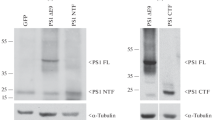
Abstract
Background
Several lines of evidence suggest that most of the early-onset forms of familial Alzheimer’s disease (FAD) are due to inherited mutations borne by a chromosome 14–encoded protein, presenilin 1 (PS1). This is likely related to an increased production of amyloid β-peptide (A β)42, one of the main components of the extracellular deposits called senile plaques that invade human cortical areas during the disease.
Materials and Methods
We set up stably transfected HEK293 cells overexpressing wild-type (wt) and various FAD-linked mutated PS1. By Western blot analysis, we examined the influence of specific proteasome inhibitors on PS1-like immunoreactivities. Furthermore, by means of metabolic labeling and immunoprecipitation with A β40 and A β42-directed specific antibodies, we assessed the effect of the inhibitors on the production of A βs by wt and mutated PS1-expressing cells transiently transfected with βAPP751.
Results
We show that two distinct proteasome inhibitors, Z-IE(Ot-Bu)A-Leucinal and lactacystin, increase in a time- and dose-dependent manner the immunoreactivities of both wt and mutated PS1. Furthermore, we demonstrate that PS1 is polyubiquitinated in these cells. Other inhibitors, ineffective on the proteasome, fail to protect wt and mutated PS1-like immunoreactivities. We also establish that the FAD-linked mutations of PS1 trigger a selective increased formation of Aβ42 as reflected by higher Aβ42 over total Aβ ratios when compared with wtPS1-expressing cells. Interestingly, this augmentation was further amplified by proteasome inhibitors in cells expressing mutated but not wtPS1.
Conclusion
Altogether, our data indicate that PS1 undergoes polyubiquitination in HEK293 cells and that the proteasome contributes to the degradation of wt and FAD-linked PS1, thereby directly influencing the Aβ production in human cells.







Similar content being viewed by others
References
Lemere CA, Lopera F, Kosik KS, et al. (1996) The E280A presenilin 1 Alzheimer mutation produces increased Aβ42 deposition and severe cerebellar pathology. Nat. Med. 2: 1146–1150.
Mann DMA, Iwatsubo T, Cairns NJ, et al. (1997) Amyloid β protein (Aβ) deposition in chromosome 14-linked Alzheimer’s disease: Predominance of Aβ42(43). Ann. Neurol. 40: 149–156.
Mann DMA, Iwatsubo T, Nochlin D, Sumi SM, Levy-Lahad E, Bird TD. (1997) Amyloid (Aβ) deposition in Chromosome 1-linked Alzheimer’s disease: The Volga German family. Ann. Neurol. 41: 52–57.
Borchelt DR, Thinakaran G, Eckman CB, et al. (1996) Familial Alzheimer’s disease-linked presenilin 1 variants elevate Aβ1-42/1-40 in vitro and in vivo. Neuron 17: 1005–1013.
Gearing M, Tigges J, Mori H, Mirra SS. (1996) Aβ40 is a major form of β-amyloid in human primates. Neurobiol. Aging 17: 903–908.
Xia W, Zhang J, Kholodenko D, et al. (1997) Enhanced production and oligomerization of the 42-residue amyloid β-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J. Biol. Chem. 272: 7977–7982.
Tomita T, Maruyama K, Saido TC, et al. (1997) The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid β protein ending at the 42nd (or 43rd) residue. Proc. Natl. Acad. Sci. USA 94: 2025–2030.
Duff K, Eckman C, Zehr C, et al. (1996) Increased amyloid-β42(43) in brains expressing mutant presenilin 1. Nature 383: 710–713.
Ancolio K, Marambaud P, Dauch P, Checler F. (1997) α-secretase-derived product of β amyloid precursor protein is decreased by presenilin 1 mutations linked to familial Alzheimer’s disease. J. Neurochem. 69: 2495–2499.
Kovacs DM, Fausett HJ, Page KJ, et al. (1996) Alzheimer-associated presenilins 1 and 2: Neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat. Med. 2: 224–229.
Cook DG, Sung JC, Golde TE, et al. (1996) Expression and analysis of presenilin 1 in a human neuronal system: Localization in cell bodies and dendrites. Proc. Natl. Acad. Sei. USA 93: 9223–9228.
Walter J, Capell A, Grünberg J, et al. (1996) The Alzheimer’s disease-associated presenilins are differentially phosphorylated proteins located predominantly within the endoplasmic reticulum. Mol. Med. 2: 673–691.
Dewji NN, Singer SJ. (1996) Specific transcellular binding between membrane proteins crucial to Alzheimer disease. Proc. Natl. Acad. Sci. USA 93: 12575–12580.
Weidemann A, Paliga K, Dürrwang U, et al. (1997) Formation of stable complexes between two Alzheimer’s disease gene products: Presenilin-2 and β-amyloid precursor protein. Nat. Med. 3: 328–332.
Marambaud P, Chevallier N, Barelli H, Wilk S, Checler F. (1997) Proteasome contributes to the α-secretase pathway of amyloid precursor protein in human cells. J. Neurochem. 68: 698–703.
Marambaud P, Lopez-Perez E, Wilk S, Checler F. (1997) Constitutive and protein kinase C-regulated secretary cleavage of Alzheimer’s β amyloid precursor protein: Different control of early and late events by the proteasome. J. Neurochem. 69: 2500–2505.
Marambaud P, Wilk S, Checler F. (1996) Protein kinase A phosphorylation of the proteasome: A contribution to the α-secretase pathway in human cells. J. Neurochem. 67: 2616–2619.
Dauch P, Champigny G, Ricci J-E, Checler F. (1997) Lack of effect of presenilin1, βAPP and their Alzheimer’s disease-related mutated forms on xenopus oocytes membrane current. Neurosci. Lett. 221: 1–4.
Thinakaran G, Borchelt DR, Lee MK, et al. (1996) Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17: 181–190.
Chevallier N, Jiracek J, Vincent B, et al. (1997) Examination of the role of endopeptidase 3.4.24.15 in Aβ secretion by human transfected cells. Br. J. Pharmacol. 121: 556–562.
Barelli H, Lebeau A, Vizzavona J, et al. (1997) Characterization of new polyclonal antibodies specific for 40 and 42 aminoacid-long amyloid β peptides: Their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer’s disease and cerebral amyloid angiopathy cases. Mol. Med. 3: 695–707.
Fenteany G, Standaert R, Lane WS, Choi S, Corey EJ, Schreiber SL. (1995) Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268: 726–731.
Pereira ME, Yu B, Wilk S. (1992) Enzymatic changes of the bovine pituitary multicatalytic proteinase complex induced by magnesium ions. Arch. Biochem. Biophys. 294: 1–8.
Ward RV, Davis JB, Gray CW, et al. (1996) Pre-senilin-1 is processed into two major cleavage products in neuronal cell lines. Neurodegeneration 5: 293–298.
De Strooper B, Beullens M, Contreras B, et al. (1997) Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer’s disease-associated presenilins. J. Biol. Chem. 272: 3590–3598.
Seeger M, Nordstedt C, Petanceska S, et al. (1997) Evidence for phosphorylation and oligomeric assembly of presenilin 1. Proc. Natl. Acad. Sci. USA 94: 5090–5094.
Walter J, Grünberg J, Capell A, et al. (1997) Proteolytic processing of the Alzheimer disease associated presenilin-1 generates an in vivo substrate for protein kinase C. Proc. Natl. Acad. Sci. USA 94: 5349–5354.
Varhavski, A. (1997) The ubiquitin system. Trends Neurosci. 22: 383–387.
Kim TW, Pettingeil WH, Hallmark OG, Moir RD, Wasco W, Tanzi RE. (1997) Endoproteolytic cleavage and proteasomal degradation of presenilin 2 in transfected cells. J. Biol. Chem. 272: 11006–11010.
Mengual E, Aritzi P, Rodrigo J, Gimenez-Amaya JM, Castano JG. (1996) Immunohistochemical distribution and electron microscopic subcellular localization of the proteasome in the rat CNS. J. Neurosci. 16: 6331–6341.
Palmer A, Rivett AJ, Thomson S, et al. (1996) Subpopulations of proteasomes in rat liver nuclei, microsomes and cytosol. Biochem. J. 316: 401–407.
Oda K, Ikehara Y, Omura S. (1996) Lactacystin, an inhibitor of the proteasome, blocks the degradation of a mutant precursor of glycosylphos-phatidyl inositol-linked protein in a pre-Golgi compartment. Biochem. Biophys. Res. Commun. 219: 800–805.
Werner ED, Brodsky JL, McCracken AA. (1996) Proteasome-dependent endoplasmic reticulum-associated protein degradation: An unconventional route to a familiar fate. Proc. Natl. Acad. Sci. USA 93: 13797–13801.
Fuller SJ, Storey E, Li QX, Smith I, Beyreuther K, Master CL. (1995) Intracellular production of βA4 amyloid of Alzheimer’s disease: Modulation by phosphoramidon and lack of coupling to the secretion of the amyloid precursor protein. Biochemistry 34: 8091–8098.
Haass C. (1996) Presenile because of presenilin: The presenilin genes and early onset Alzheimer’s disease. Curr. Opin. Neurol. 9: 254–259.
Hardy J, Allsop D. (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 12: 383–388.
Checler F. (1998) Presenilins. Mol. Neurobiol. (in press).
Checler F. (1995) Processing of the β-amyloid precursor protein and its regulation in Alzheimer’s disease. J. Neurochem. 65: 1431–1444.
Rivett AJ. (1989) The multicatalytic proteinase. Multiple proteolytic activities. J. Biol. Chem. 264: 12215–12219.
Rechsteiner M, Hoffman L, Dubiel W. (1993) The multicatalytic and 26S proteases. J. Biol. Chem. 268: 6065–6068.
Acknowledgements
We thank Drs. M. Savage and B. Greenberg (Cephalon, West Chester, PA) for generously providing us with 207 antibody and Dr. B. De Strooper (Center for Human Genetics, Leuven, Belgium) for the generous gift of the antibody (B14) directed toward the N-terminal part of PS1. We sincerely thank Dr. Sherwin Wilk for the generous gift of Z-IE(Ot-Bu)A-Leucinal and Z-L-Leucinal. We sincerely thank Drs. G. Thinakaran and S. Sisodia for generously providing us with αPS1Loop antibody and ΔE9-PS1 cDNA. We also thank J. Kervella for expert secretarial assistance, and Franck Aguila for the artwork. This work was supported by the Institut National de la Santé et de la Recherche Médicale and the Centre National de la Recherche Scientifique.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Chambon.
Rights and permissions
About this article
Cite this article
Marambaud, P., Ancolio, K., Lopez-Perez, E. et al. Proteasome Inhibitors Prevent the Degradation of Familial Alzheimer’s Disease-Linked Presenilin 1 and Potentiate Aβ42 Recovery from Human Cells. Mol Med 4, 147–157 (1998). https://doi.org/10.1007/BF03401912
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401912