Abstract
Background
The aim of this study was to determine whether crystals of hydroxyapatite (HA) or brushite (BR) formed in urine promote the epitaxial deposition of calcium oxalate (CaOx) from undiluted human urine in vitro and thereby explain the occurrence of phosphate in the core of urinary stones consisting predominantly of CaOx.
Materials and Methods
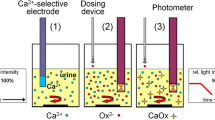
Crystals of HA, BR, and CaOx were generated from human urine and their identity confirmed by X-ray analysis. Standard quantities of each crystal were then added to separate aliquots of pooled undiluted human urine and CaOx crystallization was induced by the addition of identical loads of sodium oxalate. Crystallization was monitored by Coulter Counter and 14C-oxalate analysis and the precipitated crystals were examined by scanning electron microscopy.
Results
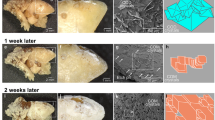
In comparison with the control to which no seeds were added, addition of CaOx crystals increased the deposition of 14C-oxalate by 23%. On the other hand, seeds of HA and BR had no effect. These findings were supported by Coulter Counter analysis, which showed that the average modal sizes of crystal particles precipitated in the presence of HA and BR seeds were indistinguishable from those in the control, whereas those deposited in the presence of CaOx were significantly larger. Scanning electron microscopy confirmed these results, demonstrating that large aggregates of CaOx dihydrates were formed in the presence of CaOx seeds, whereas BR and to a lesser extent HA seeds were scattered free on the filtration membrane and attached like barnacles on the surface of the freshly precipitated CaOx crystals.
Conclusion
Seed crystals of HA or BR do not promote CaOx deposition in urine in vitro and are therefore unlikely to influence CaOx crystal formation under physiologic conditions. However, binding of HA and BR crystals to, and their subsequent enclosure within, actively growing CaOx crystals might occur in vivo, thereby explaining the occurrence of mixed oxalate/phosphate stones.





Similar content being viewed by others
References
Sierakowski R, Finlayson B, Landes RR, Finlayson CD, Sierakowski N. (1978) The frequency of urolithiasis in hospital discharge diagnoses in the United States. Invest. Urol. 15: 438–441.
Robertson WG, Peacock M, Heyburn PJ, Hanes FA. (1980) Epidemiological risk factors in calcium stone disease. Scand. J. Urol. Nephrol. 53 (suppl): 15–28.
Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H. (1994) Demographic and geographic variability of kidney stones in the United States. Kidney Int. 46: 893–899.
Robertson WG. (2001) The changing pattern of urolithiasis in the UK and its causes. In Kok DJ, Romijn HC, Verhagen PCMS, Verkoelen CF (eds). Eurolithiasis. Maastricht, The Netherlands: Shaker Publishing; 9–11.
Clark JY, Thompson IM, Optenberg SA. (1995) Economic impact of urolithiasis in the United States. J. Urol. 154: 2020–2024.
Prien EL, Prien EL Jr. (1968) Composition and structure of urinary stone. Am. J. Med. 45: 654–672.
Elliot JS. (1973) Structure and composition of urinary calculi. J. Urol. 109: 82–83.
Modlin M. (1967) The aetiology of renal stones: a new concept arising from studies in a stone-free population. Ann. R. Coll. Surg. Engl. (Lond.) 40: 155–178.
Boyce WH, King JS Jr. (1963) Present concepts concerning the origin of matrix and stones. Ann. N. Y. Acad. Sci. 104: 563–578.
Abraham PA, Smith CL. (1987) Evaluation of factors involved in calcium stone formation. Miner. Electrolyte Metab. 13: 201–208.
Royer L. (1928) Experimental research on parallel growth on mutual orientation of crystals of different species. Bull. Soc. Fr. Mineral 51: 7–159.
Lonsdale K. (1968) Epitaxy as a growth factor in urinary calculi and gallstones. Nature 217: 5658.
Lonsdale K. (1968) Human stones: limited studies give some details of composition, rates of growth, distribution, and possible causes. Science 159: 1199–1207.
Robertson WG, Peacock M, Nordin BEC. (1968) Activity products in stone-forming and non-stone-forming urine. Clin. Sci. 34: 579–594.
Pak CYC, Holt K. (1976) Nucleation and growth of brushite and calcium oxalate in urine of stone formers. Metabolism 25: 665–673.
Smith LH, Werness PG. (1983) Hydroxyapatite—the forgotten crystal in calcium urolithiasis. Trans. Am. Clin. Climatol. Assoc. 95: 183–190.
Berland Y, Boistelle R, Olmer M. (1990) Urinary supersaturation with respect to brushite in patients suffering from calcium oxalate lithiasis. Nephrol. Dial. Transplant. 5: 179–184.
Hallson PC, Rose GA. (1976) Crystalluria in normal subjects and in stone formers with and without thiazide and cellulose phosphate treatment. Br. J. Urol. 48: 515–524.
Werness PG, Wilson JWL, Smith LH. (1984) Hydroxyapatite and its role in calcium urolithiasis. In Ryall RL, Brockis JG, Marshall VR, Finlayson B (eds). Urinary Stones. Melbourne, London, New York: Churchill Livingstone; 273–277.
Tiselius HG. (1992) Recurrent stone formation in patients treated with ESWL. J. Stone Dis. 4: 152–157.
Tiselius HG, Larsson L. (1993) Calcium phosphate: an important crystal phase in patients with recurrent calcium stone formation? Urol. Res. 21: 175–180.
Pak CYC, Hayashi Y, Arnold LH. (1976) Heterogeneous nucleation with urate, calcium phosphate and calcium oxalate. Proc. Soc. Exp. Biol. Med. 153: 83–87.
Meyer JL, Bergert JH, Smith LH. (1975) Epitaxial relationships in urolithiasis: the calcium oxalate monohydratehydroxyapatite system. Clin. Sci. Mol. Med. 49: 369–374.
Meyer JL, Bergert JH, Smith LH. (1977) Epitaxial relationships in urolithiasis: the brushite-whewellite system. Clin. Sci. Mol. Med. 52: 143–148.
Koutsoukos PG, Sheehan ME, Nancollas GH. (1981) Epitaxial considerations in urinary stone formation. II. The oxalatephosphate system. Invest. Urol. 18: 358–363.
Berg C, Tiselius HG. (1989) The effects of citrate on hydroxyapatite induced calcium oxalate crystallization and on the formation of calcium phosphate crystals. Urol. Res. 17: 167–172.
Baumann JM, Ackermann D, Affolter B. (1989) The influence of hydroxyapatite and pyrophosphate on the formation product of calcium oxalate at different pHs. Urol. Res. 17: 153–155.
Mandel NS, Mandel GS. (1981) Epitaxis between stoneforming crystals at atomic level. In Smith LH, Robertson WG, Finlayson B (eds). Urolithiasis: Clinical and Basic Research. New York: Plenum; 469–480.
Ebrahimpour A, Perez L, Nancollas GH. (1991) Induced crystal growth of calcium oxalate monohydrate at hydroxyapatite surfaces. The influence of human serum albumin, citrate and magnesium. Langmuir 7: 577–583.
Achilles W, Jockel U, Schaper A, Burk B, Riedmiller H. (1995) In vitro formation of “urinary stones”: generation of spherulites of calcium phosphate in gel and overgrowth with calcium oxalate using a new flow model of crystallization. Scanning Microsc. 9: 577–586.
Coe FL, Favus MJ, Pak CYC, Preminger GL. (1996) Kidney Stones: Medical and Surgical Management. Boston: Raven Press.
Hojgaard I, Fornander A-M, Nilsson M-A, Tiselius HG. (1998) The influence of hydroxyapatite seed on the crystallization induced by volume reduction of solutions with an ion composition corresponding to that in the distal tubule at different pH levels. Scand. J. Urol. Nephrol. 32: 311–319.
Beshensky AM, Wesson JA, Worcester EM, Snyder C, Kleinman JG. (2000) Urinary protein effects on heterogeneous nucleation of calcium oxalate on hydroxyapatite. In Rodgers AL, Hibbert BE, Hess B, Khan SR, Preminger GM (eds). Urolithiasis 2000. Cape Town, South Africa: University of Cape Town, Rondebosch; 116–118.
Stapleton AMF, Dawson CJ, Grover PK, et al. (1996) Further evidence linking urolithiasis and blood coagulation: urinary prothrombin fragment 1 is present in stone matrix. Kidney Int. 49: 880–888.
Nishio S, Hatanaka M, Takeda H, et al. (2000) Calcium phosphate crystal-associated proteins: alpha 2-HS-glycoprotein, prothrombin F1, and osteopontin. Mol. Urol. 4: 383–389.
Doyle IR, Ryall RL, Marshall VR. (1991) Inclusion of proteins into calcium oxalate crystals precipitated from human urine: a highly selective phenomenon. Clin. Chem. 37: 1589–1594.
Self PG, Pickering JG, Raven MD, Riley GG, Rosser H, Stone PA. (1987) Procedures for the control and manipulation of X-ray powder diffraction data from remote locations based on a Phillips PW1710 diffraction control system. I. Automatic control of the diffractometer—user’s manual. Glen Osmond, South Australia: CSIRO Division of Soils Technical Memo No. 75/1987.
Raven MD, Self PG. (1987) Procedures for the control and manipulation of X-ray powder diffraction data from remote locations based on a Phillips PW1710 diffraction control system. II. Handling procedures for data from the PW1710. Version 1.00. Glen Osmond, South Australia: CSIRO Division of Soils Technical Memo No. 91/1987.
Doyle IR, Ryall RL, Marshall VR. (1989) The effect of low-speed centrifugation and millipore filtration on the urinary protein content. In Walker VR, Sutton RAL, Cameron ECB, Pak CYC, Robertson WG (eds). Urolithiasis. New York: Plenum; 593–594.
Ryall RL, Harnett RM, Hibberd CM, Edyvane KA, Marshall VR. (1991) Effects of chondroitin sulphate, human serum albumin and Tamm-Horsfall mucoprotein on calcium oxalate crystallization in undiluted human urine. Urol. Res. 19: 181–188.
National Institute of Health Consensus Development Conference Statement. (1989) Prevention and treatment of kidney stones. J. Urol. 141: 804–808.
Ackermann D, Baumann JM. (1987) Chemical factors governing the state of saturation towards brushite and whewellite in urine of calcium stone formers. Urol. Res. 15: 63–65.
Robertson WG, Peacock M, Nordin BEC. (1969) Calcium crystalluria in recurrent renal-stone formers. Lancet 5: 21–24.
Strates BS, Neuman WF, Levinskas GJ. (1957) The solubility of bone mineral. II. Precipitation of near neutral solutions of calcium and phosphate. J. Phys. Chem. 61: 279–282.
Pak CYC, Eanes ED, Ruskin B. (1971) Spontaneous precipitation of brushite in urine: evidence that brushite is the nidus of renal stones originating as calcium phosphate. Proc. Natl. Acad. Sci. U.S.A. 68: 1456–1460.
Pak CYC. (1969) Physicochemical basis for formation of renal stones of calcium phosphate origin: calculation of the degree of saturation of urine with respect to brushite. J. Clin. Invest. 48: 1914–1922.
Pak CYC. (1981) Potential etiological role for brushite in the formation of calcium (renal) stones. J. Crystal Growth 53: 202–208.
Pak CYC, Skinner HCW. (1968) Ionic interaction with bone mineral. IV. Varying affinity of synthetic calcium phosphates for Ca2+. Biochem. Biophys. Acta 165: 274–282.
Ryall RL. (1996) Glycosaminoglycans, proteins, and stone formation: adult themes and child’s play. Pediatr. Nephrol. 10: 656–666.
Worcester EM, Blumenthal SS, Beshensky AM, Lewand DL. (1992) The calcium oxalate growth inhibitor protein produced by mouse kidney cortical cells in culture is osteopontin. J. Bone Miner. Res. 7: 1029–1036.
Worcester EM, Beshensky AM. (1995) Osteopontin inhibits nucleation of calcium oxalate crystals. Ann. N. Y. Acad. Sci. 760: 375–377.
Grases F, Conte A, Gil JJ. (1988) Simple method for the study of heterogeneous nucleation in calcium oxalate urolithiasis. Br. J. Urol. 61: 468–473.
Grover PK, Ryall RL. (1997) Effect of seed crystals of uric acid and monosodium urate on the crystallization of calcium oxalate in undiluted human urine in vitro. Clin. Sci. 92: 205–213.
Khan SR. (1997) Calcium phosphate/calcium oxalate crystal association in urinary stones: implications for heterogeneous nucleation of calcium oxalate. J. Urol. 157: 376–383.
Burns JR, Finlayson B. (1980) The effect of seed crystals on calcium oxalate nucleation. Invest. Urol. 18: 133–136.
Acknowledgments
The authors are indebted to Dr. A. Milnes, CSIRO, Division of Soils, Glen Osmond, South Australia, for performing the X-ray powder diffraction analyses of the seed crystals. Sincere thanks are also extended to Professor C.Y.C. Pak (University of Texas Southern Medical Center at Dallas, Dallas, Texas, USA) for providing the brushite seed crystals. This work was supported by grant 980366 from the National Health and Medical Research Council of Australia and grants from the Research Foundation of the Urological Society of Australasia, Flinders University of South Australia and Flinders 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grover, P.K., Kim, DS. & Ryall, R.L. The Effect of Seed Crystals of Hydroxyapatite and Brushite on the Crystallization of Calcium Oxalate in Undiluted Human Urine In Vitro: Implications for Urinary Stone Pathogenesis. Mol Med 8, 200–209 (2002). https://doi.org/10.1007/BF03402012
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03402012