Abstract
Background
Upon engagement of the T cell receptor for antigen, its associated CD3 proteins recruit signal transduction molecules, which in turn regulate T lymphocyte proliferation, apoptosis, and thymocyte development. Because some signal transducing molecules recruited by CD3-ε, i.e., p56lck and p59fyn, are oncogenic and since we previously found that overexpression of CD3-ε transgenes causes a block in T lymphocyte and NK cell development, we tested the hypothesis that aberrant CD3-ε signaling leads both to abnormal T lymphocyte death and lymphomagenesis.
Materials and Methods
Ten independently derived transgenic mouse lines were generated with four different genomic CD3-ε constructs. Mice either homozygous or hemizygous for each transgene were analyzed for an arrest in T lymphocyte development and for the occurrence of T cell lymphomas.
Results
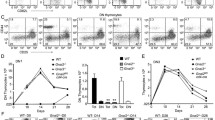
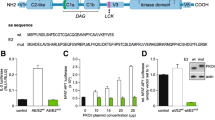
Aggressive clonal T cell lymphomas developed at very high frequencies in seven mouse lines with intermediate levels of copies of CD3-ε derived transgenes. However, these lymphomas were not found when high copy numbers of CD3-ε transgenes caused a complete block in early thymic development or when a transgene was used in which the exons coding for the CD3-ε protein were deleted. Analyses of a series of double mutant mice, tgCD3-ε × RAG-2null, indicated that lymphomagenesis was initiated in lineage-committed prothymocytes, i.e., before rearrangement of the T cell receptor genes. In addition, the transgene coding for the CD3-ε cytoplasmic domain and its transmembrane region induced a T cell differentiation signal in premalignant tgCD3-ε × RAG-2null mice.
Conclusion
The nonenzymatic CD3-ε protein acted as a potent oncogene when overexpressed early in T lymphocyte development. Lymphomagenesis was dependent on signal transduction events initiated by the cytoplasmic domain of CD3-ε.





Similar content being viewed by others
References
Korsmeyer SJ. (1993) Programed cell death: BcI-2. Important Adv. Oncol. 93: 19–28.
Vogelstein B, Kinzler KW. (1993) The multistep nature of cancer. Trends Genet. 9: 138–141.
Boettiger D. (1989) Interaction of oncogenes with differentiation programs. Curr. Top. Microbiol. Immunol. 147: 31–78.
Hall M, Peters G. (1996) Genetic alterations of cychns, cyclin-dependent kinases, and cdk inhibitors in human cancer. Adv. Cancer Res. 68: 67–109.
Seiivanova G, Wiman KG. (1995) p53: A cell cycle regulator activated by DNA damage. Adv. Cancer Res. 66: 143–180.
Leonard CJ, Canman CE, Kastan MB. (1995) The role of p53 in cell-cycle control and apoptosis: Implications for cancer. Important Adv. Oncol. 95: 33–42.
Wang JY, Knudsen ES, Welch PJ. (1994) The retinoblastoma tumor suppressor protein. Adv. Cancer Res. 64: 25–85.
Fisher RJ, Bader JP, Papas TS. (1989) Oncogenes and the mitogenic signal pathway. Important Adv. Oncol. 89: 3–27.
Balmain A, Brown K. (1988) Oncogene activation in chemical carcinogenesis. Adv. Cancer Res. 51: 147–182.
Dilworth SM, Brewster CE, Jones MD, Lanfrancone L, Pelicci G, Pelicci PG. (1994) Transformation by polyoma virus middle Tantigen involves the binding and tyrosine phosphorylation of She. Nature 367: 87–90.
Crowe AJ, McGlade J, Pawson T, Hayman MJ. (1994) Phosphorylation of the SHC proteins on tyrosine correlates with the transformation of fibroblasts and erythroblasts by the v-sea tyrosine kinase. Oncogene 9: 537–544.
Harrison-Pindik D, Susa M, Varticovski L. (1995) Association of phosphatidylinositol 3-kinase with SHC in chronic myelogeneous leukemia cells. Oncogene 10: 1385–1391.
Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Grignani F, Pawson T, Pelicci PG. (1992) A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell 70: 93–104.
Pelicci G, Lanfrancone L, Salcini AE, Romano A, Mele S, Grazia Borrello M, Segatto O, Di Fiore PP, Pelicci PG. (1995) Constitutive phosphorylation of Shc proteins in human tumors. Oncogene 11: 899–907.
Salcini AE, McGlade J, Pelicci G, Nicoletti I, Pawson T, Pelicci PG. (1994) Formation of Shc-Grb2 complexes is necessary to induce neoplastic transformation by overexpression of Shc proteins. Oncogene 9: 2827–2836.
Clevers H, Alarcon B, Wilwman T, Terhorst C. (1988) The T cell receptor/CD3 complex: A dynamic protein ensembe. Annu. Rev. Immunol. 6: 629–662.
Ashwell JD, Klusner RD. (1990) Genetic and mutational analysis of the T-cell antigen receptor. Annu. Rev. Immunol. 8: 139–167.
Letourneur F, Klausner RD. (1992) Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 ε. Science 255: 79–82.
Wegener A-MK, Letourneur F, Hoeveler A, Brocker T, Luton F, Malissen B. (1992) The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell 68: 83–95.
Terhorst C, Regueiro JR. (1992) T cell activation. In: Lachmann PJ et al. (eds) Clinical Aspects of Immunology Blackwell Science, Inc. Oxford, UK. Vol 1, pp. 447–466.
Owen JJT, Owen MJ, Williams GT, Kingston R, Jenkinson EJ. (1988) The effects of anti-CD3 antibodies on the development T-cell receptor αβ lymphocytes in embryonic thymus organ cultures. Immunology 63: 639–642.
Bentin J, Vaughan JH, Tsoukas CD. (1988) T cell proliferation induced by anti-CD3 antibodies: Requirement for a T-T cell interaction. Eur. J. Immunol. 18: 627–632.
Levelt CN, Mombaerts P, Wang B, Kohler H, Tonegawa S, Eichmann K, Terhorst C. (1995) Regulation of thymocyte development through CD3: Functional dissociation between p56lck and CD3 sigma in early thymic selection. Immunity 3: 215–222.
Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B. (1995) Altered T cell development in mice with a targeted mutation of the CD3-ε gene. EMBO J. 14: 4641–4653.
Shinkai Y, Ma A, Cheng HL, Alt FW. (1995) CD3 epsilon and CD3 zeta cytoplasmic domains can independently generate signals for T cell development and function. Immunity 2: 401–411.
Abraham KM, Levin SD, Marth JD, Forbush KA, Perlmutter RM. (1991) Thymic tumori- genesis induced by overexpression of p56lck. Proc. Natl Acad. Sci. U.S.A. 88: 3977–3981.
Anderson SJ, Levin SD, Perlmutter RM. (1994) Involvement of the protein tyrosine kinase p56lck in T cell signaling and thymocyte development. Adv. Immunol. 56: 151–178.
Wang B, Biron C, She J, Higgins K, Sunshine MJ, Lacy E, Lonberg N, Terhorst C. (1994) A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc. Natl. Acad. Sci. U.S.A. 91: 9402–9406.
Wang B, Levelt CN, Salio M, Zheng D-X, Sancho J, Liu C-P, She J, Huang M, Higgins K, Sunshine M-J, Eichmann K, Lacy L, Lonberg N, Terhorst C. (1995) Over-expression of CD36ε transgenes blocks T lymphocyte development. Int. Immunol. 7: 435–448.
Molina TJ, Kishihara K, Siderovski DP, et al. (1992) Profound block in thymocyte development in mice lacking p56lck. Nature 357: 161–164.
Levelt CN, Mombaerts P, Iglesias A, Tonegawa S, Eichmann K, (1993) Restoration of early thymocyte differentiation in T-cell receptor beta-chain-deficient mutant mice by transmembrane signaling through CD3 epsilon. Proc. Natl. Acad. Sci. U.S.A. 90: 11401–11405.
Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. (1992) Rag-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68: 855–867.
Godfrey DI, Zlotnik A. (1993) Control points in early T-cell development. Immunol. Today 14: 547–553.
Scollay R, Wilson A, D’Amico A, Kelly K, Egerton M, Pearse M, Wu L, Shortman K. (1988) Developmental status and reconstitution potential of subpopulations of murine thymocytes. Immunol. Rev. 104: 81–120.
Shinkai Y, Alt FW. (1994) CD3 epsilon-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2-/- mice in the absence of TCR beta chain expression. Int. Immunol. 6: 995–1001.
Levelt CN, Wang B, Ehrfeld A, Terhorst C, Eichmann K. (1995) Regulation of T cell receptor (TCR)-beta locus allelic exclusion and initiation of TCR-alpha locus rearrangement in immature thymocytes by signaling through the CD3 complex. Eur. J. Immunol. 25:1257–1261.
Dedera DA, Waller EK, LeBrun DP, Sen-Majumdar A, Stevens ME, Barsh GS, Cleary ML. (1993) Chimeric homeobox gene E2A-PBXI induces proliferation, apoptosis, and malignant lymphomas in transgenic mice. Cell 74: 833–843.
Winandy S, Wu P, Georgopoulos K. (1995) A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell 83: 289–299.
O’Neill HC, McGrath MS, Allison JP, Weissman IL. (1987) A subset of T cell receptors associated with L3T4 molecules mediates C6VL leukemia cell binding of its cognate retrovirus. Cell 49: 143–151.
McGrath MS, Pillemer E, Weissman IL. (1980) Murine leukaemogenesis: Monoclonal antibodies to T-cell determinants arrest T-lymphoma cell proliferation. Nature 285: 259–261.
Anderson SJ, Perlmutter RM. (1995) A signaling pathway governing early thymocyte maturation. Immunol. Today 16: 99–105.
Weiss A, Littman DR. (1994) Signal transduction by lymphocyte antigen receptors. Cell 76: 263–274.
Acknowledgments
We thank Key Higgins for technical assistance, and Drs. Clyde Dawe, Tom Benjamin and Geoffrey Cooper for a critical review of the manuscript. This work was supported by a grant from the National Institutes of Health (PO1-AI 35714).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, B., She, J., Salio, M. et al. CD3-ε Overexpressed in Prothymocytes Acts as an Oncogene. Mol Med 3, 72–81 (1997). https://doi.org/10.1007/BF03401669
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401669