Abstract
Background
Glucocorticoids are involved in the regulation of metabolic, immunological, and developmental processes. Their synthesis is tightly controlled by feedback regulation through the hypothalamus-pituitary-adrenal (HPA) axis, allowing the organism to respond to stress in an adequate manner and to adapt to new situations. Disturbance of these regulatory mechanisms leads to major human diseases. By generating mice with a targeted mutation in the glucocorticoid receptor (GR) locus, it was possible to analyze the mechanism by which glucocorticoids control the HPA axis, under conditions where at least part of the feedback control was absent early in development.
Materials and Methods
RNase-protection and in situ hybridization assays were used to compare messenger RNA (mRNA) levels of genes involved in the control of the HPA axis in both GR-mutant and wild-type animals.
Results
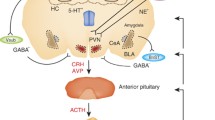
Negative feedback regulation of the HPA axis by glucocorticoids, which is established around Day E16.5 of embryonic development in wild-type mice, does not occur in GR-mutants, resulting in an increased expression of proopiomelanocortin mRNA in the anterior lobe of the pituitary and of corticotropin-releasing hormone mRNA in the paraventricular nucleus of the hypothalamus. However, the expression of both arginine vasopressin and mineralocorticoid receptor in the brain is not affected. In the neurointermediate lobe of the pituitary, expression of the proopiomelanocortin gene was inversely regulated, compared with its expression in the anterior lobe.
Conclusions
GR-dependent regulation of the HPA axis is established during fetal development, suggesting that maternal factors have an important role in influencing the HPA axis of the adult offspring.






Similar content being viewed by others
References
Beato M, Herrlich P, Schütz G. (1995) Steroid hormone receptors: many actors in search of a plot. Cell 83: 851–857.
Cole TJ, Blendy JA, Monaghan AP, et al. (1995) Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 9: 1608–1621.
Birnberg NC, Lissitzky JC, Hinman M, Herbert E. (1983) Glucocorticoids regulate proopiomelanocortin gene expression in vivo at the levels of transcription and secretion. Proc. Natl. Acad. Sci. U.S.A. 80: 6982–6986.
Autelitano DJ, Lundblad JR, Blum M, Roberts JL. (1989) Hormonal regulation of POMC gene expression. Annu. Rev. Physiol. 51: 715–726.
Young III W. (1992) Regulation of gene expression in the hypothalamus: Hybridization histochemical studies. Ciba Found. Symp. 168: 127–138.
Plotsky PM, Sawchenko PE. (1987) Hypophysial-portal plasma levels, median eminence content, and immunohistochemical staining of corticotropin-releasing factor, arginine vasopressin, and oxytocin after pharmacological adrenalectomy. Endocrinology 120: 1361–1369.
Plotsky PM, Thrivikraman KV, Meaney MJ. (1993) Central and feedback regulation of hypothalamic corticotropin-releasing factor secretion. Ciba Found. Symp. 172: 59–84.
Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. (1993) The functional neuroanatomy of corticotropin-releasing factor. Ciba Found. Symp. 172: 5–29.
Michelsohn AM, Anderson DJ. (1992) Changes in competence determine the timing of two sequential glucocorticoid effects on sympathoadrenal progenitors. Neuron 8: 589–604.
Chomczynski P, Sacchi N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162: 156–159.
Kaestner KH, Ntambi JM, Kelly TJ, Lane MD. (1989) Differentiation-induced gene expression in 3T3-L1 preadipocytes. A second differentially expressed gene encoding stearoyl-CoA desaturase. J. Biol. Chem. 264: 14755–14761.
Tamura T, Sumita K, Fujino I, et al. (1991) Striking homology of the ‘Variable’ N-terminal as well as the ‘conserved core’ domains of the mouse and human TATA-factors (TFIID). Nucleic Acids Res. 19: 3861–3865.
Wilkinson DG (ed). (1992) In Situ Hybridization. A Practical Approach. Oxford University Press, New York.
Keegan CE, Herman JP, Karolyi IJ, O’Shea KS, Camper SA, Seasholtz AF. (1994) Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology 134: 2547–2555.
Hyodo S, Yamada C, Takezawa T, Urano A. (1992) Expression of pro vasopressin gene during ontogeny in the hypothalamus of developing mice. Neuroscience 46: 241–250.
Ang HL, Carter DA, Murphy D. (1993) Neuron-specific expression and physiological regulation of bovine vasopressin transgenes in mice. EMBO J. 12: 2397–2409.
Cullinan WE, Herman JP, Watson SJ. (1993) Ventral subicular interaction with the hypothalamic paraventricular nucleus: Evidence for a relay in the bed nucleus of the stria terminalis. J. Comp. Neurol. 332: 1–20.
Joels M, de Kloet R. (1994) Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog. Neurobiol. 43: 1–36.
Drouin J, Sun YL, Chamberland M, et al. (1993) Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J. 12: 145–156.
Dupouy JP, Chatelain A. (1984) In-vitro effects of corticosterone, synthetic ovine corticotrophin releasing factor and arginine vasopressin on the release of adrenocorticotrophin by fetal rat pituitary glands. J. Endocrinol. 101: 339–344.
Lugo IL, Pintar JE. (1996) Ontogeny of basal and regulated secretion from POMC cells of the developing anterior lobe of the rat pituitary gland. Dev. Biol. 1: 95–109.
Autelitano DJ, Clements JA, Nikolaidis I, Canny BJ, Funder JW. (1987) Concomitant dopaminergic and glucocorticoid control of pituitary proopiomelanocortin messenger ribonucleic acid and beta-endorphin levels. Endocrinology 121: 1689–1696.
Lugo DI, Pintar JE. (1996) Ontogeny of basal and regulated proopiomelanocortin-derived peptide secretion from fetal and neonatal pituitary intermediate lobe cells: Melanotrophs exhibit transient glucocorticoid responses during development. Dev. Biol. 173: 110–118.
Chen CL, Dionne FT, Roberts JL. (1983) Regulation of the proopiomelanocortin mRNA levels in rat pituitary by dopaminergic compounds. Proc. Natl. Acad. Sci. U.S.A. 80: 2211–2215.
Lindley SE, Lookingland KJ, Moore KE. (1990) Dopaminergic and beta-adrenergic receptor control of alpha-melanocyte-stimulating hormone secretion during stress. Neuroendocrinology 52: 46–51.
Makino S, Smith MA, Gold PW. (1995) Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology 136: 3299–3309.
Muglia L, Jacobson L, Dikkes P, Majzoub JA. (1995) Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 373: 427–432.
Herman JP, Adams D, Prewitt C. (1995) Regulatory changes in neuroendocrine stressintegrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61: 180–190.
Holmes MC, Yau JL, French KL, Seckl JR. (1995) The effect of adrenalectomy on 5-hydroxytryptamine and corticosteroid receptor subtype messenger RNA expression in rat hippocampus. Neuroscience 64: 327–337.
Reul JM, Stec I, Wiegers GJ, et al. (1994) Prenatal immune challenge alters the hypothalamic-pituitary-adrenocortical axis in adult rats. J. Clin. Invest. 93: 2600–2607.
Acknowledgments
We would like to thank Dagmar Bock and Anja Schwäger for expert technical assistance and Werner Fleischer for oligonucleotide synthesis and photographical artwork. We are grateful to Drs. Klaus H. Kaestner, A. Paula Monaghan, and Wolfgang Schmid for helpful discussions, and to Dr. Klaus H. Kaestner for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft through SFB 229, the Fonds der Chemischen Industrie, the BMFT project 0310681, and European Community Grant BI02-CT93-0319.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reichardt, H.M., Schütz, G. Feedback Control of Glucocorticoid Production is Established during Fetal Development. Mol Med 2, 735–744 (1996). https://doi.org/10.1007/BF03401657
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401657