Abstract
The effect of a prebiotic (fructooligosaccharides) or a synbiotic components (prebiotic and probiotic) on the viability, proteolysis and antioxidant properties of probiotic and synbiotic yogurt during 28 days of storage at 4 °C has been investigated. Yogurt starters in conjunction with either probiotic bacteria Lactobacillus plantarum CFR 2194, Lactobacillus fermentum CFR 2192 and/or fructooligosaccharides (FOS) were used for yogurt preparation. Titratable acidity and pH of all yogurt samples followed a similar pattern of increase or decrease during storage. Proteolysis in synbiotic yogurts was found to be significantly (P < 0.05) higher in comparison with that of control. The addition of prebiotics had no effect (P = 0.17888) on the viability of yogurt starters during cold storage. No observable changes in the viability of probiotic cultures in probiotic groups. However, supplementation of FOS affected the growth significantly (P < 0.05) in promoting the growth of L. plantarum and L. fermentum. Antioxidant activities, the index of nutritional value of yogurt, were monitored. Results showed that the DPPH-radical-scavenging activity (85 %) in synbiotic yogurt containing L. plantarum and FOS was significantly higher (P < 0.05) in comparison with that of control yogurt (72 %). Total phenolics and the ferric reducing power were highest in synbiotic yogurts in comparison with that of other test samples during the entire period of storage. Addition of selected probiotics with FOS thus resulted in an improved functionality of yogurt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continued efforts are being made to improve the health status of human by modulating the intestinal microbiota using live microbial adjuncts, probiotics. Probiotic organisms require a vehicle to reach the site of action, the gastrointestinal (GI) tract of the human body. The vehicle is generally a food product, which contains these live bacteria. The products should have a good shelf-life and should have a cell count more than 106 cfu/mL till the end of storage. The product should also go through the harsh conditions of gastric acid and bile salts before it reaches the GI tract. Scientific evidence suggests that probiotic bacteria consumed at a level of 109–1011 cfu/day can decrease the incidence and severity of some intestinal disorders [41]. In the current market scenario, dairy products such as yogurt, fermented milk and cheese dominate the probiotic food sector.
A variety of fermented milk products are produced throughout the world, among which yogurt (or yoghurt) is most popular. The worldwide production of fermented milk products probably exceeds 20 million tones [16]. The popularity of fermented milks is due at least in part to various health claims and therapeutic benefits that have been associated with some of these products. It is generally assumed that consumption of probiotic yogurt should be more than 100 g/day containing more than 106 cfu/mL [32]. In the past two decades, there has been a significant increase in the popularity of yogurt emphasizing the incorporation of Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium animalis ssp. lactis and Bifidobacterium longum ssp. longum [29, 36]. The conventional yogurt starter bacteria Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus lack the ability of surviving the passage through the GI tract and consequently do not play a role in the human gut. Many studies suggest that consumption of synbiotic products has a greater beneficial effect on the human health than probiotic or prebiotic products [14, 15, 31, 34]. A combination of probiotic and prebiotic in a single food is shown to improve the survival of probiotic bacteria during the storage of the product and also during the passage along the intestinal tract. Moreover, the synbiotic product may allow an efficient implantation of probiotic bacteria in the colon, because prebiotic has a stimulating effect on the growth and/or activities of the exogenous and the endogenous bacteria [31]. In synbiotic fermented milks, the strains of L. acidophilus, L. casei and Bifidobacterium ssp. (B. animalis, B. bifidum, B. breve, B. infantis and B. longum) are widely used as probiotic, whereas fructooligosaccharides, galactooligosaccharides, lactulose, inulin-derived products etc. are widely used as prebiotics [17, 39].
L. plantarum CFR 2194 and L. fermentum CFR 2192 used in the present study are isolates from kanjika, a rice-based ayurvedic fermented food product [30]. The organisms under study has shown some of the important probiotic properties like acid and bile tolerance, ability for the production of vitamin B12 and significant antagonistic activity against the intestinal pathogen like Escherichia coli, Listeria monocytogens and Salmonella [23, 24]. Besides the probiotic properties, the antioxidative ability of lactic acid bacteria, including yogurt starters, has been reported [19, 20, 22]. The antioxidative activity of some Lactobacillus strains used as food components and probiotics may have a substantial impact on human health [22, 28]. To assess such possibilities, the present study focuses on the characterization and antioxidative functionality of probiotic and synbiotic yogurt samples during refrigerated storage for 28 days.
Materials and Methods
Microorganisms and Culture Conditions
The standard starters namely S. thermophilus (ST) ATCC19258 and L. delbrueckii ssp. bulgaricus (LB) CFR 2028 along with probiotic L. plantarum (LP) CFR 2194 and L. fermentum (LF) CFR 2192, isolated from kanjika, [23, 24, 30] were used for the yogurt preparation. The cultures were stored at −60 °C in de Man-Rogosa Sharpe (MRS) broth (Hi-media, India), supplemented with 40 % (v/v) glycerol as a cryoprotectant. Prior to use, the cultures [1 % (v/v)] were transferred to MRS broth: LB was incubated at 40 °C and ST, LP and LF were incubated at 37 °C for 12 h. The active cultures after two successive transfers were further inoculated [1 % (v/v)] to 10 mL aliquots of reconstituted skim milk medium (RSM) supplemented with glucose (2 %), yeast extract (1 %) and incubated for 4–6 h at 37 °C before inoculation into milk. Fructooligosaccharides (FOS) of 70°B [containing 90–93 % (w/w) FOS] was used for the preparation of synbiotic yogurt.
Yogurt Preparation and Storage
Fresh, pasteurized milk containing 3 % fat collected from local market was used for the preparation of yogurt. The milk was preheated to 63 °C for 30 min, at which stage the FOS (1 g/100 mL) was added, followed by cooling to 40 °C before inoculation. The milk was divided into 3 groups and 5 different portions (Table 1), and 100 mL of same was poured into each of the polystyrene cups under aseptic conditions. This was followed by inoculation with ST (7.92 log cfu ml−1), LB (7.38 log cfu ml−1), LP (7.51 log cfu ml−1) and LF (7.42 log cfu ml−1), each at 1 % (v/v). The preparation was mixed thoroughly and kept for incubation at 40 °C for 6–8 h. After incubation, yogurt samples were stored at 4 °C for 28 days. Samples were drawn at weekly intervals up to fourth week.
Determination of Viability
The colony counts of LB, ST were determined as described elsewhere [10, 37]. The viability of ST and LB was determined using M17 agar medium (Hi-media, India) (aerobic incubation at 37 °C for 48 h) and reinforced clostridia agar (RCA) (Hi-media, India) (anaerobic incubation at 42 °C for 48 h), respectively. LP was enumerated on Lactobacillus plantarum selective medium (LPSM), under anaerobic incubation at 37 °C for 48 h [5]. LF was enumerated on Columbia Agar Base (CAB) (Hi-media, India) at pH 5.1, supplemented with 0.5 g cysteine, 5 g raffinose, 2 g Li–Cl and 3 g sodium propionate per litter [7]. Plates were incubated at 37 °C for 48 h under anaerobic conditions.
Chemical Analyses
The pH values of yogurt samples were measured using a pH meter (Fisher Scientific, model 955, India). Titratable acidity (TA) was determined by titration with 0.1 N NaOH solution and expressed as percent lactic acid [2]. TA and pH were measured on a weekly basis during storage of yogurt samples.
Texture Analysis
The gel strength of yogurt samples was determined at 4–6 °C by penetration measurements (Stevens-L.F.R.A. Texture Analyser, CNS Farnell, Borehamwood, UK). The instrument was adjusted to the following conditions: cylindrical probe, probe area 5.07 cm2; penetration speed, 1.0 mm/s; penetration distance, 20 mm into surface. Gel strength was determined in triplicate and expressed as N/cm2 of probe area.
Color Analysis
The color values of yogurt samples were measured using a Hunter Lab color measuring system (Lab scan XE, Hunter Ass. Lab, Virginia, USA), using the L*, a*, b* color scheme. The L*, a*, b* values represent brightness/darkness, green/red and yellow/blue, respectively [18]. The operating conditions were illuminant D65 and 10° observer. An average of 5 values was taken per replication. The values represent an average of three readings.
Determination of Proteolytic Activity
The extent of proteolysis was determined by measuring the liberated amino acids and peptides using the o-phthaldialdehyde (OPA) method of Leclerc et al. [21] with some modifications. Yogurt samples (2.50 mL) were mixed with trichloroacetic acid (0.75 %; 5 mL), and the mixture was filtered using a filter paper (Whatman No. 1). To the permeate (150 μL), OPA reagent (3 mL) was added and the absorbance of the solution was measured spectrophotometrically (UV-1601, Shimadzu Corporation, Japan) at 340 nm after 2 min at room temperature (28 ± 2 °C). The proteolytic activity of these bacterial cultures was expressed as the free amino groups measured at 340 nm as a difference in absorbance between probiotic, synbiotic and control batches.
Antioxidant Activity Assay
Measurement of DPPH Free Radical–Scavenging Activity
The antioxidant activity of each yogurt sample was determined as the ability of the extract to scavenge 1,1- diphenyl-2-picrylhydrazyl (DPPH) radicals. A 0.1 mM DPPH radical solution in 95 % ethanol was prepared. Ethanolic DPPH solution (800 μL) was mixed with 0.2 mL of yogurt sample or 95 % ethanol (control), vortexed well and incubated for 30 min at RT (28 ± 2 °C). The samples were centrifuged for 5 min at 13,000 rpm at RT, and the absorbance of samples was measured spectrophotometrically at 517 nm. The antioxidant activity was expressed as percentage (%) DPPH scavenging = [(control absorbance − sample absorbance)/(control absorbance) × 100].
Determination of Total Phenolics
The method of Zheng and Wang [40] was used for the determination of total phenolic compounds in yogurt samples using Folin–Ciocalteu reagent (FCR) and gallic acid as standard. The sample (0.1 mL) was mixed with 0.9 mL of distilled water and was incubated for 2 h at room temperature (28 ± 2 °C) in a shaking water bath. To this, FCR reagent (1 mL) (1:2 dilution) and 10 % Na2CO3 (2 mL) were added. The mixture was centrifuged at 20,000×g for 20 min, and the supernatant was decanted and filtered through filter paper (Whatman No. 1). The absorbance of the clear supernatant solution was measured at 765 nm. Experiments were carried in triplicates. Results were expressed as milligrams gallic acid equivalent; (GAE) mg/100 mL extract.
Measurement of Ferric Reducing Antioxidant Power (FRAP)
The total antioxidant potential of the sample was determined by the ferric reducing ability (FRAP assay) as a measure of the ‘antioxidant power’ [6]. To the freshly prepared FRAP solution (3 mL), 100 μL of sample was added and incubated at 37 °C for 10 min. The absorbance of reaction mixture was measured at 593 nm. FRAP values were calculated with reference to a standard curve [ferrous sulphate (FeSO47H2O) solutions (0.1–3.0 mM/L)], and results were expressed as mg Fe2+/100 mL (FRAP value).
Statistical Analysis
The experiments were organized as a randomized blocked split-plot in time design, exploring the influence of prebiotics and time as the main effects. All experiments were carried out in triplicates. Results were analyzed using the general linear model (GLM) procedure of the SAS system [33]. The level of significance is presented at P ≤ 0.05.
Results and Discussion
Effect of FOS on the Antioxidant Properties of Yogurt Samples
The DPPH-scavenging activity of yogurt samples is shown in Table 2. The probiotic and synbiotic yogurt samples had a higher antioxidant potential when compared to control yogurt. In synbiotic yogurt samples containing L. plantarum and L. fermentum, the DPPH radical inhibition was 85 and 82 %, respectively, at day 1 when compared to that of control sample (72 %) and the values were higher throughout the storage period. These results indicate that the metabolic end products of LAB, resulting from the selective utilization of FOS, might be contributing to the higher antioxidant potential in comparison with that of control sample.
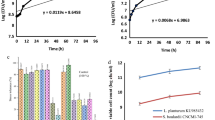
The total soluble phenolics of the yogurt samples are presented in Fig. 1. As can be seen from the results, the total phenolics in the synbiotic yogurt samples (C, D) were found to be higher (P < 0.05) when compared with that of control sample. However, the total phenolics decreased gradually during 1–28 days of storage period. The total phenolics in control yogurt decreased to 170 mg GAE/100 mL by 28 days of storage from an initial value of 238 mg GAE/100 mL. The total phenolics in the synbiotic yogurt samples (C, D) were considerably higher (262 and 258 mg GAE/100 mL), but were also found to decrease to 195 and 191 mg GAE/100 mL, respectively, by 28 days of storage. The increased total phenolic content in synbiotic yogurt could be due to the fermentative activity of the probiotics. The observed values of total phenolics in the present study are much higher in comparison with an earlier report, where the phenolic content of milk fermented with L. bulgaricus and L. acidophilus was found to be 9.7 mg/100 mL and 9.5 mg/100 mL, respectively [3]. The results thus indicate the higher and selective fermentative ability of the selected probiotics.
Effect of storage time on phenolic compound content in yogurt samples. Error bars represent a pooled standard error of the mean, SEM = 0.02 mg/100 mL. The significant difference in different samples when compared to that of control at respective time intervals was analyzed and indicated as P ≤ 0.05. Abbreviations are as per Table 1
The results of FRAP assay are presented in Fig. 2. The antioxidant power was significantly higher (P < 0.05) in synbiotic yogurt samples (C, D) and however was found to decrease from an initial value of 45 and 42 mg Fe2+/100 mL, respectively, from day 1 of storage to 37.3 and 34 mg Fe2+/100 mL by 28 days storage. The antioxidant power of control yogurt was 35.3 mg Fe2+/100 mL at day 1 of storage then decreased to 26.6 mg Fe2+/100 mL by 28 days storage (Fig. 2). A previous study had reported that the antioxidant power of yogurt supplemented with 10 % date palm syrup was 43.3 – 33.5 mg Fe2+/100 mL at 12 days storage [13]. Significant correlation was observed between the total phenolics and the antioxidant power (FRAP assay; r = 0.853). The results are in agreement with earlier findings by Benzie and Szeto [6].
Effect of storage time on antioxidant power (FRAP value) of yogurt samples. Error bars represent a pooled standard error of the mean, SEM = 0.03 mg/100 mL. The significant difference in different samples when compared to that of control at respective time intervals was analyzed and indicated as P ≤ 0.05. Abbreviations are as per Table 1
Viability of Lactic Acid Bacteria During Storage
The viability of yogurt starters and probiotics is presented in Table 3. The addition of prebiotics in general had no observable (P = 0.17888) effect on the viability of the yogurt starters ST and LB, which is in accordance with earlier report [38]. There were minimal differences in the viability of ST in all batches; however, the slight increase observed during storage was insignificant (P > 0.05). The viable cell counts of LB declined from 7.89 to 6.73 log cfug−1 in control yogurts; however, it was maintained ~7 log cfug−1 in probiotic and synbiotic yogurt samples throughout the storage. There were no observable changes in the viability of probiotic cultures in probiotic batches. However, supplementation of FOS resulted in a significant (P < 0.05) increase in the total count of LP and LF from 9.16 and 9.17 log cfug−1 to 9.52 and 9.45 log cfug−1, respectively. The results obtained (Table 3) are in agreement with our earlier study [25], indicating the ability of selected prebiotics to stimulate the growth and viability of probiotics. The viable cell counts of all probiotics by the end of 28 days of storage were ≥9 log cfug−1, and thus, the yogurt developed could be considered as a probiotic product. Addition of FOS could either act as an additional nutrient or modify the unfavorable environmental influences, resulting in improved probiotic viability [11, 25, 26].
Changes in pH and TA
Changes in pH and TA during refrigerated storage of yogurt samples are shown in Table 4. Both synbiotic samples with L. plantarum and L. fermentum showed a significant (P < 0.05) decrease in pH by 14 days of storage and slight increase with further storage (21 & 28 days of storage), when compared with that of probiotic and normal yogurt. The observed change in the pH of the test samples during storage was found to be similar to that of synbiotic low-fat yogurt containing inulin [29]. Synbiotic yogurt samples showed significant (P < 0.05) increase in TA by the end of 14 days concomitant with decrease in pH. Increase in TA was not significant (P > 0.05) with further storage. From these observations, it could be said that FOS did not influence the postacidification in yogurts.
The nutritional and physiologic value of the yogurt is attributed due to the production of lactic acid during the fermentation process of milk to yogurt. It has been argued that protein from yogurt is more easily digested than the protein from milk, as bacterial predigestion of milk proteins in yogurt may occur [35]. Generally, a high TA value and low pH indicate a higher bacterial activity and an increased acid production. During fermentation, acid production results in finer coagulation of casein, which may also contribute to the greater protein digestibility [1]. This argument is supported by evidence of a higher content of free amino acids, especially proline and glycine, in yogurt than in milk. Moreover, the acidic pH of yogurt ionizes calcium and thus facilitates intestinal calcium uptake [4]. The low pH of yogurt also may reduce the inhibitory effect of dietary phytic acid on calcium bioavailability [1].
Changes in Extent of Proteolysis
During fermentation, milk proteins are hydrolyzed by extracellular proteinases produced by lactic acid bacteria (LAB) and would result in an increase in the amount of free amino groups [8]. Proteolytic activity of mixed culture supplemented with prebiotic FOS during prolonged cold storage was estimated by the determination of free amino groups using the OPA method (Fig. 3). The extent of proteolysis was similar in probiotic and synbiotic samples until day 14. Thereafter, it was higher (P < 0.05) in synbiotic samples than in probiotic samples. Donkor et al. [12] observed an improvement of proteolytic activity in yogurt samples by probiotic organisms in presence of selected prebiotics. Hence, the enhancement of the liberation of peptides and amino acids is related to the proteolytic activity of potential probiotic lactobacilli, which is improved in the presence of FOS. Over the storage period of 28 days, the probiotic and synbiotic yogurt samples showed continued increase in extent of proteolysis being significant (P < 0.05) at day 7, 14, 21 and 28 for synbiotic sample, whereas for probiotic the changes were significant at day 7 and 14. There was a significant difference (P < 0.05) in proteolytic activity between the control yogurt and the probiotic and synbiotic yogurt samples; this is a further indication of the proteolytic activity of the probiotic organisms. Ramchandran and Shah [29] observed significantly higher proteolysis in synbiotic low-fat yogurt containing inulin as a prebiotic.
Changes in extent of proteolysis (A340) in control, probiotic and synbiotic yogurt samples stored at 4 °C for 28 days. Error bars represent a pooled standard error of the mean, SEM = 0.02 Abs340nm. Values were significantly different when compared to control sample at P ≤ 0.05. Abbreviations of samples are as per Table 1
Changes in Gel Strength and Color
The gel strength of the yogurt samples is presented in Online Resource 1. The yogurt samples showed an increase in gel strength of up to 0.54 N/cm2 for probiotic yogurt samples and up to 0.65 N/cm2 for synbiotic yogurt samples (Online Resource 1). This suggests that supplementation of milk with FOS could result in increased firmness, agreeing in line with the studies by Oliveira et al. [27], wherein they have supplemented milk with inulin. According to Damin et al. [9], firmness in commercial brands of yogurts in Brazil ranges from 0.32 to 0.79 N at 5 °C. In the case of control yogurt, the gel strength increased slightly during the first week of storage at 4–6 °C, while gel strength increased considerably during the first 2 weeks of storage in probiotic and synbiotic yogurt samples. A slight yellowness was found in the synbiotic yogurt sample, due to the presence of FOS (Online Resource 2).
The above results clearly indicate the role of probiotics and prebiotics, in providing a unique functionality during yogurt preparation. The supplementation of prebiotic FOS during the preparation of yogurt resulted improved viability of LP and LF during 28 days of storage. The use of probiotic cultures in conjunction with prebiotics resulted in the appreciable proteolytic activity likely improving the growth of selected probiotics. In addition, the yogurt samples exhibited higher antioxidant activities in the presence of FOS and probiotics. There was a good correlation between total phenol content and the FRAP values of the yogurt samples. The total phenol content and antioxidant capacity were highest in synbiotic yogurt samples. Higher antioxidant activity could be due to the metabolic end products of selective utilization of FOS by the probiotics.
References
Adolfsson O, Meydani SN, Russell RM (2004) Yogurt and gut function. Am J Clin Nutr 80(2):245–256
AOAC (1984) Official methods of analysis, 14th edn. Association of Official Analytical Chemists, Washington
Apostolidis E, Kwon YI, Ghaedian R, Shetty K (2007) Fermentation of milk and soymilk by lactobacillus bulgaricus and lactobacillus acidophilus enhances functionality for potential dietary management of hyperglycemia and hypertension. Food Biotechnol 21:217–236
Bronner F, Pansu D (1999) Nutritional aspects of calcium absorption. J Nutr 129:9–12
Bujalance C, Jimenez-Valera M, Moreno E, Ruiz-Bravo A (2006) A selective differential medium for Lactobacillus plantarum. J Microbiol Met 66:572–575
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma as a measure of antioxidant power. The FRAP assay. Anal Biochem 239:70–76
Champagne CP, Roy D, Lafond A (1997) Selective enumeration of Lactobacillus casei in yogurt-type fermented milks based on a 15 °C incubation temperature. Biotechnol Tech 11(8):567–569
Christensen JE, Dudley EG, Pederson JA, Steele JL (1999) Peptidases and amino acid catabolism in lactic acid bacteria. Ant van Leeuwenhoek 75(1–4):217–246
Damin MR, Almeida KE, Minowa E (2006) Propriedades físico-químicas e viabilidade de Streptococcus salivarius subsp. bulgaricus em diferentes marcas de iogurtes comerciais no período final da vida de prateleira. Revista Leite E Derivados 9:20–30
Dave RI, Shah NP (1996) Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus, and bifidobacteria. J Dairy Sci 79:1529–1536
Desai AR, Powell IB, Shah NP (2004) Survival and activity of probiotic lactobacilli in skim milk containing prebiotics. J Food Sci 69(1):57–60
Donkar ON, Nilmini SLI, Stolic P, Vasiljevic T, Shah NP (2007) Survival and activity of selected probiotic organisms in set-type yogurt during cold storage. Int Dairy J 16(1):1181–1189
Gad AS, Kholif AM, Sayed AF (2010) Evaluation of the nutritional value of functional yogurt resulting from combination of date palm syrup and skim milk. Amer J Food Technol 5(4):250–259
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125(6):1401–1412
Gmeiner M, Kneifel W, Kulbe KD, Wouters R, De Boever P, Nollet L, Verstraete W (2000) Gmeiner M, Kneifel W, Kulbe KD, Wouters R, De Boever P, Nollet L, Verstraete W (2000) Influence of a synbiotic mixture consisting of Lactobacillus acidophilus 74-2 and a fructooligosaccharide preparation on the microbial ecology sustained in a simulation of the human intestinal microbial ecosystem (SHIME reactor). Appl Microbiol Biotechnol 53:219–223
IDF (1995) Consumption statistics for milk and milk products. Int Dairy Fed Bull (301), Brussels
Klaenhammer TR, Kullen MJ (1999) Selection and design of probiotics. Int J Food Microbiol 50(1–2):45–57
Krishnamurthy S, Kantha HJ (2005) Food color measurement: Instrumentation and Techniques. J Instr Soc India 35(2):227–238
Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, Kilk A (2002) Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 72:215–224
Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M (2003) Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr 90(2):449–456
Leclerc PL, Gauthier SF, Bachelard H, Santure M, Roy D (2002) Antihypertensive activity of casein-enriched milk fermented by Lactobacillus helveticus. Int Dairy J 12:995–1004
Lin MY, Chang FY (2000) Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci 45:1617–1622
Madhu AN, Giribhattanavar P, Prapulla SG (2010) Probiotic lactic acid bacterium from kanjika as a potential source of vitamin B12: evidence from LC-MS, immunological and microbiological techniques. Biotechnol Lett 32:503–506
Madhu AN, Awasthi SP, Reddy KBPK, Prapulla SG (2011) Impact of Freeze and Spray drying on the retention of probiotic properties of Lactobacillus fermentum: An in vitro evaluation model. Int J Microbiol Res 2(3):243–251
Madhu AN, Prapulla SG (2012) In vitro fermentation of prebiotics by lactobacillus plantarum CFR 2194: selectivity, viability and effect of metabolites on β-glucuronidase activity. World J Microbiol Biotechnol 28:901–908
Makras L, Van Acker G, De Vuyst L (2005) Lactobacillus casei subsp. Casei 8700:2 degrades inulin-type fructans exhibiting different degrees of polymerization. Appl Environ Microbiol 71(11):6531–6537
Oliveira RPD, Perego P, Converti A, Oliveira MND (2009) The effect of inulin as a prebiotic on the production of probiotic fibre-enriched fermented milk. Int J Dairy Technol 62(2):195–203
Oxman T, Shapira M, Diver A, Klein R, Avazov N, Rabinowitz B (2000) A new method of long-term preventive cardioprotection using Lactobacillus. Am J Physiol 278:H1717–H1724
Ramachandran L, Shah NP (2010) Characterization of functional, biochemical and textural properties of synbiotic low-fat yogurts during refrigerated storage. LWT Food Sci Technol 43:819–827
Reddy PKKB, Raghavendra P, Girishkumar B, Misra MC, Prapulla SG (2007) Screening of probiotic properties of lactic acid bacteria isolated from Kanjika, an ayurvedic lactic acid fermented product: an in vivo evaluation. J Gen Appl Microbiol 53:207–213
Roberfroid MB (1998) Prebiotics and synbiotics—concepts and nutritional properties. Brit J Nutr 80:197–202
Rybka S, Kailaspathy K (1995) The survival of culture bacteria in fresh and freeze dried AB yogurts. Aus J Dairy Technol 50:3480–3484
SAS (1996) SAS/STAT software: changes and enhancements through release 6.11. SAS Inst Inc., Cary
Schaafsma G, Meuling WJ, van Dokkum W, Bouley C (1998) Effects of a milk product, fermented by Lactobacillus acidophilus and with fructo-oligosaccharides added, on blood lipids in male volunteers. Eur J Clin Nutr 52(6):436–440
Shahani KM, Chandan RC (1979) Nutritional and healthful aspects of cultured and culture-containing dairy foods. J Dairy Sci 62:1685–1694
Sheu SJ, Hwang WZ, Chiang YC, Lin WH, Chen HC, Tsen HY (2010) Use of Tuf gene-based primers for the PCR detection of probiotic bifidobacterium species and enumeration of bifidobacteria in fermented milk by cultural and quantitative real-time PCR methods. J Food Sci 75(8):521–527
Tharmaraj N, Shah NP (2003) Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus and Propionibacteria. J Dairy Sci 86:2288–2296
Vasiljevic T, Kealy T, Mishra VK (2007) Effects of β-glucan addition to a probiotic containing yogurt. J Food Sci 72(7):405–411
Ziemer CJ, Gibson GR (1998) An overview of probiotics, prebiotics, and synbiotics, in the functional food concept. Perspectives and future strategies. Int Dairy J 8:473–479
Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agri Food Chem 49:5165–5170
Zubillaga M, Weill R, Postaire EC, Caro R, Boccio J (2001) Effect of probiotics and functional foods and their use in different diseases. Nutr Res 21:569–579
Acknowledgments
A. N. Madhu is thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, India for the award of Senior Research Fellowship. The authors thank Director, CFTRI, for supporting the work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Madhu, A.N., Amrutha, N. & Prapulla, S.G. Characterization and Antioxidant Property of Probiotic and Synbiotic Yogurts. Probiotics & Antimicro. Prot. 4, 90–97 (2012). https://doi.org/10.1007/s12602-012-9099-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-012-9099-6