Abstract
We describe trends in fast, high resolution elemental imaging by laser ablation–inductively coupled plasma mass spectrometry (LA–ICPMS). Recently developed low dispersion LA cells deliver quantitative transport of ablated aerosols within 10 ms and also provide enhanced sensitivity compared to conventional LA cells because the analyte ion signal becomes less diluted during aerosol transport. When connected to simultaneous ICPMS instruments, these low dispersion LA cells offer a platform for high speed and high lateral resolution shot-resolved LA–ICPMS imaging. Here, we examine the current paradigms of LA–ICPMS imaging and discuss how newly developed LA cell technology combined with simultaneous ICPMS instrumentation is poised to overcome current instrumental limitations to deliver faster, higher resolution elemental imaging.

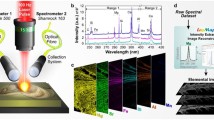
On means for obtaining high-speed, high-resolution, multielemental images is to combine new lowdispersion laser-ablation cell technology with an inductively coupled plasma time-of-flight mass spectrometer (ICP-TOFMS). Here, we show three selected-isotope LA-ICP-TOFMS images of a hetereogeneous Opalinus clay sample
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a growing trend in laser ablation–inductively coupled plasma mass spectrometry (LA–ICPMS) imaging toward higher lateral resolution and faster image acquisition times. Several LA–ICPMS imaging studies with lateral resolutions at or below 5 μm have recently been reported for both the analysis of soft biological tissues [1, 2] and hard materials [3–6]. Motivations for this improved resolution include the need to measure subcellular element distributions in tissues, to quantitatively image microdomains and heterogeneities in geological samples, and to examine man-made materials, such as archeological objects or semiconductor electronics [7–9]. Concurrent with the push toward higher lateral resolution imaging is a move toward high speed LA–ICPMS imaging, both to facilitate acceptable high resolution image acquisition times and to reduce costs. Further, research emphasizes that high resolution and low acquisition time ICPMS imaging should not come at the expense of the already well-established advantages of LA–ICPMS, namely, the quantitative determination of multiple isotopes, from trace-to-major concentrations. In this article, we discuss the emergence of low dispersion LA cell technology for high resolution LA–ICPMS imaging and how this technology dictates a shift toward simultaneous multi-element ICPMS instrumentation. To provide context, we present brief surveys of current LA–ICPMS imaging strategies and the requirements and challenges of high resolution LA–ICPMS imaging. Recent results of low dispersion LA and high resolution multi-elemental LA–ICPMS imaging are provided as evidence of this growing trend. We envision that high lateral resolution LA–ICPMS imaging will be a future direction of elemental imaging; however, realizing this goal requires ICPMS instrumentation fit for the task.
Basics of the LA–ICPMS imaging experiment
Laser ablation (LA) was first developed as a solid-sample introduction technique for ICPMS in 1985 [10] and has since been applied broadly for the quantification of trace to major elements and isotopes in solid samples [11, 12]. Figure 1 is a schematic diagram of a general LA–ICPMS imaging setup. In LA–ICPMS, a solid sample is enclosed in a chamber filled with inert gas and a pulsed laser beam is used to eject (ablate) minute quantities of material from the sample surface. Ablated aerosol is then transferred online, via a carrier gas stream, to an ICPMS instrument for elemental and/or isotopic analysis. To obtain an LA–ICPMS elemental image, a sample is scanned underneath the pulsed laser beam at a known speed while time-dependent ICPMS signals are collected. ICPMS signals are matched to ablation position and then assembled into a 2D array and projected onto a false-color scale to construct one or more isotope-specific images.
Schematic diagram of LA–ICPMS imaging experiment. In LA–ICPMS imaging, a sample is scanned underneath a fixed-position pulsed laser (translation stage not shown) and consecutive horizontal line scans are taken to measure pixel intensities across each row of the elemental image. LA can be operated in either a pulse-resolved mode, in which the ablated aerosol from each laser shot is quantitatively transferred to the ICPMS between laser shots, or in a continuous-scan mode, in which the laser ablates at a rate faster than the transport time of the aerosols and a quasi-steady-state signal is obtained that varies smoothly as analyte abundance changes across a sample surface. In Fig. 1, pulse-resolved mode signals are depicted as analyte signal spikes, whereas the continuous-scan data is a steady flat-topped signal
The simplicity of LA–ICPMS is appealing; one simply shoots a laser at a surface and transfers ablated aerosols into the ICPMS system. However, several issues—such as matrix-dependent ablation rates, ablated particle size distributions, and elemental fractionation—must be understood and accounted for to use LA–ICPMS as a quantitative method [13, 14]. To this end, many researchers have performed fundamental and applied LA–ICPMS studies on the use of various laser systems, gas mixtures for ablation, and calibration methods. Research in all of these areas continues to advance analysis by LA–ICPMS approaches. For the interested reader, reports on the current state of the art and best practices for quantitative LA–ICPMS analysis are treated in a number of review and tutorial articles [11, 12, 15]. Apart from the phenomena that affect quantification in LA–ICPMS, many additional factors impact the ability to attain a high resolution LA–ICPMS image. For instance, laser beam focus and shape, X-Y motor stage precision and accuracy, sample surface flatness, sample matrix composition, ablated aerosol transport characteristics, and the speed and sensitivity of the mass analyzer can all influence the lateral resolution achievable. Detailed evaluation of all these parameters is beyond the scope of this article. Instead, we discuss how newly developed fast LA technology and high speed multi-elemental ICPMS instrumentation can overcome fundamental challenges of aerosol transport and detection to present a clear pathway to high speed, high resolution LA–ICPMS imaging.
Imaging modes for LA–ICPMS: advantages and limitations
LA–ICPMS imaging is typically achieved either in a “continuous-scan” or a “spot-resolved” imaging mode. Because these two approaches offer different performance in terms of image resolution, elemental concentration accuracy, detection limits, and time of analysis, their advantages and limitations are described here within the context of high speed, high resolution LA–ICPMS imaging.
In continuous-scan imaging—which is the most common approach—the laser is fired at a high repetition rate and the sample is slowly scanned underneath the focused laser spot. The period between laser shots is often much shorter than the washout time of the ablation cell, which causes the composition of ablated aerosols delivered to the ICPMS system to be “pulse-mixed.” The ICPMS signal is collected in a time-resolved manner and the laser scan speed is adjusted so that distance covered in a single mass scan is equal to the LA spot size (i.e., one pixel) [16]. In this way, a number of overlaid laser ablation events comprise the signal for each pixel, but the set of LA events across which each m/z is measured are not the same. The collection of cumulative (pulse-mixed) signals from multiple ablation events for each pixel of an elemental image allows more material to be ablated (provided the sample is relatively thick) and, thus, for a given laser spot size can improve detection limits and reduce spectral intensity skew error [17, 18]. However, negative side effects of this approach include the loss of depth resolution, as well as image blurring and/or inaccurate concentration measurement due to pulse-to-pulse mixing. To overcome the effects of pulse-to-pulse mixing, several researchers have attempted to deconvolve pulse-mixed LA–ICPMS signals [19–22]; however, in practice, inconsistent LA peak profiles attained across heterogeneous surfaces and insensitivity to low abundance signal variation limit the usefulness of pulse deconvolution. For high resolution quantitative ICPMS imaging, resolution uncertainty caused by pulse-to-pulse mixing precludes the continuous-scan imaging approach.
As an alternative to continuous-scan imaging, in spot-resolved imaging, LA is applied at a grid of positions across the sample surface, often with ablation craters arranged edge-to-edge. Here, ICPMS signals can be unequivocally assigned to an LA position because the effects of pulse-to-pulse mixing are eliminated by waiting for the ablated aerosol signal to decline to background between laser spots [23–25]. This approach is more time consuming than the continuous-scan method because the delay time between LA positions can be up to several seconds. Apart from slow image collection times, measurements can suffer from spectral intensity skew error if a scanning MS instrument is used and the elemental signal intensities from each spot are not constant across the mass scan time. For high resolution LA–ICPMS imaging, the spot-resolved approach is preferred because image blurring is minimized; to overcome time-of-analysis limitations, fast aerosol transport LA systems can be used.
Instrumentation for high resolution LA–ICPMS imaging: past and present
The most straightforward way to obtain high lateral resolution is to use small laser ablation spots. Currently, round LA spots with diameters down to 1 μm are possible for UV nanosecond laser ablation [1], and near-field LA has been investigated to deliver ablation below the diffraction limit [15]. However, as LA spot size decreases, the amount of material ablated decreases rapidly (as the square of the radius), so that high sensitivity detection is required even to measure signals from major or minor components. Moreover, because resolution in LA–ICPMS imaging depends on the accurate correlation of LA position to transient ICPMS signals, the temporal structure of the ablated aerosols and the method for measuring the resultant signals play an important role in the quality of the elemental image obtained.
Mass analyzers
The most common instrumental setups for LA–ICPMS make use of quadrupole or sector-field mass spectrometers (QMS or SFMS) that detect ions of a single mass-to-charge (m/z) value at a time. Though the extreme sensitivities of modern QMS and SFMS instruments enable low absolute detection limits (attograms) [26], sequential m/z determination has a number of limitations for the detection of transient signals. First, precision is limited because of ICP flicker noise. Second, elemental signals can be biased relative to one another because ion signals of disparate m/z are measured at different points along the temporal profile (i.e., spectral intensity skew error). Third, relatively long acquisition times are required for multi-elemental determinations. In fact, these limitations have been well discussed. In the conclusions of his pioneering LA–ICPMS paper, Gray comments:
“Because of the transient nature of the signal, a scanning instrument is at a disadvantage for quantitative measurements. Although scanning at high rates enables acceptable ratios to be obtained on isotopes over a small mass range, over a wider scan significant changes in the ion concentration in the plasma can occur between elements in different parts of the mass range [10].”
Since 1985, ICP–QMS and SFMS instruments have improved remarkably: they are now more sensitive, and support faster dwell times, mass-switching times, and settling times. These advances have helped maintain the market dominance of scanning-based ICPMS instruments and make them fit-for-purpose for almost all applications. Modern ICP–QMS instruments can deliver complete mass spectrum scans in about 90 s with 500-μs dwell times, while still preserving moderate sensitivity [27]. Most often, ICP–QMS instruments are operated in a peak-hopping mode for multi-element LA–ICPMS imaging. In this mode, dwell times for each analyte isotope must be optimized to deliver required detection limits; the fastest mass cycle times reported for trace-element bio-imaging with a narrow element menu with 13 m/z values is about 240 ms [16]. These scan speeds, even though much faster than those available on Gray’s system, still require compromises to be made between high spatial resolution, measurement accuracy, and time of analysis. Moreover, the speed of scanning MS technology faces a physical limitation: mass scanning simply cannot be faster than the transport times of ions through the mass-separation element [28]. In their review article on LA–ICPMS microanalysis, Heinrich et al. aptly explain the dilemma of LA analysis with scanning-based MS:
“Multi-element analysis of a limited amount of sample material demands a trade-off between signal intensity and signal duration, for optimal quantification of integrated intensities. A large cell volume (e.g., 20–100 cm3) leads to relatively long signals of low intensity, permitting a greater number of measurements on each element over the duration of the signal, and thus a potentially more representative and reproducible analysis [29].”
Modern LA–ICPMS imaging approaches—especially with the continuous-scan imaging approach—have been designed to compensate for the scanning nature of ICP–QMS and SFMS instruments; however, with these systems, reports with laser spot sizes below 15 μm are rare, and routine images are collected at a pixel generation rate of ~1 s/pixel (4 Hz is the fastest reported) [16].
Many limitations of scanning-type ICPMS instruments are obviated when multichannel ICPMS instruments are employed. Simultaneous multi-elemental ion detection has been shown to reduce multiplicative noise through signal ratioing, to eliminate spectral intensity skew error, and to offer improved measurement duty cycles for multi-element analysis [18, 30]. Currently, there are two ICPMS instrument designs available that deliver complete, simultaneous elemental coverage: ICP–time-of-flight mass spectrometry (ICP–TOFMS) and the ICP–Mattauch-Herzog mass spectrograph (ICP–MHMS) [31, 32]. Again, these instrument configurations are not new; Hieftje et al. discussed the benefits of simultaneous TOFMS detection for the analysis of transients by ICPMS in 2001:
“When sampling devices such as flow injection, laser ablation, electrothermal vaporization, or chromatography are employed, the user must choose between broad elemental or isotopic coverage and signal-to-noise ratio (S/N). In turn, compromised S/N means lower precision or poorer detection limits…. [A] time-of-flight mass spectrometer, provides excellent detection limits, resolving power better than commercial quadrupole mass filters, precision of at least 0.02 % rsd in a ratioing mode, and extraordinarily high speed for use with transient sampling devices [33].”
While the use of ICP–TOFMS for the analysis of transient LA signals has indeed been investigated previously [34–36], broad application of this method was stymied by the insufficient sensitivity of early ICP–TOFMS instrumentation and low mass spectrum generation rates due to signal averaging technology of the time [35]. Importantly, these limitations are not fundamental to ICP–TOFMS, but technology-limited. Recent advances have resulted in ICP–TOFMS instrumentation that delivers simultaneous full-spectrum detection at high speed, medium mass resolution, moderate-to-high sensitivity, and over four orders of magnitude simultaneous dynamic range [30, 37]. The performance characteristics of this recent ICP–TOFMS technology encourages us to look “back to the future” and consider the potential of this instrumentation combined with low dispersion LA technology for elemental imaging.
Low dispersion LA
In LA, the amount of time it takes for ablated aerosol to be swept out of the ablation cell and through the sample transfer tubing is called the washout time. In conventional large-format single-chamber LA cells, the transient profile of transported aerosols follows an exponential decay, in which the time constant is controlled by the internal volume of the aerosol transport system. Washout times on the order of 0.5–30 s are typical for such cells. However, there are several reasons to move beyond these conventional cell designs and toward low dispersion LA cells for LA–ICPMS imaging studies. First, high speed aerosol transfer eliminates the effects of pulse-to-pulse mixing, which leads to more precise and quantitative assignment of MS signals to LA position. Second, fast aerosol transport improves measurement sensitivity (cps/ppm) because the instantaneous concentration of analyte in the ICP is increased [38, 39]. In this way, the time-dependent concentration of analyte from a small laser ablation spot can be on par with the in-plasma concentration available with a larger laser spot and a conventional LA cell. Finally, fast aerosol transport improves the time of analysis for spot-resolved LA–ICPMS imaging.
Much work in recent years has focused on minimizing the duration of LA-produced signals. Figure 2 presents an overview of recent LA cell designs used to achieve fast aerosol transport and Table 1 lists the performance characteristics of these cells and others to illustrate this trend in fast-washout LA cell design. The most straightforward way to reduce aerosol transport time is to reduce the internal volume of the ablation chamber and aerosol transfer tubing. Here, in-torch LA serves as an extreme example: with the LA cell and transfer tubing eliminated, in-torch LA–ICPMS peak widths between 1 and 3 ms have been achieved [39, 40]. Unfortunately, in-torch LA is not applicable to imaging because there is no room for lateral scanning with a stage motor or for a large sample. Recently, LA cells with laminar gas flows have been used to efficiently entrain aerosol to improve ablated particle uptake and reduce aerosol transport times [20, 41]. Further advancements have come from the development of “active” ablated aerosol transport, in which cell geometry and flow rates are used to create gas pressure gradients across the LA cell to extract and confine ablated aerosol particles to reduce dispersion [42–44]. To date, the fastest aerosol transport times have been obtained with low volume, in-line aerosol transport systems that sweep ablated aerosol directly from the LA cell to the ICP torch without additional gas mixing or bends in tubing; these systems provide full width 1 % maximum (FW0.01 M) signal peak widths of 5–10 ms for laser spot sizes of 1–10 μm [3, 5, 45] and are highlighted in Fig. 2.
Schematic diagrams of three recent low dispersion LA cells; diagrams were redrawn from their original publications for clarity. a A half-open in-tube LA cell with very small available inner volume and a high helium flow rate orthogonal to ablation expansion axis provides LA peak widths (FW0.01 M) of ~6 ms. (Adapted from [3] with permission of The Royal Society of Chemistry.) b The “tube” LA cell is a two-volume cell in which the sample is housed in a large chamber filled with helium and ablated aerosol transport occurs in a small inner volume tube with a stream of argon carrier gas orthogonal to the ablation axis. This system delivers complete aerosol transport with a peak width (FW0.01 M) of less than 10 ms. The two-volume design makes the system ideal for imaging large samples and allows the sample to be scanned without significantly affecting the gas dynamics of aerosol transport. (Adapted with permission from [5]. Copyright 2015 American Chemical Society.) c The two-volume low dispersion LA cell developed in the group of Prof. B. Sharp consists of a small “sniffer” cell (top diagram) housed in a helium-filled chamber (lower diagram). Ablated aerosol is transferred from sniffer cell in helium carrier gas through narrow-bore tubing directly to the base of the plasma. This cell produces FW0.01 M single-shot peak widths of 5 ms; moreover, it is well suited for imaging experiments because a sample can be scanned underneath the stationary sniffer cell. (Adapted with permission from [45]. Copyright 2015 American Chemical Society)
Low dispersion LA–ICPMS: high resolution and high speed imaging
High speed and high resolution actually go hand in hand: low dispersion LA eliminates pulse-to-pulse mixing and enables use of smaller LA spot sizes while also significantly improving time of analysis for pulse-resolved imaging. In Fig. 3, we demonstrate the single-shot elemental sensitivity enhancement gained by low dispersion LA with an aerosol transport time of 10 ms versus more conventional LA with a washout of ~500 ms.
Signal enhancement of 238U+ in terms of counts per time-of-flight extraction for the low dispersion tube cell (green) versus a conventional LA cell with a washout time of ~500 ms (purple). The peak height of the low dispersion LA–ICP–TOFMS signal is roughly 50 times greater than the long-washout signal; the inset shows a zoom-in of the signal intensity axis to visualize the broader 500-ms-long signal. Both signals were acquired for the ablation of NIST SRM 610 glass, with an LA diameter of 10 μm, equal fluence, and on the same ICP–TOFMS instrument
Because the signal is detected across a shorter time period, less noise is integrated, which increases the S/N ratio of the measurement. With this low dispersion LA setup, single-shot detection limits are in the single-digit ppm level for a 10-μm-diameter round LA spot, which translates to an absolute detection limit of 60 attograms for 238U (even though the ICP–TOFMS instrument used here offers modest single-isotope sensitivity compared to state-of-the-art ICP–QMS and SFMS instruments) [5]. Extremely fast aerosol transport times require equally fast ICPMS measurement. For scanning-based ICPMS instruments, only one or two isotopes could be measured within a transient profile of 10 ms, and so while sensitivity and detection limits can be boosted by low dispersion LA, the multi-element capacity of LA–ICPMS imaging is compromised [3, 45]. For ICP–TOFMS, there is no compromise between transient speed and number of analyte elements measureable, so signal enhancement by low dispersion LA is an effective method to overcome limitations of instrument sensitivity and to allow quantitative detection of analytes from increasingly small LA spots.
In Fig. 4, we present a summary of recent multi-element LA–ICP–TOFMS imaging studies performed in our lab with a low dispersion “tube” LA cell and a prototype TOFMS instrument [6]. In this study, a lateral resolution (LA spot diameter) of 5 μm and a laser repetition rate of 20 Hz were used for single-shot pulse-resolved LA–ICPMS imaging of a cesium-infiltrated Opalinus clay rock sample. At a repetition rate of 20 Hz, no pulse-to-pulse mixing occurs so that the integrated signal of every isotope is representatively measured from each laser shot and the lateral resolution is fixed at 5 μm. Moreover, because all elements are measured for each pixel, a 100 % oxide normalization approach can be applied for pixel-by-pixel quantification. In terms of improvement of the speed of analysis, the full-spectrum LA–ICP–TOFMS imaging approach is roughly 1800 times faster than state-of-the-art full-spectrum LA–ICP–QMS imaging if the acquisition speed is calculated as the number of pixels recorded per unit time, and at least five times faster than high speed LA–ICP–QMS imaging with a limited element menu. While low dispersion LA–ICPMS has thus far only been applied to high resolution elemental imaging studies (pixel size ≤ 10 μm), this approach should also be amenable to courser resolution (10–50 μm spot size) LA–ICPMS imaging studies to allow for extremely fast image acquisition. Importantly, the concurrent time-of-analysis and single-shot quantification improvements demonstrated with ICP–TOFMS are simply not available on scanning-based ICPMS instruments. To obtain high lateral resolution multi-element imaging with a scanning-based MS, the signal must be temporally stretched, and the mass analyzer must provide relatively higher sensitivity to overcome dilution of the ablated aerosol. To date, low dispersion LA combined with scanning-based ICPMS instruments has only been used to monitor a single isotope, and here the lack of an internal standard eliminates the possibility of quantification [3, 4]. While low dispersion LA combined with scanning-based ICPMS instruments has proven useful for proof-of-principle demonstrations, almost all real-world applications demand multi-element imaging.
Summary of high speed, high resolution LA–ICP–TOFMS imaging of a cesium-infiltrated Opalinus clay rock sample [6]. a Data for each two-dimensional LA–ICP–TOFMS image is collected as series of time-resolved TOFMS signals, with each line scan separated by a narrow delay time. Within each line scan, the laser is fired such that each laser shot ablates at a new location (pixel) directly adjacent to the preceding spot. Together, the ablation positions in each line scan create a rectangular grid of edge-to-edge ablation locations spread across the sample surface. In this case, the laser was fired at a repetition rate of 20 Hz, TOFMS signal was recorded at a full-spectrum generation rate of 333 Hz to provide several data points across each LA–produced signal, and the complete 5000-pixel image (500 × 250 μm) was acquired within 7.5 min. b Selected TOFMS signals from a single spot-resolved line scan of the elemental image demonstrate baseline separation between each LA signal. The three isotopic signals are characteristic of three domains of the cesium-infiltrated Opalinus clay sample, namely, the domains of clay (indicated by Al), calcium carbonate (Ca), and pyrite (Fe); while only three elements are shown, all isotope signals are available. c Selected 2D-LA–ICP–TOFMS single-isotope intensity images obtained from integrating the ion counts from each LA shot; the white rectangles highlight the image row developed from the time trace shown in b. d Quantification by 100 % oxide normalization is used to calculate concentration at each pixel. A 3D quantified LA–ICP–TOFMS is shown for Cs2O, which is a minor abundance element of the Opalinus clay sample and is localized to the clay domain. This 100,000-pixel 3D elemental image was collected in a real lab time of less than 3 h
Outlook
High spatial resolution and high speed multi-elemental LA–ICPMS imaging offers attractive performance characteristics for a number of applications in areas such as bio-imaging and geological imaging. However, the continued development of this approach and a future transition from technical demonstration to research applications requires a shift in the ICPMS instrumentation paradigm. ICP–TOFMS instrumentation for transient analyses such as multiplexed cytometry, single nanoparticle detection, or isotope-tagged tissue imaging has already gained considerable attention [1, 37, 46].
While the benefits of high resolution elemental imaging are certainly application-dependent, other advantages of the approach presented here, such as reduced costs by high speed imaging, are clear and compelling. However, speed-of-analysis improvements alone are not enough to shift from the paradigm of scanning-based ICPMS instruments. Instead, it is the unique measurement capabilities of low dispersion LA combined with simultaneous ICPMS measurements that will drive the continued development of fast, sensitive, multichannel ICPMS instrumentation. To date, ICP–TOFMS instruments have been proven to be up to this challenge, but in the future, other simultaneous mass analyzer designs, such as MHMS, multi-collector ICPMS, or distance-of-flight MS [47], might be attractive alternatives.
At the beginning of this article, we stated that a goal of high resolution LA–ICPMS imaging is to provide needed resolution without sacrificing the other well-established benefits of LA–ICPMS. Currently, low dispersion LA–ICP–TOFMS offers a best detection limit of around 100 ppm for a 1-μm LA spot diameter, whereas LA–ICP–QMS offers a detection limit of ~8 ppm for the measurement of only a single isotope [3]. To maintain the advantages of LA–ICPMS as a trace-element imaging method, continued improvements to absolute sensitivity through both mass analyzer developments and controlled sample introduction schemes are required. Here, recently developed (and commercially available) next-generation ICP–TOFMS instruments that offer higher sensitivity and better mass resolution should play a key role in the development of high resolution quantitative LA–ICPMS imaging [48]. Quantitative elemental imaging of biological and inorganic samples with this next-generation ICP–TOFMS instrumentation will further indicate the potential of this technology to be applied across disciplines such as medicine, biology, geology, archeology, etc. In addition to advanced simultaneous ICPMS instrumentation, implementation of “routine” high resolution LA–ICPMS imaging will require commercial LA systems designed to deliver small LA spots (down to 1 μm) in combination with low dispersion LA cells and precise lateral scanning stages. As low dispersion LA technologies and the benefits of high resolution LA–ICPMS imaging are quickly becoming established, we expect these advances to be a driving factor for LA systems released in the near future. Finally, fundamental research into spot-resolved quantification strategies for high resolution LA–ICPMS of multi-domain surfaces, multi-elemental three-dimensional LA imaging, and incorporation of new femtosecond LA technologies all have potential to further advance high resolution elemental imaging.
The development of simultaneous multichannel ICPMS instruments and low dispersion LA cells can be thought of as a kind of co-evolution: recent advances in each system have boosted the performance of the other and also reinforced its significance. This trend is expected to continue. Together, low dispersion LA and high speed simultaneous ICPMS detection provide elemental imaging characteristics that are not (and are not foreseen to be) available elsewhere; thus, as demand for high resolution, high speed elemental imaging increases, so too will the necessity of this instrumentation.
References
Giesen C, Wang HAO, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11(4):417–22.
Wang HA, Grolimund D, Giesen C, Borca CN, Shaw-Stewart JR, Bodenmiller B, et al. Fast chemical imaging at high spatial resolution by laser ablation inductively coupled plasma mass spectrometry. Anal Chem. 2013;85(21):10107–16.
Van Malderen SJM, van Elteren JT, Vanhaecke F. Development of a fast laser ablation-inductively coupled plasma-mass spectrometry cell for sub-μm scanning of layered materials. J Anal At Spectrom. 2015;30(1):119–25.
Van Malderen SJ, van Elteren JT, Vanhaecke F. Submicrometer imaging by laser ablation-inductively coupled plasma mass spectrometry via signal and image deconvolution approaches. Anal Chem. 2015;87(12):6125–32.
Gundlach-Graham AW, Burger M, Allner S, Schwarz G, Wang HAO, Gyr L, et al. High-speed, high-resolution, multi-elemental LA–ICP–TOFMS imaging: Part I. instrumentation and two-dimensional imaging of geological samples. Anal Chem. 2015;87(16):8250–8.
Burger M, Gundlach-Graham A, Allner S, Schwarz G, Wang HAO, Gyr L, et al. High-speed, high-resolution, multielemental LA–ICP–TOFMS imaging: Part II. critical evaluation of quantitative three-dimensional imaging of major, minor, and trace elements in geological samples. Anal Chem. 2015;87(16):8259–67.
Kindness A, Sekaran CN, Feldmann J. Two-dimensional mapping of copper and zinc in liver sections by laser ablation-inductively coupled plasma mass spectrometry. Clin Chem. 2003;49(11):1916–23.
Woodhead JD, Hellstrom J, Hergt JM, Greig A, Maas R. Isotopic and elemental imaging of geological materials by laser ablation inductively coupled plasma‐mass spectrometry. Geostand Geoanal Res. 2007;31(4):331–43.
Sabine Becker J. Imaging of metals in biological tissue by laser ablation inductively coupled plasma mass spectrometry (LA–ICP–MS): state of the art and future developments. J Mass Spectrom. 2013;48(2):255–68.
Gray AL. Solid sample introduction by laser ablation for inductively coupled plasma source mass spectrometry. Analyst. 1985;110(5):551–6.
Günther D, Hattendorf B. Solid sample analysis using laser ablation inductively coupled plasma mass spectrometry. TrAC Trends Anal Chem. 2005;24(3):255–65.
Hattendorf B, Günther D. Laser ablation inductively coupled plasma mass spectrometry (LA–ICPMS). Handbook of spectroscopy. Weinheim: Wiley-VCH; 2014. p. 647–98.
Longerich HP, Günther D, Jackson SE. Elemental fractionation in laser ablation inductively coupled plasma mass spectrometry. Fresenius J Anal Chem. 1996;355(5–6):538–42.
Kuhn H-R, Guillong M, Günther D. Size-related vaporisation and ionisation of laser-induced glass particles in the inductively coupled plasma. Anal Bioanal Chem. 2004;378(4):1069–74.
Russo RE, Mao X, Gonzalez JJ, Zorba V, Yoo J. Laser ablation in analytical chemistry. Anal Chem. 2013;85(13):6162–77.
Lear J, Hare D, Adlard P, Finkelstein D, Doble P. Improving acquisition times of elemental bio-imaging for quadrupole-based LA–ICP–MS. J Anal At Spectrom. 2012;27(1):159–64.
Holland JF, Enke CG, Allison J, Stults JT, Pinkston JD, Newcome B, et al. Mass spectrometry on the chromatographic time scale: realistic expectations. Anal Chem. 1983;55(9):997A–1012.
Schilling GD, Andrade FJ, Barnes JH, Sperline RP, Denton MB, Barinaga CJ, et al. Continuous simultaneous detection in mass spectrometry. Anal Chem. 2007;79(20):7662–8.
Bleiner D, Belloni F, Doria D, Lorusso A, Nassisi V. Overcoming pulse mixing and signal tailing in laser ablation inductively coupled plasma mass spectrometry depth profiling. J Anal At Spectrom. 2005;20(12):1337–43.
Bleiner D, Gunther D. Theoretical description and experimental observation of aerosol transport processes in laser ablation inductively coupled plasma mass spectrometry. J Anal At Spectrom. 2001;16(5):449–56.
Plotnikov A, Vogt C, Wetzig K, Kyriakopoulos A. A theoretical approach to the interpretation of the transient data in scanning laser ablation inductively coupled plasma mass spectrometry: consideration of the geometry of the scanning area. Spectrochim Acta B. 2008;63(4):474–83.
Triglav J, van Elteren JT, Šelih VS. Basic modeling approach to optimize elemental imaging by laser ablation ICPMS. Anal Chem. 2010;82(19):8153–60.
Wang HAO, Grolimund D, Van Loon LR, Barmettler K, Borca CN, Aeschlimann B, et al. Quantitative chemical imaging of element diffusion into heterogeneous media using laser ablation inductively coupled plasma mass spectrometry, synchrotron micro-x-ray fluorescence, and extended x-ray absorption fine structure spectroscopy. Anal Chem. 2011;83(16):6259–66.
Elteren J, Izmer A, Sala M, Orsega EF, Selih VS, Panighello S, et al. 3D laser ablation-ICP–mass spectrometry mapping for the study of surface layer phenomena – a case study for weathered glass. J Anal At Spectrom. 2013;28:994–1004.
Chirinos JR, Oropeza DD, Gonzalez JJ, Hou HM, Morey M, Zorba V, et al. Simultaneous 3-dimensional elemental imaging with LIBS and LA–ICP–MS. J Anal At Spectrom. 2014;29(7):1292–8.
Jakubowski N, Prohaska T, Rottmann L, Vanhaecke F. Inductively coupled plasma- and glow discharge plasma-sector field mass spectrometry Part I. Tutorial: fundamentals and instrumentation. J Anal At Spectrom. 2011;26(4):693–726.
Wehe C, Thyssen G, Herdering C, Raj I, Ciarimboli G, Sperling M, et al. Elemental bioimaging by means of fast scanning laser ablation-inductively coupled plasma-mass spectrometry. J Am Soc Mass Spectrom. 2015;26(8):1274–82.
Dziewatkoski MP, Daniels LB, Olesik JW. Time-resolved inductively coupled plasma mass spectrometry measurements with individual, monodisperse drop sample introduction. Anal Chem. 1996;68(7):1101–9.
Heinrich C, Pettke T, Halter W, Aigner-Torres M, Audétat A, Günther D, et al. Quantitative multi-element analysis of minerals, fluid and melt inclusions by laser-ablation inductively-coupled-plasma mass-spectrometry. Geochim Cosmochim Acta. 2003;67(18):3473–97.
Borovinskaya O, Hattendorf B, Tanner M, Gschwind S, Gunther D. A prototype of a new inductively coupled plasma time-of-flight mass spectrometer providing temporally resolved, multi-element detection of short signals generated by single particles and droplets. J Anal At Spectrom. 2013;28(2):226–33.
Myers DP, Li G, Yang P, Hieftje GM. An inductively coupled plasma–time-of-flight mass spectrometer for elemental analysis. Part I: optimization and characteristics. J Am Soc Mass Spectrom. 1994;5(11):1008–16.
Burgoyne TW, Hieftje GM, Hites RA. Design and performance of a plasma-source mass spectrograph. J Am Soc Mass Spectrom. 1997;8(4):307–18.
Hieftje GM, Barnes JH, Grøn OA, Leach AM, McClenathan DM, Ray SJ, et al. Evolution and revolution in instrumentation for plasma-source mass spectrometry. Pure Appl Chem. 2001;73(10):1579–88.
Mahoney PP, Li G, Hieftje GM. Laser ablation-inductively coupled plasma mass spectrometry with a time-of-flight mass analyser. J Anal At Spectrom. 1996;11(6):401–5.
Bleiner D, Hametner K, Günther D. Optimization of a laser ablation-inductively coupled plasma “time of flight” mass spectrometry system for short transient signal acquisition. Fresenius J Anal Chem. 2000;368(1):37–44.
Leach AM, Hieftje GM. Standardless semiquantitative analysis of metals using single-shot laser ablation inductively coupled plasma time-of-flight mass spectrometry. Anal Chem. 2001;73(13):2959–67.
Borovinskaya O, Gschwind S, Hattendorf B, Tanner M, Günther D. Simultaneous mass quantification of nanoparticles of different composition in a mixture by microdroplet generator-ICPTOFMS. Anal Chem. 2014;86(16):8142–8.
Leach AM, Hieftje GM. Factors affecting the production of fast transient signals in single shot laser ablation inductively coupled plasma mass spectrometry. Appl Spectrosc. 2002;56(1):62–9.
Liu XR, Horlick G. In-situ laser-ablation sampling for inductively-coupled plasma-atomic emission-spectrometry. Spectrochim Acta B. 1994;50(4–7):537–48.
Tanner M, Gunther D. In torch laser ablation sampling for inductively coupled plasma mass spectrometry. J Anal At Spectrom. 2005;20(9):987–9.
Tabersky D, Nishiguchi K, Utani K, Ohata M, Dietiker R, Fricker MB, et al. Aerosol entrainment and a large-capacity gas exchange device (Q-GED) for laser ablation inductively coupled plasma mass spectrometry in atmospheric pressure air. J Anal At Spectrom. 2013;28(6):831–42.
Pisonero J, Fliegel D, Gunther D. High efficiency aerosol dispersion cell for laser ablation-ICP–MS. J Anal At Spectrom. 2006;21(9):922–31.
Gurevich EL, Hergenroder R. A simple laser ICP–MS ablation cell with wash-out time less than 100 ms. J Anal At Spectrom. 2007;22(9):1043–50.
Lindner H, Autrique D, Pisonero J, Gunther D, Bogaerts A. Numerical simulation analysis of flow patterns and particle transport in the HEAD laser ablation cell with respect to inductively coupled plasma spectrometry. J Anal At Spectrom. 2010;25(3):295–304.
Douglas DN, Managh AJ, Reid HJ, Sharp BL. A high-speed, integrated ablation cell and dual concentric injector plasma torch for laser ablation-inductively coupled plasma-mass spectrometry. Anal Chem. 2015;87(22):11285–94.
Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal Chem. 2009;81(16):6813–9822.
Gundlach-Graham A, Dennis EA, Ray SJ, Enke CG, Barinaga CJ, Koppenaal DW, et al. First inductively coupled plasma-distance-of-flight mass spectrometer: instrument performance with a microchannel plate/phosphor imaging detector. J Anal At Spectrom. 2013;28(9):1385–95.
Borovinskaya O, Tanner M, Cubison M, Günther D (2015) A new commercial ICP–TOFMS for the anaylsis of nanoparticles. European winter conference on plasma spectrochemistry, Münster, Germany, 23 February 2015.
Acknowledgments
Alex Gundlach-Graham would like to acknowledge financial support through the Marie Curie International Incoming Fellowship: the research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 624280. A.G.-G. thanks Gunnar Schwarz for helpful critiques and comments on an early version of this manuscript. We would also like the acknowledge the work of Marcel Burger, Steffen Allner, Luzia Gyr, and Dr. Hao Wang for contribution to collection and analysis of the LA–ICP–TOFMS data presented here, and Dr. Daniel Grolimund for providing the cesium-infiltrated Opalinus clay sample.
Compliance with ethical standards
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection featuring Young Investigators in Analytical and Bioanalytical Science with guest editors S. Daunert, A. Baeumner, S. Deo, J. Ruiz Encinar, and L. Zhang.
Rights and permissions
About this article
Cite this article
Gundlach-Graham, A., Günther, D. Toward faster and higher resolution LA–ICPMS imaging: on the co-evolution of LA cell design and ICPMS instrumentation. Anal Bioanal Chem 408, 2687–2695 (2016). https://doi.org/10.1007/s00216-015-9251-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9251-8