HCC. The detailed clinical toxicity data collected may serve as an illness script for treating interventional radiologists and medical oncologists when formulating treatment plans. By having an expected patient side effect course, physicians can better inform patients what they may expect in the short and long term after Y90-RE. Critically, this study was able to determine a NTL dose threshold that predicts severe clinical toxicities at 3 and 6 months after lobar treatment. For this patient cohort, the threshold found, 112 Gy, can serve as a dosimetric planning ceiling. This study’s reported threshold is slightly higher than other published tumor dose thresholds of 100 Gy in patients undergoing lobar glass-based Y90-RE treatment.
This study has several limitations, including its retrospective design, length of study period beginning in 2013, lack of standardization in Y90-RE treatment protocols, the use of SPECT instead of PET/CT for dosimetry analysis, lack of censorship for subsequent therapy after Y90, and relatively limited clinical follow-up. Despite these limitations, a significant NTL dose threshold was found to predict severe treatment-related AE in patients with HCC undergoing Y90-RE with glass microspheres in a lobar fashion. Future, prospective, studies are required to confirm the proposed NTL dose threshold in this study and to further confirm its ability to maximize radioembolization tumor effect while minimizing patient AE after Y90-RE.
References
Garin E, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(1):17–29.
Shah RM, et al. Prognostic factors of unresectable hepatocellular carcinoma treated with yttrium-90 radioembolization: results from a large cohort over 13 years at a single center. J Gastrointest Oncol. 2021;12(4):1718–31.
Moreno-Luna LE, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36(3):714–23.
Lance C, et al. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22(12):1697–705.
Hilgard P, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52(5):1741–9.
Salem R, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497-507.e2.
El Fouly A, et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35(2):627–35.
Gabr A, et al. Correlation of Y90-absorbed radiation dose to pathological necrosis in hepatocellular carcinoma: confirmatory multicenter analysis in 45 explants. Eur J Nucl Med Mol Imaging. 2021;48(2):580–3.
Levillain H, et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol Imaging. 2021;48(5):1570–84.
Camacho JC, et al. (90)Y Radioembolization: multimodality imaging pattern approach with angiographic correlation for optimized target therapy delivery. Radiographics. 2015;35(5):1602–18.
Package Insert: TheraSphere Yttrium-90 Glass Microspheres. [cited 6 February 2022]; https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwi_h-qxxuz1AhVkjYkEHSDnBocQFnoECAMQAQ&url=https%3A%2F%2Fwww.accessdata.fda.gov%2Fcdrh_docs%2Fpdf%2Fh980006c.pdf&usg=AOvVaw3DTs9oqnqZ43-boZ5FwCzu.
Villalobos A, et al. Tumor-to-normal ratio relationship between planning technetium-99 macroaggregated albumin and posttherapy Yttrium-90 Bremsstrahlung SPECT/CT. J Vasc Interv Radiol. 2021;32(5):752–60.
Bolch WE, et al. MIRD pamphlet No 17: the dosimetry of nonuniform activity distributions–radionuclide S values at the voxel level medical internal radiation dose committee. J Nucl Med. 1999;40(1):11s–36s.
Bolch WE, et al. MIRD pamphlet No 21: a generalized schema for radiopharmaceutical dosimetry–standardization of nomenclature. J Nucl Med. 2009;50(3):477–84.
Elsayed M, et al. Comparison of Tc-99m MAA planar versus SPECT/CT imaging for lung shunt fraction evaluation prior to Y-90 radioembolization: are we overestimating lung shunt fraction? Cardiovasc Intervent Radiol. 2021;44(2):254–60.
Knešaurek K. An estimate of (90)Y dosimetry for bremsstrahlung SPECT/CT imaging in liver therapy with (90)Y microspheres. Eur J Radiol. 2021;139: 109698.
Potrebko PS, et al. SPECT/CT image-based dosimetry for Yttrium-90 radionuclide therapy: application to treatment response. J Appl Clin Med Phys. 2018;19(5):435–43.
Nelson A, Swallen A, Dewaraja Y. Evaluation of a voxel-based yttrium-90 (Y-90) dose calculation method for Bremsstrahlung SPECT using a liver phantom. J Nucl Med. 2016;57(supplement 2):306–306.
Skanjeti A, et al. Selective internal radiation therapy of hepatic tumors: morphologic and functional imaging for voxel-based computer-aided dosimetry. Biomed Pharmacother. 2020;132: 110865.
Sankhla T, et al. Role of resin microsphere y90 dosimetry in predicting objective tumor response, survival and treatment related toxicity in surgically unresectable colorectal liver metastasis: a retrospective single institution study. Cancers (Basel). 2021;13(19):4908.
Program, N.C.T.E. Common terminology criteria for adverse events (CTCAE). 2020 [cited 6 February 2022]; https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
Oken MM, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–55.
Garin E, et al. Boosted selective internal radiation therapy with 90Y-loaded glass microspheres (B-SIRT) for hepatocellular carcinoma patients: a new personalized promising concept. Eur J Nucl Med Mol Imaging. 2013;40(7):1057–68.
Chiesa C, et al. Radioembolization of hepatocarcinoma with 90Y glass microspheres: development of an individualized treatment planning strategy based on dosimetry and radiobiology. Eur J Nucl Med Mol Imaging. 2015;42(11):1718–38.
Garin E, et al. High impact of macroaggregated albumin-based tumour dose on response and overall survival in hepatocellular carcinoma patients treated with (90) Y-loaded glass microsphere radioembolization. Liver Int official J Int Assoc Study Liver. 2017;37(1):101–10.
Chan KT, et al. Hepatotoxic dose thresholds by positron-emission tomography after yttrium-90 radioembolization of liver tumors: a prospective single-arm observational study. Cardiovasc Intervent Radiol. 2018;41(9):1363–72.
South CD, et al. Yttrium-90 microsphere induced gastrointestinal tract ulceration. World J Surg Oncol. 2008;6:93.
Riaz A, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20(9):1121–30.
Lam M et al. A global evaluation of advanced dosimetry in transarterial radioembolization of hepatocellular carcinoma with Yttrium-90: the TARGET study. Eur J Nucl Med Mol Imaging. 2022;49(10):3340–52.
Padia SA, et al. Comparison of positron emission tomography and bremsstrahlung imaging to detect particle distribution in patients undergoing yttrium-90 radioembolization for large hepatocellular carcinomas or associated portal vein thrombosis. J Vasc Interv Radiol. 2013;24(8):1147–53.
Funding
This article was funded by Sirtex Medical and Boston Scientific Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nima Kokabi, MD, receives research support from SIRtex Medical. Nima Kokabi, MD receives educational grant from Boston Scientific. Bill S. Majdalany, MD, serves on the scientific advisory board of Balt Medical. The remaining authors have no conflicts of interests to disclose.
Compliance with Ethical Standards
This study was IRB-approved, and, due to the retrospective nature of this work, no formal consent was required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Oral Presentation at the 2022 SIR Annual Scientific Meeting.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Webster, L.A., Villalobos, A., Cheng, B. et al. Correlation of Non-tumoral Liver Dose with Treatment-Related Adverse Events in Patients with Hepatocellular Carcinoma Treated with Glass-Based Yttrium-90 Radioembolization. Cardiovasc Intervent Radiol 46, 60–68 (2023). https://doi.org/10.1007/s00270-022-03314-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-022-03314-9