Abstract
Background
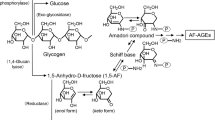
The Maillard reaction that leads to the formation of advanced glycation end-products (AGE) plays an important role in the pathogenesis of angiopathy in diabetic patients and in the aging process. Recently, it was proposed that AGE were not only created by glucose, but also by dicarbonyl compounds derived from the Maillard reaction, autoxidation of sugars and other metabolic pathways of glucose. In this study, we developed four types of non-carboxymethyllysine (CML) anti-AGE antibodies that recognized proteins modified by incubation with short chain sugars and dicarbonyl compounds.
Materials and Methods
AGE-modified serum albumins were prepared by incubation of rabbit serum albumin with glyceraldehyde, glycolaldehyde, methylglyoxal or glyoxal. After immunization of rabbits, four types of AGE-specific antisera were obtained that were specific for the AGE modification. To separate non-CML AGE antibodies (Ab) (non-CML AGE-Ab-2, -3, -4, and -5), these anti-AGE antisera were subjected to affinity chromatography on a matrix coupled with four kinds of AGE bovine serum albumin (BSA) or CML-BSA. These non-CML AGE antibodies were used to investigate the AGE content of serum obtained from diabetic patients on hemodialysis.
Results
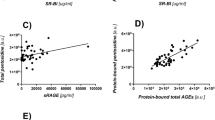
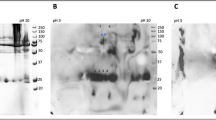
Characterization of the four types of non-CML AGE antibodies obtained by immunoaffinity chromatography was performed by competitive ELISA and immunoblot analysis. Non-CML AGE-Ab-2 cross-reacted with the protein modified by glyceraldehyde or glycolaldehyde. Non-CML AGE-Ab-3 and -Ab-4 specifically cross-reacted with protein modified by glycolaldehyde and methylglyoxal, respectively. Non-CML AGE-Ab-5 cross-reacted with protein modified with glyoxal as well as methylglyoxal and glycolaldehyde. Three kinds of non-CML AGE (AGE-2, -4, and -5) were detected in diabetic serum as three peaks with apparent molecular weights of 200, 1.15, and 0.85 kD; whereas, AGE-3 was detected as two peaks with apparent molecular weights of 200 and 0.85 kD.
Conclusion
We propose that various types of non-CML AGE are formed by the Maillard reaction, sugar autoxidation and sugar metabolism. These antibodies enable us to identify such compounds created by the Maillard reaction in vivo.








Similar content being viewed by others
References
Makita Z, Bucala R, Reyfield EJ, et al. (1994) Reactive glycosylation endproducts in diabetic uremia and treatment of renal failure. Lancet 343: 1519–1522.
Vlassara H, Bucala R, Striker L. (1994) Pathogenic effects of AGEs: biochemical, biologic, and clinical implications for diabetes and aging. Lab. Invest. 70: 138–151.
Brownlee M. (1995) Advanced protein glycosylation in diabetes and aging. Ann. Rev. Med. 46: 223–234.
Vlassara H. (1997) Recent progress in advanced glycation end products and diabetic complications. Diabetes 46 (Suppl. 2): S19–S25.
Thorpe SR, Baynes JW. (1996) Role of the Maillard reaction in diabetes mellitus and diseases of aging. Drugs Aging 9: 69–77.
Wells-Knecht KJ, Zyzak DV, Litchfield SR, Thorpe SR, Baynes JW. (1995) Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry 34: 3702–3709.
Wells-Knecht MC, Thorpe SR, Baynes JW. (1995) Pathways of formation of glycoxidative products during glycation of collagen. Biochemistry 34: 15134–15141.
Thornalley PJ. (1990) The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J. 269: 1–11.
Thornalley PJ. (1996) Pharmacology of methylglyoxalase: formation, modification of proteins and nucleic acids, and enzymatic detoxification- a role in pathogenesis and antiproliferative chemotherapys. Gen. Pharmacol. 27: 565–573.
McLellan AC, Thornalley PJ, Ben J, Sonksen PH. (1994) Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complication Clin. Sci. 87: 21–29.
McLellan AC, Phillips SA, Thornalley PJ. (1992) The assay of methylglyoxal in biological systems by derivatization with 1,2-diamino-4,5-dimethoxybenzene. Anal. Biochem. 206: 17–23.
Haik GM, Lo TW, Thornalley PJ (1994) Methylglyoxal concentration and glyoxalase activities in the human lens. Exp. Eye Res. 59: 497–500.
Glomb MA, Monnier VM. (1995) Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J. Biol. Chem. 270: 10017–10026.
Takeuchi M, Makita Z, Yanagisawa K, Kameda Y, Koike T. (1999) Detection of noncarboxy-methyllysine and carboxymethyllysine advanced glycation end products (AGE) in serum of diabetic patients. Mol. Med. 5: 393–405.
Ikeda K, Higashi T, Sano H, et al. (1996) N-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry 35: 8075–8083.
Ahmed MU, Frye EB, Degenhardt TP, Thorpe SR, Baynes JW. (1997) N-(Carboxyethyl)ly-sine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 324: 565–570.
Wells-Knecht KJ, Brinkmann E, Wells-Knecht MC, et al. (1996) New biomarkers of Maillard reaction damage to proteins. Nephrol. Dial. Transplant. 11(Suppl 5): 41–47.
Requena JR, Fu M-X, Ahmed MU, Jenkins AJ, Lyons TJ, Thorpe SR. (1996) Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol. Dial. Transplant. 11(Suppl 5): 48–53.
Odani H, Shinzato T, Matsumoto Y, Usami J, Maeda K. (1999) Increase in three α, β-dicarbonyl compound levels in human uremic plasma: specific in vivo determination of intermediates in advanced Maillard reaction. Biochem. Biophys. Res. Commun. 256: 89–93.
Anderson MM, Hazen SL, Hsu FF, Heinecke JW. (1997) Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein: a mechanism for the generation of highly reactive α-hydroxy and α, β-unsaturated aldehydes by phagocytes at sites of inflammation. J. Clin. Invest. 99: 424–432.
Shamsi FA, Partal A, Sady C, Glomb MA, Nagaraj RH. (1998) Immunological evidence for methylglyoxal-derived modifications in vivo. J. Biol. Chem. 273: 6928–6936.
Uchida K, Khor OT, Oya T, Osawa T, Yasuda Y, Miyata T. (1997) Protein modification by a Maillard reaction intermediate methylglyoxal immunochemical detection of fluorescent 5-methylimidazolone derivatives in vivo. FEBS Lett. 410: 313–318.
Oya T, Hattori N, Mizuno Y, et al. (1999) Methylglyoxal modification of protein: chemical and immunochemical characterization of methylglyoxal-arginine adducts. J. Biol. Chem. 274: 18492–18502.
Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. (1998) Role of the Maillard reaction in aging of tissue proteins: advanced glycation end product-dependent increase in imidazolium crosslinks in human lens proteins. J. Biol. Chem. 273: 18714–18719.
Degenhardt TP, Thorpe SR, Baynes JW. (1998) Chemical modification of proteins by methylglyoxal. Cell Mol. Biol. 44: 1139–1145.
Baynes JW, Thorpe SR. (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48: 1–9.
Acknowledgments
We are grateful to Dr. Satoshi Miyata (Kobe University School of Medicine) for providing pyrraline-BSA and to Dr. Toshio Miyata (Tokai University School of Medicine) for providing pentosidine-BSA. These studies were supported in part by a Grant-in-Aid for Scientific Research (#09470204) to Zenji Makita from the Japanese Ministry of Education, Science, Sports, and Culture, and by a Health Science Research Grant (#H10-Chozyu-033) to Zenji Makita from the Japanese Ministry of Health and Welfare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Bucala.
Rights and permissions
About this article
Cite this article
Takeuchi, M., Makita, Z., Bucala, R. et al. Immunological Evidence that Non-carboxymethyllysine Advanced Glycation End-products Are Produced from Short Chain Sugars and Dicarbonyl Compounds in vivo. Mol Med 6, 114–125 (2000). https://doi.org/10.1007/BF03401779
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401779