Abstract
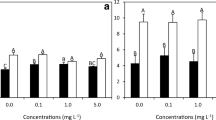
Bacteria and marine macroalgae form close associations, while various bacteria affect the morphogenesis and growth of macroalgae. Hyphomonas strains exhibit normal morphogenetic activity in protoplasts of the red alga Pyropia yezoensis (nori). However, the effects of the bacteria on the growth of Pyropia from protoplast cells to regenerated thalli remain unknown. Here, we assessed the growth of P. yezoensis and Pyropia tenera using combined cultures of three Hyphomonas strains (LNM10-16, SCM-2, and LNM-9) and three algal media (artificial seawater with vitamins, artificial seawater, and natural seawater) over 7 weeks. Third week after culture, the three Hyphomonas strains showed almost similar levels of normal growth activity for both Pyropia species. However, at 7 weeks, significant differences were observed among the three Hyphomonas strains in terms of length, length-to-width ratio, and normal morphology of Pyropia thalli. LNM10-16 significantly promoted the thalli length and length-to-width ratios of both Pyropia species in artificial seawater without vitamins and natural seawater, compared with the other two Hyphomonas strains. P. yezoensis cultured in artificial seawater with vitamins showed a much higher demand for LNM10-16 in development of the thalli length than P. tenera. These results may be explained by differences in the growth activities of Hyphomonas strains and the nutrient requirements of Pyropia species. Furthermore, the bacteria were more specifically attached to the rhizoid surfaces of both species. This study is the first to reveal that Hyphomonas strains affect the growth of Pyropia species by attaching to their rhizoids.






Similar content being viewed by others
Data Availability
The 16S rRNA gene sequence data are available in the GenBank under the accession numbers LC765679, AB758565, and AB758567.
Abbreviations
- ASW:
-
Artificial seawater medium without vitamin mixtures
- ASW + V:
-
Artificial seawater medium with vitamin mixtures
- NSW:
-
90% Natural seawater
References
Mumford TF, Miura A (1988) Porphyra as food: cultivation and economics. In: Lembi CA, Waaland JR (eds) Algae and human affairs. Cambridge University Press, Cambridge, pp 87–117
Miura A (1984) A new variety and a new form of Porphyra (Bangiales, Rhodophyta) from Japan: Porphyra tenera Kjellman var. tamatsuensis Miura, var. nov. and P. yezoensis Ueda form. narawaensis Miura, form. nov. J Tokyo Univ Fish 71:1–37
Miura A (1988) Taxonomic studies of Porphyra species cultivated in Japan, referring to their transition to the cultivated variety. J Tokyo Univ Fish 75:311–325
Ministry of the Environment Government of Japan (2020) Japanese red lists 2020. http://www.env.go.jp/press/107905.html (in Japanese)
Fujita Y, Saito M (1990) Protoplast isolation and fusion in Porphyra (Bangiales, Rhodophyta). Hydrobiologia 204(205):161–166
Mizukami Y, Okauchi M, Kito H, Ishimoto S, Ishida T, Fuseya M (1995) Culture and development of electrically fused protoplasts from red marine algae, Porphyra yezoensis and P. suborbiculata. Aquaculture 132:361–367
Fujita Y, Migita S (1985) Isolation and culture of protoplasts from some seaweeds. Bull Fac Fish Nagasaki Univ 57:39–45 (in Japanese)
Araki T, Aoki T, Kitamikado M (1987) Preparation and regeneration of protoplasts from wild-type of Porphyra yezoensis and green variant of P. tenera. Nippon Suisan Gakkaishi 53:1623–1627 (in Japanese)
Provasoli L (1958) Effect of plant hormones on Ulva. Biol Bull 144:375–384
Provasoli L, Pintner IJ (1980) Bacteria induced polymorphism in an axenic laboratory strain of Ulva lactuca (Chlorophyceae). J Phycol 16:196–201
Tatewaki M, Provasoli L, Pintner IJ (1983) Morphogenesis of Monostroma oxyspermum (Kütz.) Doty (Chlorophyceae) in axenic culture, especially in bialgal culture. J Phycol 19:409–416
Spoerner M, Wichard T, Bachhuber T, Stratmann J, Oertel W (2012) Growth and thallus morphogenesis of Ulva mutabilis (Chlorophyta) depends on a combination of two bacterial species excreting regulatory factors. J Phycol 48:1433–1447
Wichard T (2015) Exploring bacteria-induced growth and morphogenesis in the green macroalga order Ulvales (Chlorophyta). Front Plant Sci 6:86
Yamazaki A, Nakanishi K, Saga N (1998) Axenic tissue culture and morphogenesis in Porphyra yezoensis (Bangiales, Rhodophyta). J Phycol 34:1082–1087
Yamazaki A, Sekida S, Hanzawa N, Okuda K, Saga N (2000) Factors in growth and morphogenesis recovery under axenic conditions in Porphyra yezoensis (Bangiales, Rhodophyta), especially the effect on symbiotic bacteria in conditioned media. Bull Inst Oceanic Res Develop Tokai Univ 21:57–76 (in Japanese)
Mori S, Yamazaki A, Matsuyama-Serisawa K, Fukuda S, Mizuta H, Saga N (2004) Effect of two symbiotic bacteria for growth of Porphyra yezoensis (Rhodophyta, Bangiales) in axenic culture. Aquacult Sci 52:239–244 (in Japanese)
Handayani M, Sasaki H, Matsuda R, Takechi K, Takano H, Takio S (2014) Characterization of an epiphytic bacterium Neptunomonas sp. BPy-1 on the gametophytes of a red alga Pyropia yezoensis. Ame J Plant Sci 5:3652–3661
Matsuda R, Handayani ML, Sasaki H, Takechi K, Takano H, Takio S (2018) Production of indoleacetic acid by strains of the epiphytic bacteria Neptunomonas spp. isolated from the red alga Pyropia yezoensis and the seagrass Zostera marina. Arch Microbiol 200:255–265
Fukui Y, Abe M, Kobayashi M, Ishihara K, Oikawa H, Yano Y, Satomi M (2012) Maritalea porphyrae sp. nov., isolated from a red alga (Porphyra yezoensis), and transfer of Zhangella mobilis to Maritalea mobilis comb. nov. Int J Syst Evol Microbiol 62:43–48
Fukui Y, Abe M, Kobayashi M, Saito H, Oikawa H, Yano Y, Satomi M (2013) Algimonas porphyrae gen. nov., sp. nov., a member of the family Hyphomonadaceae, isolated from the red alga Porphyra yezoensis. Int J Syst Evol Microbiol 63:314–320
Fukui Y, Abe M, Kobayashi M, Saito H, Oikawa H, Yano Y, Satomi M (2013) Polaribacter porphyrae sp. nov., isolated from the red alga Porphyra yezoensis, and emended descriptions of the genus Polaribacter and two Polaribacter species. Int J Syst Evol Microbiol 63:1665–1672
Fukui Y, Kobayashi M, Saito H, Oikawa H, Yano Y, Satomi M (2013) Algimonas ampicilliniresistens sp. nov., isolated from the red alga Porphyra yezoensis, and emended description of the genus Algimonas. Int J Syst Evol Microbiol 63:4407–4412
Fukui Y, Abe M, Kobayashi M, Shimada Y, Saito H, Oikawa H, Yano Y, Satomi M (2014) Sulfitobacter porphyrae sp. nov., isolated from the red alga Porphyra yezoensis. Int J Syst Evol Microbiol 64:438–443
Fukui Y, Abe M, Kobayashi M, Satomi M (2015) Sulfitobacter pacificus sp. nov., isolated from the red alga Pyropia yezoensis. Antonie Van Leeuwenhoek 107:1155–1163
Nakamura Y, Sasaki N, Kobayashi M et al (2013) The first symbiont-free genome sequence of marine red alga, susabi-nori (Pyropia yezoensis). Plos One 8:e57122
Fukui Y, Abe M, Kobayashi M, Yano Y, Satomi M (2014) Isolation of Hyphomonas strains that induce normal morphogenesis in protoplasts of the marine red alga Pyropia yezoensis. Micob Ecol 68:556–566
Provasoli L, Carlucci AF (1974) Vitamins and growth regulators. In: Stewart WDP (ed) Algal Physiology and Biochemistry. Blackwell Scientific Publications, Oxford, pp 741–787
Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG (2005) Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438:90–93
Yamada S, Shibata Y, Takayama M, Narita Y, Sugawara K, Fukuda M (1996) Content and characteristics of vitamin B12 in some seaweeds. J Nutr Sci Vitaminol 42:497–505
Yamada S, Sasa-Kamasuzu M, Yamada K, Fukuda M (1996) Release and uptake of vitamin B12 by asakusanori (Porphyra tenera) seaweed. J Nutr Sci Vitaminol 42:507–515
Takenaka S, Takubo K, Watanabe F, Tanno T, Tsuyama S, Nanano Y, Tamura Y (2003) Occurrence of coenzyme forms of vitamin B12 in a cultured purple laver (Porphyla yezoensis). Biosci Biotechnol Biochem 67:2480–2482
Ogata E (1970) On a new algal culture medium SWM-III. Bull Jpn Soc Phycol 18:171–173 (in Japanese)
Ghaderiardakani F, Califano G, Mohr JF, Abreu MH, Coates JC, Wichard T (2019) Analysis of algal growth- and morphogenesis-promoting factors in an integrated multi-trophic aquaculture system for farming Ulva spp. Aquacult Environ Interact 11:375–391
Motomura T, Hishinuma T (1997) Methods for immunofluorescence observation on the algal cytoskeleton. Jpn J Phycol 45:175–181 (in Japanese)
Polne-Fuller M, Gibor A (1984) Developmental studies in Porphyra. I. Blade differentiation in Porphyra perforata as expressed by morphology, enzymatic digestion, and protoplast regeneration. J Phycol 20:609–616
Polne-Fuller M, Biniaminov M, Gibor A (1984) Vegetative propagation of Porphyra perforata. Hydrobiologia 116(117):308–313
Polne-Fuller M, Gibor A (1987) Calluses and callus-like growth in seaweeds: induction and culture. Hydrobiologia 151(152):131–138
Notoya M (1999) ‘Seed’ production of Porphyra spp. by tissue culture. J Appl Phycol 11:105–110
Ar Gall E, Chiang Y-M, Kloareg B (1993) Isolation and regeneration of protoplasts from Porphyra dentata and Porphyra crispata. Eur J Phycol 28:277–283
Waaland JR, Dickson LG, Watson BA (1990) Protoplast isolation and regeneration in the marine red alga Porphyra nereocystis. Planta 181:522–528
Provasoli L, McLaughlin JJA, Droop MR (1957) The development of artificial media for marine algae. Archiv ftir Mikrobiologie 25:392–428
Iwasaki H (1965) Nutritional studies of the edible seaweed Porphyra tenera I. The influence of different B12 analogues, plant hormones, purines and pyrimidines on the growth of Conchocelis. Plant Cell Physiol 6:325–336
Niwa K, Kikuchi N, Aruga Y (2005) Morphological and molecular analysis of the endangered species Porphyra tenera (Bangiales, Rhodophyta). J Phycol 41:294–304
De Clerck O, Kao S-M, Bogaert KA et al (2018) Insights into the evolution of multicellularity from the sea lettuce genome. Curr Biol 28:2921–2933
Alsufyani T, Califano G, Deicke M, Grueneberg J, Weiss A, Engelen AH, Kwantes M, Mohr JF, Ulrich JF, Wichard T (2020) Macroalgal-bacterial interactions: identification and role of thallusin in morphogenesis of the seaweed Ulva (Chlorophyta). J Exp Bot 71:3340–3349
Dhiman S, Ulrich JF, Wienecke P, Wichard T, Arndt H-D (2022) Stereoselective total synthesis of (–)-thallusin for bioactivity profiling. Angew Chem Int Ed 61:e202206746
Matsuo Y, Imagawa H, Nishizawa M, Shizuri Y (2005) Isolation of an algal morphogenesis inducer from a marine bacterium. Science 307:1598
Yamamoto H, Takagi Y, Yamasaki N, Mitsuyama T, Kasai Y, Imagawa H, Kinoshita Y, Oka N, Hiraoka M (2018) Syntheses of thallusin analogues and their algal morphogenesis-inducing activities. Tetrahedron 74:7173–7178
Nakanishi K, Nishijima M, Nomoto AM, Yamazaki A, Saga N (1999) Requisite morphologic interaction for attachment between Ulva pertusa (Chlorophyta) and symbiotic bacteria. Mar Biotechnol 1:107–111
Singh RP, Mantri VA, Reddy CRK, Jha B (2011) Isolation of seaweed-associated bacteria and their morphogenesis-inducing capability in axenic cultures of the green alga Ulva fasciata. Aquat Biol 12:13–21
Tapia JE, González B, Goulitquer S, Potin P, Correa JA (2016) Microbiota influences morphology and reproduction of the brown alga Ectocarpus sp. Front Microbiol 7:197
Tanabe Y, Okazaki Y, Yoshida M, Matsuura H, Kai A, Shiratori T, Ishida K, Nakano S, Watanabe MM (2015) A novel alphaproteobacterial ectosymbiont promotes the growth of the hydrocarbon-rich green alga Botryococcus braunii. Sci Rep 5:10467
Kessler RW, Weiss A, Kuegler S, Hermes C, Wichard T (2018) Macroalgal-bacterial interactions: role of dimethylsulfoniopropionate in microbial gardening by Ulva (Chlorophyta). Mol Ecol 27:1808–1819
Chisholm JRM, Dauga C, Ageron E, Grimont PAD, Jaubert JM (1996) ‘Roots’ in mixotrophic algae. Nature 381:382
Zhang Y, Yan X-H, Aruga Y (2013) The sex and sex determination in Pyropia haitanensis (Bangiales, Rhodophyta). Plos One 8:e73414
Ouriques LC, Schmidt ÉC, Bouzon ZL (2012) The mechanism of adhesion and germination in the carpospores of Porphyra spiralis var. amplifolia (Rhodophyta, Bangiales). Micron 43:269–277
Alsufyani T, Weiss A, Wichard T (2017) Time course exo-metabolomic profiling in the green marine macroalga Ulva (Chlorophyta) for identification of growth phase-dependent biomarkers. Mar Drugs 15:14
Quintero EJ, Busch K, Weiner RM (1998) Spatial and temporal deposition of adhesive extracellular polysaccharide capsule and fimbriae by Hyphomonas strain MHS-3. Appl Environ Microbiol 64:1246–1255
Langille SE, Weiner RM (1998) Spatial and temporal deposition of Hyphomonas strain VP-6 capsules involved in biofilm formation. Appl Environ Microbiol 64:2906–2913
Morikawa T, Mine T, Tsuge K (2019) Effects of laver density in laver culture nets on dried laver production. Aquacult Sci 67:257–264 (in Japanese)
Abe M, Tara C, Fujiki S, Kawasaki S, Murase N (2022) Effects of soaking in arginine or ornithine immediately after conchospores adhere to substrate in adherence strength and growth of Neopyropia yezoensis. Aquacult Sci 70:179–191 (in Japanese)
Acknowledgements
We are grateful to Dr. K. Kimura for valuable advice about the observation of fluorescence microscopy. We also thank N. Hatano for assisting with the experiments.
Funding
This work was supported by the project of Global Warming Countermeasures from Ministry of Agriculture, Forestry and Fisheries (Grant Number JPJ005317).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, and performed material preparation, data collection, and analysis. Youhei Fukui wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fukui, Y., Abe, M. & Kobayashi, M. Effects of Hyphomonas Strains on the Growth of Red Algae Pyropia Species by Attaching Specifically to Their Rhizoids. Microb Ecol 86, 2502–2514 (2023). https://doi.org/10.1007/s00248-023-02257-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02257-z