Abstract
Background
Insights in the herpesvirus-cell interactions are of general cell biology interest, especially to studies of intracellular transport, and of considerable significance in the efforts to generate drugs, vaccines, and gene therapy. However, the pathway of virus particle egress and maturation is a contentious issue.
Materials and Methods
The intracellular transport was inhibited in cultured herpes simplex virus type 1 (HSV-1) infected human fibroblasts by brefeldin A (BFA). The virus-cell interactions including the viral envelopment, transport of HSV-1 virions, and transport of viral glycoprotein D (gD-1) and glycoprotein C (gC-1) were studied by titration assay, immunoblot, immunofluorescence light microscopy, and immunogold electron microscopy of cryosections.
Results
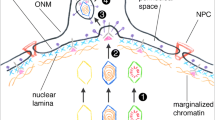
gD-1 and gC-1 were synthesized and normally transported to the plasma membranes of untreated HSV-1 infected host cells. BFA (1 µg/ml medium) effectively blocked the transport of the glycoproteins to the plasma membranes and affected the tubulin and vimentin of the cytoskeleton. Viral particles and glycoproteins accumulated in the perinuclear space and the endoplasmic reticulum of BFA treated cells. Withdrawal of BFA influence up to 9 hr resulted in restored tubulin and vimentin, transport of glycoproteins to the plasma membranes, and steady release of infectious viral particles to the extracellular space superior to the cellular assembly of new virions. The ultrastructural data presented support that the primary envelopment of viral particles occur at the nuclear membranes containing immature glycoproteins followed by multiple de-envelopments and re-envelopments of the virions during the transport and maturation in the endoplasmic reticulum and the Golgi complex.
Conclusions
BFA-induced changes include the cytoskeleton with significant effect on HSV-1 maturation and egress. The data support a multiple-step envelopment of HSV-1 in a common pathway of glycoprotein synthesis and virion egress.







Similar content being viewed by others
References
Nii S. (1992) Electron microscopic study on the development of herpesviruses. J. Electron. Microsc. 41: 414–423.
Stryer L. (1981) Biochemistry, 2nd ed. New York: Freeman.
Tufaro F, Snider MD, McKnight SL. (1987) Identification and characterization of a mouse cell mutant defective in the intracellular transport of glycoproteins. J. Cell. Biol. 105: 647–657.
Doms RW, Lamb RA, Rose JK, Helenius A. (1993) Folding and assembly of viral membrane proteins. Virology 193: 545–562.
Roizman B, Sears AE. (1996) Herpes simplex viruses and their replication. In Fields BN, Knipe DM, Howley PM, et al. (eds). Fields Virology, 3rd ed. Philadelphia: Lippincott-Raven; 2231–2295.
Ward PL, Campadelli-Fiume G, Avitabile E, Roizman B. (1994) Localization and putative function of the UL20 membrane protein in cells infected with herpes simplex virus 1. J. Virol. 68: 7406–7417.
Rajcáni J, Vojvodová A. (1998) The role of herpes simplex virus glycoproteins in the virus replication cycle. Acta Virol. 42: 103–118.
Minson AC. (1994) Interactions of herpes simplex viruses with the host cell. Biochem. Soc. Trans. 22: 298–301.
Ligas MW, Johnson DC. (1988) A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62: 1486–1494.
Olofsson S. (1992) Carbohydrates in herpesvirus infections. APMIS Suppl. 27 100: 84–95.
Compton T, Courtney RJ. (1984) Virus-specific glycoproteins associated with the nuclear fraction of herpes simplex virus type 1-infected cells. J. Virol. 49: 594–597.
Compton T, Courtney RJ. (1984) Synthesis, processing, and localization of herpes simplex virus type 1 glycoproteins, gB and gC. In Rapp F (ed). Herpesvirus. New York, NY, USA, Alan R. Liss; 343–358.
Di Lazzaro C, Campadelli-Fiume G, Torrisi MR. (1995) Intermediate forms of glycoconjugates are present in the envelope of herpes simplex virions during their transport along the exocytic pathway. Virology 214: 619–623.
van Genderen IL, Brandimarti R, Torrisi MR, Campadelli G, van Meer G. (1994) The phospholipid composition of extracellular herpes simplex virions differs from that of host cell nuclei. Virology 200: 831–836.
Enquist LW, Husak PJ, Banfield BW, Smith GA. (1998) Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51: 237–347.
Jensen HL, Norrild B. (2002) Morphologic, immunohistochemical, immunologic, ultrastructural, and time-related study of herpes simplex virus type 1-infected cultured human fibroblasts. AIMM, 10: 71–81.
Bedows E, Rao KMK, Welsh MJ. (1983) Fate of microfilaments in Vero cells infected with measles virus and herpes simplex virus type 1. Mol. Cell Biol. 3: 712–719.
Jones NL, Lewis JC, Kilpatrick BA. (1986) Cytoskeletal disruption during human cytomegalovirus infection of human lung fibroblasts. Eur. J. Cell Biol. 41: 304–312.
Norrild B, Lehto V-P, Virtanen I. (1986) Organization of cytoskeleton elements during herpes simplex virus type 1 infection of human fibroblasts: an immunofluorescence study. J. Gen. Virol. 67: 97–105.
Jensen HL, Rygaard J, Norrild B. (1995) A time-related study of brefeldin A effects in HSV-1 infected cultured human fibroblasts. APMIS 103: 530–539.
Jensen HL, Norrild B. (2000) The effects of cell passages on the cell morphology and the outcome of herpes simplex virus type 1 infection. J. Virol. Meth. 84: 139–152.
Nii S, Morgan C, Rose HM. (1968) Electron microscopy of herpes simplex virus. II. Sequence of development. J. Virol. 2: 517–536.
Jensen HL, Norrild B. (1998) Herpes simplex virus type 1-infected human embryonic lung cells studied by optimized immunogold cryosection electron microscopy. J. Histochem. Cytochem. 46: 487–496.
von Härri E, Loeffler W, Sigg HP, Stähelin H, Tamm C. (1963) Über die isolierung neuer stoffwechselprodukte aus Penicillium brefeldianum Dodge. Helv. Chim. Acta 46: 1235–1243.
Misumi Y, Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y. (1986) Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 261: 11398–11403.
Pelham HRB. (1991) Multiple targets for brefeldin A. Cell 67: 449–451.
Klausner RD, Donaldson JG, Lippincott-Schwartz J. (1992) Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116: 1071–1080.
Ulmer JB, Palade GE. (1991) Effects of brefeldin A on the Golgi complex, endoplasmic reticulum and viral envelope glycoproteins in murine erythroleukemia cells. Eur. J. Cell Biol. 54: 38–53.
Pereira L, Klassen T, Baringer JR. (1980) Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect. Immun. 29: 724–732.
Koga J, Chatterjee S, Whitley RJ. (1986) Studies on herpes simplex virus type 1 glycoproteins using monoclonal antibodies. Virology 151: 385–389.
Eisenberg RJ, Long D, Ponce de Leon M, et al. (1985) Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J. Virol. 53: 634–644.
Isola VJ, Eisenberg RJ, Siebert GR, Heilman CJ, Wilcox WC, Cohen GH. (1989) Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 63: 2325–2334.
Lausch RN, Staats H, Oakes JE, Cohen GH, Eisenberg RJ. (1991) Prevention of herpes keratitis by monoclonal antibodies specific for discontinuous and continuous epitopes on glycoprotein D. Invest. Ophthalmol. Vis. Sci. 32: 2735–2740.
Grøndahl-Hansen J, Ralfkiær E, Nielsen LS, Kristensen P, Frentz G, Danø K. (1987) Immunohistochemical localization of urokinase- and tissue-type plasminogen activators in psoriatic skin. J. Invest. Dermatol. 88: 28–32.
Jensen H, Broholm N, Norrild B. (1995) Low-fading immunofluorescence with propidium iodide contrast compared with immunogold light microscopy in whole cells and semithin cryosections. J. Histochem. Cytochem. 43: 507–513.
Jensen HL, Norrild B. (1999) Easy and reliable doubleimmunogold labelling of herpes simplex virus type-1 infected cells using primary monoclonal antibodies and studied by cryosection electron microscopy. Histochem. J. 31: 525–533.
Ejercito PM, Kieff ED, Roizman B. (1968) Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2: 357–364.
Schägger H, von Jagow G. (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166: 368–379.
Cheung P, Banfield BW, Tufaro F. (1991) Brefeldin A arrests the maturation and egress of herpes simplex virus particles during infection. J. Virol. 65: 1893–1904.
Chatterjee S, Sarkar S. (1992) Studies on endoplasmic reticulum-Golgi complex cycling pathway in herpes simplex virus-infected and Brefeldin A-treated human fibroblast cells. Virology 191: 327–337.
Koyama AH, Uchida T. (1994) Inhibition by brefeldin A of the envelopment of nucleocapsids in herpes simplex virus type 1-infected Vero cells. Arch. Virol. 135: 305–317.
Dasgupta A, Wilson DW. (2001) Evaluation of the primary effect of brefeldin A treatment upon herpes simplex virus assembly. J. Gen. Virol. 82: 1561–1567.
Eggers M, Bogner E, Agricola B, Kern HF, Radsak K. (1992) Inhibition of human cytomegalovirus maturation by brefeldin A. J. Gen. Virol. 73: 2679–2692.
Gershon AA, Sherman DL, Zhu Z, Gabel CA, Ambron RT, Gershon MD. (1994) Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68: 6372–6390.
Whealy ME, Card JP, Meade RP, Robbins AK, Enquist LW. (1991) Effects of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65: 1066–1081.
Doms RW, Russ G, Yewdell JW. (1989) Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell Biol. 109: 61–72.
Urbani L, Simoni RD. (1990) Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J. Biol. Chem. 265: 1919–1923.
Miller SG, Carnell L, Moore H-PH. (1992) Post-Golgi membrane traffic: brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J. Cell Biol. 118: 267–283.
Hammond C, Helenius A. (1994) Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J. Cell Biol. 126: 41–52.
Nuchtern JG, Bonifacino JS, Biddison WE, Klausner RD. (1989) Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature 339: 223–226.
Qiu Z, Tufaro F, Gillam S (1995) Brefeldin A and monensin arrest cell surface expression of membrane glycoproteins and release of rubella virus. J. Gen. Virol. 76: 855–863.
Maynell LA, Kirkegaard K, Klymkowsky MW. (1992) Inhibition of poliovirus RNA synthesis by brefeldin A. J. Virol. 66: 1985–1994.
Stannard LM, Himmelhoch S, Wynchank S. (1996) Intranuclear localization of two envelope proteins gB and gD, of herpes simplex virus. Arch. Virol. 141: 505–524.
Browne H, Bell S, Minson T, Wilson DW. (1996) An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70: 4311–4316.
Kousoulas KG, Bzik DJ, DeLuca N, Person S. (1983) The effect of ammonium chloride and tunicamycin on the glycoprotein content and infectivity of herpes simplex virus type 1. Virology 125: 468–474.
Pfeffer SR, Rothman JE. (1987) Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu. Rev. Biochem. 56: 829–852.
Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. (1989) Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56: 801–813.
Lippincott-Schwartz J, Donaldson JG, Schweizer A et al. (1990) Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell 60: 821–836.
Donaldson JG, Lippincott-Schwartz J, Bloom GS, Kreis TE, Klausner RD. (1990) Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J. Cell Biol. 111: 2295–2306.
Orci L, Tagaya M, Amherdt M et al. (1991) Brefeldin A, a drug that blocks secretion, prevents the assembly of nonclathrin-coated buds on Golgi cisternae. Cell 64: 1183–1195.
Pietschmann SM, Gelderblom HR, Pauli G. (1989) Compartment-specific immunolocalization of conserved epitopes of the glycoprotein gB of herpes simplex virus type 1 and bovine herpes virus type 2 in infected cells. Arch. Virol. 108: 1–17.
Roizman B, Furlong D. (1974) The replication of herpesviruses. In Fraenkel-Conrat H, Wagner RR (eds). Comprehensive Virology 3: Reproduction. DNA Animal Viruses. New York: Plenum; 229–403.
Fishman PH, Curran PK. (1992) Brefeldin A inhibits protein synthesis in cultured cells. FEBS Lett. 314: 371–374.
Avitabile E, Gaeta SD, Torrisi MR, Ward PL, Roizman B, Campadelli-Fiume G. (1995) Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J. Virol. 69: 7472–7482.
Sodeik B, Ebersold MW, Helenius A. (1997) Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136: 1007–1021.
Elliott G, O’Hare P. (1998) Herpes simplex virus type 1 tegument protein VP22 induces the stabilization and hyperacetylation of microtubules. J. Virol. 72: 6448–6455.
Holland DJ, Miranda-Saksena M, Boadle RA, Armati P, Cunningham AL. (1999) Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73: 8503–8511.
Granzow H, Klupp BG, Fuchs W, Veits J, Osterrieder N, Mettenleiter TC. (2001) Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75: 3675–3684.
Skepper JN, Whiteley A, Browne H, Minson A. (2001) Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment->deenvelopment->reenvelopment pathway. J. Virol. 75: 5697–5702.
Acknowledgments
This work was supported by grants from the Danish Cancer Society and by funds from the Health Insurance “danmark,” Frands Køhler Nielsen and wife, Erik Hørslev and wife, Birgit Hørslev, E. Danielsen and wife, Anna and Jakob Jakobsen, Valdemar and Thyra Foersom, Torben and Alice Frimodt, Aage Bang, and the LEO Foundation.
We are grateful to G.H. Cohen and R.J. Eisenberg (University of Pennsylvania, Philadelphia), S. Chatterjee (University of Alabama, Birmingham), and L. Pereira (University of California, San Francisco) for generous gifts of monoclonal antibodies. Cryosection and electron microscopy facilities were kindly made available by Professor O. Norén and Professor H. Sjöström (University of Copenhagen, Denmark).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jensen, H.L., Norrild, B. Temporal Morphogenesis of Herpes Simplex Virus Type 1-Infected and Brefeldin A-Treated Human Fibroblasts. Mol Med 8, 210–224 (2002). https://doi.org/10.1007/BF03402013
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402013