Abstract
Background
Mutations in the presenilin (PSEN) genes are responsible for the majority of early-onset Alzheimer disease (AD) cases. PSEN1 is a component of a high molecular weight, endoplasmic reticulum, membrane-bound protein complex, including β-catenin. Pathogenic PSEN1 mutations were demonstrated to have an effect on β-catenin and glycogen synthase kinase-3β(GSK-3β), two members of the wingless Wnt pathway. The nuclear translocation and the stability of β-catenin, and the interaction between GSK3β and PSEN1 were influenced.
Materials and Methods
Stably transfected human embryonic kidney (HEK) 293 cells overexpressing wild-type (wt) and mutant (mt) PSEN1, treated with and without LiCl, were used to isolate cytoplasmic and nuclear fractions. By Western blot analysis, endogenous β-catenin levels were examined. By analyzing cytosolic fractions of PSEN1, transfected and nontransfected HEK 293 cells, and total brain extracts of AD patients and controls, we evaluated the effect of PSEN1 overexpression on β-catenin stability. Finally, we analyzed the effect of pathogenic PSEN1 mutations on the interaction between PSEN1 and GSK3β by co-immunoprecipitation experiments.
Results
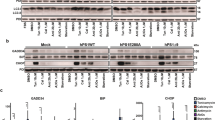
We report reduced nuclear translocation of β-catenin in cells stably expressing I143T, G384A, and T113-114ins PSEN1. The G384A PSEN1 mutation showed a similar pronounced effect on nuclear translocation of β-catenin, as reported for processing of amyloid precursor protein (APP) into amyloid β(Aβ). Overexpression of PSEN1 and the presence of pathogenic mutations in PSEN1 had no significant effect on the stability of β-catenin. Nonspecific binding of overexpressed PSEN1 to endogenous GSK3β was observed when GSK3β was immuno-precipitated. Immunoprecipitation of PSEN1 in cells overexpressing PSEN1 and in native cells, however, did not result in co-immunoprecipitation of endogenous GSK3β.
Conclusion
Our results further establish the nuclear translocation assay of β-catenin as an adequate alternative for traditional Aβ measurement to evaluate the effect of PSEN1 mutations on biochemical processes. We detected no significant effect of overexpressed wt or mt PSEN1 on the stabilty of β-catenin. Finally, co-immunoprecipitation between PSEN1 and GSK3β was not observed in our experimental setup.





Similar content being viewed by others
References
Cruts M, Van Broeckhoven C. (1998) Molecular genetics of Alzheimer’s disease. Ann. Med. 30: 560–565.
Kovacs DM, Fausett HJ, Page KJ, et al. (1996) Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat. Med. 2: 224–229.
Lah JJ, Heilman CJ, Nash NR, et al. (1997) Light and electron microscopic localization of presnilin-1 in primate brain. J. Neurosci. 17: 1971–1980.
Annaert WG, Levesque L, Craessaerts K, et al. (1999) Presenilin 1 controls γ-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J. Cell Biol. 147: 277–294.
Li J, Xu M, Zhou H, et al. (1997) Alzheimer pre-senilins in the nuclear membrane, interphase kinetochores, and centrosomes suggest a role in chromosome segregation. Cell 90: 917–927.
Thinakaran G, Borchelt DR, Lee MK, et al. (1996) Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17: 181–190.
Thinakaran G, Harris CL, Ratovitski T, et al. (1997) Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J. Biol. Chem. 272: 28415–28422.
Yu G, Chen F, Levesque G, et al. (1998) The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains (β catenin. J. Biol. Chem. 273: 16470–16475.
Capell A, Grunberg J, Pesold B, et al. (1998) The proteolytic fragments of the Alzheimer’s disease-associated presenilin-1 form heterodimers and occur as a 100–150-kDa molecular mass complex. J. Biol. Chem. 273: 3205–3211.
Thinakaran G, Regard JB, Bouton CM, et al. (1998) Stable association of presenilin derivatives and absence of presenilin interactions with APP. Neurobiol. Dis. 4: 438–453.
Steiner H, Capell A, Pesold B, et al. (1998) Expression of Alzheimer’s disease-associated presenilin-1 is controlled by proteolytic degradation and complex formation. J. Biol. Chem. 273: 32322–32331.
Van Gassen G, De Jonghe C, Pype S, et al. (1999) Alzheimer’s disease associated presenilin 1 interacts with HC5 and ZETA, subunits of the catalytic 20S proteasome. Neurobiol. Dis. 6: 376–391.
Borchelt DR, Thinakaran G, Eckman CB, et al. (1996) Familial Alzheimer’s disease-linked pre-senilin 1 variants elevate Aβ1-42/1-40 ratio in vitro and in vivo. Neuron 17: 1005–1013.
De Strooper B, Saftig P, Craessaerts K, et al. (1998) Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391: 387–390.
De Strooper B, Annaert W, Cupers P, et al. (1999) A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522.
Song W, Nadeau P, Yuan M, et al. (1999) Proteolytic release and nuclear translocation of Notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc. Natl. Acad. Sci. U.S.A. 96: 6959–6963.
Nishimura M, Yu G, Levesque G, et al. (1999) Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of β-catenin, a component of the presenilin protein complex. Nat. Med. 5: 164–169.
Wodarz A, Nusse R. (1998) Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14: 59–88.
Hall AC, Lucas FR, Salinas PC. (2000) Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 2000 100: 525–535.
Takashima A, Murayama M, Murayama O, et al. (1998) Presenilin 1 associates with glycogen synthase kinase-3β and its substrate τ. Proc. Natl. Acad. Sci. U.S.A. 95: 9637–9641.
Tesco G, Kim TW, Diehlmann A, et al. (1998) Abrogation of the presenilin 1/β-catenin interaction and preservation of the heterodimeric presenilin 1 complex following caspase activation. J. Biol. Chem. 273: 33909–33914.
Zhang Z, Hartmann H, Do VM, et al. (1998) Destabilization of β-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395: 698–702.
Kang DE, Soriano S, Frosch MP, et al. (1999) Presenilin 1 facilitates the constitutive turnover of β-catenin: differential activity of Alzheimer’s disease-linked PS1 mutants in the β-catenin-signaling pathway. J. Neurosci. 19: 4229–4237.
Zhou J, Liyanage U, Medina M, et al. (1997) Pre-senilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport. 8: 1489–1494.
Tanahashi H, Tabira T. (1999) Isolation of human Δ-catenin and its binding specificity with presenilin 1. Neuroreport. 10: 563–568.
Levesque G, Yu G, Nishimura M, et al. (1999) Presenilins interact with armadillo proteins including neural-specific plakophilin-related protein and β-catenin. J. Neurochem. 72: 999–1008.
De Jonghe C, Cras P, Vanderstichele H, et al. (1999) Evidence that Aβ42 plasma levels in pre-senilin-1 mutation carriers do not allow for prediction of their clinical phenotype. Neurobiol. Dis. 6: 280–287.
De Jonghe C, Cruts M, Rogaeva EA, et al. (1999) Aberrant splicing in the presenilin-1 intron 4 mutation causes presenile Alzheimer’s disease by increased Aβ42 secretion. Hum. Mol. Genet. 8: 1529–1540.
Dermaut B, Cruts M, Slooter AJ, et al. (1999) The Glu318Gly substitution in presenilin 1 is not causally related to Alzheimer disease. Am. J. Hum. Genet. 64: 290–292.
Hendriks L, Thinakaran G, Harris CL, et al. (1997) Processing of presenilin 1 in brains of patients with Alzheimer’s disease and controls. Neuroreport. 8: 1717–1721.
Cruts M, Backhovens H, Wang SY, et al. (1995) Molecular genetic analysis of familial early-onset Alzheimer’s disease linked to chromosome 14q24.3. Hum. Mol. Genet. 4: 2363–2371.
Murayama O, Tomita T, Nihonmatsu N, et al. (1999) Enhancement of amyloid β 42 secretion by 28 different presenilin 1 mutations of familial Alzheimer’s disease. Neurosci. Lett. 265: 61–63.
Wolfe MS, Xia W, Ostaszewski BL, et al. (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and β-secretase activity. Nature 398: 513–517.
Fagotto F, Gluck U, Gumbiner BM. (1998) Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr. Biol. 8: 181–190.
Acknowledgement
The authors want to thank Sofie Van Gestel for statistical assistance. This work was supported by the Fund for Scientific Research-Flanders (FWO-F), the Interuniversity Attraction Poles (IUAP p4/17), the International Alzheimer’s Research Foundation (IARF), and the Focused Giving Program of Johnson & Johnson. This work was further supported by grants of the Canadian Genetic Disease Network, the Medical Research Council of Canada, the Howard Hughes Medical Research Foundation (PH St GH), and the Department of Medicine Post-Doctoral fellowship award (MN). GVG is a grant holder of the Institute for Science and Technology (IWT). CDJ is a postdoctoral fellow at the FWO-F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Gassen, G., De Jonghe, C., Nishimura, M. et al. Evidence that the β-catenin Nuclear Translocation Assay Allows for Measuring Presenilin 1 Dysfunction. Mol Med 6, 570–580 (2000). https://doi.org/10.1007/BF03401795
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401795