Abstract
Background
Typically, a diagnosis of diabetes mellitus is based on elevated circulating blood glucose levels. In an attempt to discover additional markers for the disease and predictors of prognosis, we undertook the characterization of HbA1d3 in diabetic and normal patients.
Material and Methods
PolyCAT A cation exchange chromatography and liquid chromatography-mass spectroscopy was utilized to separate the α- and β-globin chains of HbA1d3 and characterize their presence in normal and diabetic patients.
Results
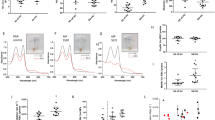
We report the characterization of HbA1d3 as a glutathionylated, minor hemoglobin subfraction that occurs in higher levels in diabetic patients (2.26 ± 0.29 %) than in normal individuals (1.21 ± 0.14%, p < 0.001). The α-chain spectrum displayed a molecular ion of m/z 15126 Da, which is consistent with the predicted native mass of the HbA0 α-globin chain. By contrast, the mass spectrum of the β-chain showed a mass excess of 307 Da (m/z = 16173 Da) versus that of the native HbA0 β-globin chain (m/z = 15866 Da). The native molecular weight of the modified β-globin chain HbA0 was regenerated by treatment of HbA1d3 with dithiothreitol, consistent with a glutathionylated adduct.
Conclusions
We propose that HbA1d3 (HbSSG) forms normally in vivo, and may provide a useful marker of oxidative stress in diabetes mellitus and potentially other pathologic situations.





Similar content being viewed by others
References
Dryer DG, Dunn JA, Thorpe SR, et al. (1993). Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Invest. 91: 2463–2469.
Ledl F, Schleicher E. (1990). The Maillard reaction in food and in human body-New results in chemistry, biochemistry and medicine. Angew. Chem. Int. Ed. Engl. 6: 565–594.
Brownlee M, Cerami A, Vlassara H. (1998). Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 318: 1315–1321.
Bucala R, Cerami A. (1992). Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 23: 1–34.
Rahbar S. (1968). Hemoglobin H disease in two Iranian families: Clin. Chim. Acta. 22: 296–299.
Koenig RJ, Blobstein SH, Cerami A. (1977). Structure of carbohydrate of hemoglobin AIc. J. Biol. Chem. 252: 2992–2997.
Makita Z, Vlassara H, Cerami A, Bucala R. (1992). Immunochemical detection of advanced glycosylation end products in vivo. J. Biol. Chem. 267: 5133–5138.
Schleicher E, Wagner E, Nerlich AG. (1997). Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J. Clin. Invest. 99: 457–468.
Makita Z, Vlassara H, Rayfield E, et al. (1992). Hemoglobin-AGE: a circulating marker of advanced glycosylation. Science 258: 651–653.
Wolffenbuttel BH, Giordano D, Founds HW, Bucala R: (1996). Long-term assessment of glucose control by hemoglobin-AGE measurement. Lancet 347: 513–515.
Gandhi CR, Roy Chowdhury RD. (1979). Effect of diabetes mellitus on sialic acid & glutathione content of human erythrocytes of different ages. Indian J. Exp. Biol. 17: 585–587.
Jain SK, McVie R. (1994). Effect of glycemic control, race (white versus black), and duration of diabetes on reduced glutathione content in erythrocytes of diabetic patients. Metabolism 43: 306–309.
Ciuchi E, Odetti P, Prando R. (1996). Relationship between glutathione and sorbitol concentrations in erythrocytes from diabetic patients: Metabolism 45: 611–613.
Murakami K, Kondo T, Ohtsuka Y, Fujiwara Y, Shimada M, Kawakami Y. (1989). Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism 38: 753–758.
Murakami K. (1991). Glutathione metabolism in erythrocytes from patients with diabetes mellitus. Hokkaido Igaku Zasshi 66: 29–40.
Blakytny R, Harding JJ. (1992). Glycation (non-enzymic glycosylation) inactivates glutathione reductase. Biochem J 288: 303–307.
Birchmeier W, Tuchschmid PE, Winterhalter KH. (1973). Comparison of human hemoglobin A carrying glutathione as a mixed disulfide with the naturally occurring human hemoglobin A. Biochemistry 12: 3667–3672.
Wodak SJ, De Coen JL, Edelstein SJ, Demarne H, Beuzard Y. (1986). Modification of human hemoglobin by glutathione. III. Perturbations of hemoglobin conformation analyzed by computer modeling. J. Biol. Chem. 261: 14717–14724.
Garel MC, Domenget C, Caburi-Martin J, Prehu C, Galacteros F, Beuzard Y. (1986). Covalent binding of glutathione to hemoglobin. I. Inhibition of hemoglobin S polymerization. J. Biol. Chem. 261: 14707–14724.
Niketic V, Beslo D, Raicevic S, Sredic S, Stojkovic M. (1992). Glutathione adduct of hemoglobin (Hb ASSG) in hemolysates of patients on long-term antiepileptic therapy. Int. J. Biochem. 24: 503–507.
Al-Abed Y, Mitsuhashi T, Li H, et al. (1999). Inhibition of advanced glycation endproduct formation by acetaldehyde: role in the cardioprotective effect of ethanol. Proc. Natl. Acad. Sci. U.S.A. 96: 2385–2390.
Niwa T, Naito C, Mawjood AHM, Imai K. (2000). Increased glutathionyl hemoglobin in diabetes mellitus and hyperlipidemia demonstrated by liquid chromatography/electrospray ionization-mass spectrometry. Clin. Chem. 46: 82–88.
Naito C, Kajita M, Niwa TJ. (1999). Determination of glutathionyl hemoglobin in hemodialysis patients using electrospray ionization liquid chromatography-mass spectrometry. Chromatogr. B 731: 121–124.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Abed, Y., VanPatten, S., Li, H. et al. Characterization of a Novel Hemoglobin-Glutathione Adduct That Is Elevated in Diabetic Patients. Mol Med 7, 619–623 (2001). https://doi.org/10.1007/BF03401868
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401868