Abstract
Executive control could be involved in neural capacity, which corresponds to the modulation of neural activity with increased task difficulty. Thus, by exploring the P300—an electrophysiological correlate of working memory—we examined the role played by executive control in both the age-related decline in working memory and neural capacity in aging. Event-related potentials (ERPs) were recorded while younger and older participants performed a Sternberg task with two set sizes (2 vs. 6 items), allowing us to calculate a neural capacity index. Participants also completed two control tasks (Stroop and 3-back tests), which were used to calculate a composite executive control index. Results indicated that working memory performance decreased with aging and difficulty. At the neural level, results indicated that the P300 amplitude varied with aging and also with task difficulty. In the low difficulty condition, frontal P300 amplitude was higher for older than for younger adults, whereas in the high difficulty condition, the amplitude of frontal and parietal P300 did not differ between both age groups. Results also suggest that task difficulty led to a decrease in parietal amplitude in both age groups and to an increase in frontal amplitude in younger but not older adults. Both executive control and frontal neural capacity mediated the age-related variance in working memory for older adults. Moreover, executive control mediated the age-related variance in the frontal neural capacity of older adults. Thus, the present study suggests a model for older adults in which executive control deficits with advancing age lead to less efficient frontal recruitment to cope with task difficulty (neural capacity), which in turn has a negative impact on working memory functioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The purpose of the present study was to explore the role of executive control in both the age-related decline in working memory and in neural capacity associated with working memory in aging. Working memory is a cognitive process involving short-term online storage of information and the control processes enabling this information to be maintained and manipulated (Baddeley, 2003; Baddeley & Hitch, 1974). Recent models of working memory suggest that an executive attention component plays a role in regulating the interplay of storage and processing in working memory (Cowan, 1998, 1999; Engle et al., 1999). Executive attention enables the maintenance of task-relevant goals, the suppression of less pertinent responses, and the creation of new bindings (Kane & Engle, 2003; McCabe et al., 2010). This attentional component relies on executive control functioning, which refers to a set of fluid processes underlying complex cognitive operations in order to attain a goal in an adaptive and flexible manner (Elliott, 2003). McCabe et al. (2010) suggested that executive attention control is a common component of working memory functioning and executive functioning. At the neural level, working memory functioning depends on a frontoparietal network involving three brain areas playing differential roles: 1) the prefrontal cortex (PFC) involved in executive control processes, such as active maintenance and manipulation of information; 2) the anterior cingulate cortex, which plays an attentional control role and is involved in monitoring operations allowing adaptation to the task demands (Osaka et al., 2003); and 3) the parietal cortex (Chai et al., 2018; Kim et al., 2015), which is involved in storage operations and the allocation of attentional resources (Owen et al., 2005).
It is well known that aging is associated with working memory deficits (Bopp & Verhaeghen, 2005; Braver & West, 2008; Park et al., 2002; Smith et al., 2001; Verhaeghen & Salthouse, 1997). The efficacy of executive control processes also declines with aging (Craik & Bialystok, 2006), which is considered to be one of the main factors explaining cognitive impairments in aging (Braver & Barch, 2002; Braver et al., 2005; West, 1996). According to the executive deficit hypothesis (West, 1996), age-related cognitive decline is the result of impaired functioning of the prefrontal cortex, which supports top-down executive control processes and is particularly affected by age-related neural changes (Lara & Wallis, 2015; Miller & Cohen, 2001; Raz, 2000). In line with the executive hypothesis, several studies have shown that working memory decline is partly explained by age-related deficits in executive control processes (Brébion et al., 1997; Chaytor & Schmitter-Edgecombe, 2004; Guerrero et al., 2021; Oberauer, 2005; Oberauer & Kliegl, 2001; Shimamura & Jurica, 1994; Touron et al., 2010; Zuber et al., 2019).
In fact, working memory operations relying on the executive control processes would be impaired because of the prefrontal damage in aging. In line with this idea, the context processing hypothesis suggested that aging is linked to less efficient context processing (Braver & Barch, 2002; Braver et al., 2001, 2005). Context processing is a control mechanism supported by the lateral prefrontal cortex (PFC), whereby context information serves as a cue for attentional allocation, suppression of irrelevant information, and maintenance of task-relevant information. For instance, in recognition tasks (classically used to assess working memory decline in aging), the lateral PFC is thought to generate an efficient representation of the context and of the task-directed goal (e.g., “if the probe was in the memory set then press the target button; if not press the nontarget button”) (Braver & West, 2008). It also would underlie active maintenance processes allowing these representations to be highly accessible (e.g., maintaining the memory set over the delay period) (Braver & Barch, 2002; Miller & Cohen, 2001). The context processing hypothesis proposes that prefrontal alterations in aging would result in a less efficient capacity to maintain, represent, and update context information relevant to the task goal. Age-related deficits would be greater in situations requiring representations to be maintained and updated over time to respond efficiently (Braver & Barch, 2002; Braver et al., 2001, 2005). Context processing would play an important role in tasks in which representations must be maintained despite interferences, as when the memory load increases.
Several studies suggest that age-related working memory deficits increase as cognitive demands increase: for instance, when the memory load in recognition working memory tasks become higher (Nagel et al., 2009; Rypma et al., 2007; Speer & Soldan, 2015; Störmer et al., 2013; Zarahn et al., 2007). When the memory load increases, supplementary executive control operations (e.g., updating and inhibition) must be engaged to cope with interference of additional items and maintain information over repeated comparisons (Holtzer et al., 2009). Consequently, executive control processes would be involved in operations to cope with the task demands. Given that executive control processes decline with aging, older adults would have more difficulty coping with the increase in task demands. Isingrini et al. (2015) showed that older adults need to engage control processes more than younger adults to maintain effective working memory performance, particularly at low difficulty levels, whereas at high difficulty levels, executive control is only involved in working memory performance for younger adults (Isingrini et al., 2015).
Neuroimaging studies have shown that neural activity related to working memory decreases with age in some brain regions, reflecting neural impairment (Cabeza et al., 2004; Rypma et al., 2001). For instance, in line with the hypothesis that working memory in aging is linked to age-related executive control decline, Rypma and D’Esposito (2000) found decreased activity in the dorsolateral prefrontal cortex of older adults. Rypma et al. (2002) suggested that upregulation of the dorsolateral cortex reflects strategically controlled processes (e.g., binding) enabling the maintenance of a high load in working memory. However, older adults also exhibit higher neural activity than younger adults during working memory tasks (Cabeza et al., 2018; Grady et al., 1994; Park et al., 2003; Reuter-Lorenz et al., 1999; for a review see Reuter-Lorenz & Lustig, 2005), reflecting neural reorganization. Neural reorganization patterns in aging can take the form of activity that is distributed more symmetrically (Hemispheric Asymmetry Reduction in OLDer adults; HAROLD model; Cabeza, 2002) or more anteriorly (Posterior Anterior Shift in Aging: PASA model; Davis et al., 2008) and has been interpreted as reflecting either compensatory mechanisms or inefficient neural functioning (Dennis & Cabeza, 2008; Park et al., 2003).
Thanks to their precise temporal resolution, ERPs have been used to explore the time course of neural activity related to working memory in aging. Some of these studies have focused on the P300 component, considered as an electrophysiological index of working memory updating processes (Donchin & Coles, 1988). It corresponds to a positive deflection that is maximal in the parietal scalp region in younger adults; a peak latency of approximately 300 and 700 ms P300 amplitude would reflect resource allocation for stimulus evaluation (Polich, 1996; Wickens et al., 1983). With aging, the P300 amplitude changes differentially at parietal and frontal sites. At the parietal site, amplitude decreases (Lubitz et al., 2017; McEvoy et al., 2001; Pinal et al., 2015a; Saliasi et al., 2013; Speer & Soldan, 2015; Wild-Wall et al., 2011) or remains stable (Daffner et al., 2010), whereas at the frontal site it increases, leading to a reduction of the predominance of parietal P300 observed in young adults (Fabiani et al., 1998; Fjell & Walhovd, 2001; Friedman et al., 1993, 1997; van Dinteren et al., 2014). This pattern is consistent with the PASA model (Davis et al., 2008), which postulates that the decrease in posterior neural activity observed during aging is coupled with a neural activity increase in anterior areas.
The functional role of this frontal activity increase and the conditions in which it is exhibited remain to be determined. Some studies show that it is linked to lower working memory performance (inefficient neural functioning: Saliasi et al., 2013; Schmitt et al., 2014), whereas others found the opposite pattern (compensation role: Kopp et al., 2014; Lubitz et al., 2017). According to Friedman et al. (1997), the more frontal orientation of the P300 in aging reflects poor frontal functioning. Older adults who exhibit frontal deficits overrecruit this region to counteract higher susceptibility to interference, lower working memory capacity, and greater difficulty maintaining information over time. The predominant frontal distribution of the P300 has been observed in both younger and older adults during an oddball task for new stimuli (Fabiani & Friedman, 1995; Friedman et al., 1993). However, repeated exposure to the stimuli leads to a decrease in frontal P300 amplitude only in younger adults but not for older ones. Repeated experience with the stimuli would enable younger adults to generate and maintain a good representation of the stimuli in working memory, leading to reduced frontal recruitment (Friedman et al., 1997). Moreover, Friedman et al. (1997) suggested that the frontally oriented P300 distribution is similar to that elicited by task-irrelevant stimuli. Thus, in line with the context processing hypothesis (Braver & Barch, 2002; Braver et al. 2001, 2005), the age-related increase in frontal P300 amplitude would reflect the difficulty of older adults to maintain an efficient representation of the task-relevant context and to inhibit interference and irrelevant information (Friedman, 2003; Friedman et al., 1997).
Several studies suggest that the P300 amplitude is modulated not only by aging but also by the task demands (Gevins et al., 1996; Lubnitz et al., 2017; McEvoy et al., 2001; Saliasi et al., 2013; Speer & Soldan, 2015; Wild-Wall et al., 2011). These studies have revealed that the parietal amplitude of the P300 decreases with task difficulty in both older and younger adults. When task difficulty increases, more resources must be engaged to maintain and manipulate working memory, leaving fewer resources available for stimulus evaluation (McEvoy et al., 1998; Morgan et al., 2008). Interestingly, Daffner et al. (2010) found that the frontal amplitude of the P300 was globally higher for older than younger adults during a working memory task. However, frontal P300 amplitude was modulated differentially by difficulty in the two age groups. In fact, frontal P300 amplitude increased significantly with task difficulty only for younger adults. Thus, younger adults recruited additional neural resources at frontal sites to cope with the task difficulty. In line with these results, Pinal et al. (2015b) showed that the predominance of parietal over frontal P300 was lower for older than younger adults, and only under a low load condition. In other words, older adults recruited more frontal resources under the low load condition than younger adults. These results are coherent with the Compensation-Related Utilization of Neural Circuits Hypothesis (CRUNCH; Reuter-Lorenz & Cappell, 2008), which proposes that neural overactivation in aging could reflect differential sensitivity to task difficulty in younger and older adults. Thus, given that older adults exhibit less efficient processing than younger adults, they must engage additional neural activity in tasks with low levels of difficulty to compensate for cognitive decline. However, at higher levels of task difficulty, neural resource limits would be reached only for the older adults, leading to underactivation and to greater performance decline. Younger adults would rely on additional neural activity to respond to the increasing difficulty, enabling them to maintain good performance.
This neural regulation with task demands reflects neural capacity, which is defined as the degree to which a neural network can reach its maximum activation level in order to meet increasing task difficulty (Barulli & Stern, 2013; Stern, 2009; Steffener & Stern, 2012). According to Holtzer et al. (2009), neural capacity deficits in aging trigger working memory impairments. In contrast to younger adults, older adults exhibit overactivation at low levels of difficulty and underactivation linked to cognitive impairment at higher levels (Cappell et al., 2010; Carp et al., 2010; Schneider-Garces et al., 2010). Thus, they are less able to upregulate neural activity to cope with task demands due to diminished neural capacity. According to Speer and Soldan (2015), high neural capacity reflects the ability to boost neural activity and implement supplementary stimulus processing, which would help maintain efficient performance. While the biological mechanisms underlying age-related changes in neural capacity are not yet well known, they could be linked to structural and functional brain changes (e.g., diminished neural connectivity, white matter degeneration; Speer & Soldan, 2015). Susceptibility to age-related neurocognitive impairment varies widely among older adults, some maintaining a high level of cognitive and neural functioning (Christensen et al., 1999; Habib et al., 2007; Steffener & Stern, 2012). It is therefore possible that the neural capacity of some older adults remains stable or declines less. As neural capacity underlies cognitive performance, older adults with greater neural capacity would exhibit less cognitive decline, as they would be able to use neural activity more efficiently to deal with the additional processes required by increasing task difficulty (Barulli & Stern, 2013; CER-TP 2018; Steffener & Stern, 2012).
Speer and Soldan (2015) found that neural capacity related to working memory can vary according to some individual characteristics. They used a Sternberg task (Sternberg, 1966) with three memory set sizes (1, 4, and 7 items) to explore whether a cognitive reserve index (estimated by premorbid intelligence, vocabulary, and education level) was associated with parietal P300 functioning. They computed a neural capacity index corresponding to the mean change in P300 amplitude between set sizes. They found a correlation between the neural capacity index and the cognitive reserve index, such that individuals with a high reserve level exhibited smaller parietal P300 amplitude decrease as task difficulty increased, reflecting a more efficient modulation of neural activity with difficulty. In that study, the P300 was only assessed at the parietal site, and one of its main limitations is that the upregulation of the P300 in aging was not investigated at the frontal site. Moreover, as far as we know, there has been no investigation to date of the involvement of executive control processes on the upregulation of P300 with memory load (reflecting neural capacity).
In the present study, we were interested precisely in the link between working memory related to neural capacity and executive control processes in aging. Executive control is not only involved in working memory functioning but also is linked to brain functioning. Some studies have revealed that older adults with a high executive level exhibit less structural (Kochunov et al., 2009) and functional modifications with aging than those with a low level (Burzynska et al., 2011). Interestingly, Angel et al. (2016) showed that older adults with a high executive control level upregulated neural activity with task difficulty more efficiently than those with a low level, allowing them to maintain good episodic memory performance. Thus, older adults with a high executive control level exhibited greater neural capacity than those with a low level. As far as we know, no study has directly explored the link between executive control processes and neural capacity in working memory. Some evidence suggests that executive control can modulate age-related changes in the P300 component. For example, some studies (Fabiani et al., 1998; West et al., 2010) have shown that neural reorganization, reflected by a frontal-maximal P300 distribution, is observed mainly in older adults with a low executive control level. However, Daffner et al. (2005), consistent with the compensation hypothesis, found that older adults with a high level of performance on a series of neuropsychological tests, including executive tasks, did not exhibit cognitive decline when performing a novelty and target processing task and showed larger frontal P300 amplitude than the younger adults. Thus, the role played by this frontal over-recruitment in aging remains to be clarified.
Aims and hypotheses
The main goal of this study was to explore whether individual variability in executive control can explain neural capacity deficits in aging, which in turn would account for working memory deficits. To this end, ERPs were recorded while young and older participants performed a Sternberg working memory task (Sternberg, 1966) with two memory set sizes (2 and 6 items). This task has been widely used in the aging literature to explore age-related behavioral and neural changes in working memory functioning (Rypma et al., 2002, 2005; Schneider-Garces et al., 2010; Speer & Soldan, 2015; Zarahn et al., 2007). One of the main advantages of this paradigm is that the set size can be manipulated by varying the number of items to encode, making it possible to assess behavioral and neural changes as a function of increasing memory load. Moreover, this task involves both maintaining and processing operations, as participants have to hold a series of letters in memory, compare them to a target and subsequently decide whether the target was included in the memory set. Executive control processes would thus play a key role in performing this task: first by enabling attention to be focused on task relevant information despite interference; second, by updating memory over trials and suppressing irrelevant information, thereby preventing proactive interference and enabling representations to be updated according to changes in context; and finally, by enabling the task goal to be maintained over time and cognitive processing to be adapted to the task demands. In the present study, we assessed executive control functioning by computing an executive control index using an inhibition (Stroop, Stroop, 1935) and an updating (3-back) measure. These two processes are thought to play a key role in the capacity to maintain and update accurate representation of the task goal over time (Braver & Barch, 2002; Braver et al., 2001, 2005). Deficits in context processing in older adults would trigger greater susceptibility to interference as well as difficulty updating and switching representations according to changes in context. Zuber et al. (2019) explored the involvement of three executive control dimensions (inhibition, updating, and shifting) in age-related working memory decline. They found that differences in performance were related to inhibition and updating but not to shifting. At the behavioral level, we expected to confirm the classic effects of aging (Gevins et al., 1996; McEvoy et al., 1998; Schneider-Garces et al., 2010; Speer & Soldan, 2015) and set size (Gevins et al., 1996; McEvoy et al., 1998; Schneider-Garces et al., 2010; Speer & Soldan, 2015) on working memory performance and also the classic age-related decline in executive control (see for a review Collette & Salmon, 2014; Craik & Bialystok, 2006, 2008; Isingrini, 2001; Reuter-Lorenz et al., 2016). At the electrophysiological level, in line with previous studies, we expected that the parietal P300 amplitude would be lower for older than for younger adults and in the 6-item than 2-item memory set (Lubitz et al., 2017; McEvoy et al., 2001; Saliasi et al., 2013; Speer & Soldan, 2015; Wild-Wall et al., 2011). Moreover, consistent with the PASA model (parietal to frontal shift) and with previous studies, we expected to observe a more frontally distributed P300 in older than younger adults (Fabiani et al., 1998; Fjell & Walhovd, 2001; Friedman et al., 1993, 1997; van Dinteren et al., 2014). According to Friedman et al. (Friedman, 2003; Friedman et al., 1997), frontal P300 over-recruitment in aging is linked to frontal damage and reflects age-related deficits in the capacity to maintain an efficient stimuli representation in working memory and inhibit irrelevant information. In line with the main aim of this study and with previous studies (Daffner et al., 2010; Pinal et al., 2015b), we also expected to observe that frontal P300 amplitude would increase with difficulty more in younger than in older adults, reflecting neural capacity decline with aging. Due to their neurocognitive deficits, older adults would have more difficulty engaging additional neural resources to cope with a higher task load.
Additionally, to explore the hypothesis that executive control is involved in age-related changes in working memory and neural capacity, we computed a neural capacity index at both parietal and frontal sites. In line with previous studies (Heinzel et al., 2014; Speer & Soldan, 2015), we measured neural capacity by estimating neural changes with increasing task difficulty. In fact, in the present study we proposed a novel index, which allowed us to compute the proportion of neural change with increasing task difficulty relative to individual differences in the low-load condition. Thus, in the present study, neural capacity corresponded to the ratio between the P300 amplitude change with memory load increase and the P300 amplitude at the low-level load. This index would thus provide a more accurate estimation of neural modulation with task difficulty, independently of differences in the low-load condition. This index was used in the correlational and regression analyses.
In line with the main goal of this study, correlational and regression analyses were performed to explore the role played by executive control in both working memory performance and neural capacity in aging. For these analyses, we computed mean working memory performance across the two memory loads to obtain an overall estimation of working memory functioning. At the behavioral level, given that executive control processes play a key role in working memory functioning, we expected that working memory functioning would be linked to executive control level, but differentially for younger and older adults. In accordance with Isingrini et al. (2015), we expected that older adults would need to engage control processes more than younger adults in order to maintain efficient working memory functioning and to maintain performance with increasing task demands. Moreover, in line with the executive hypothesis (West, 1996) and with the context processing hypothesis (Braver & Barch, 2002; Braver et al., 2001, 2005), suggesting that working memory decline in aging is triggered by less efficient functioning of control processes, we predicted that the control level would mediate the age-related variance in behavioral working memory performance. Previous studies have already explored the role played by the age-related decline in executive control in working memory dysfunction (Guerrero et al., 2021). However, no study has yet explored the link between neural capacity and executive control functioning and its involvement in age-related working memory deficits. According to Cabeza et al. (2005), the link between executive control processes and neural capacity would be accounted by two different effects: psychogenic or neurogenic effects. Psychogenic effects refer to changes in the brain triggered by cognitive change; in other words, executive control deficits in aging would lead to less efficient neural capacity. By contrast, neurogenic effects occur when brain change leads to cognitive change; in other words, neural capacity change in aging would cause executive control deficits. In the present study, we explored which of these effects best explains the working memory deficits in aging. Because executive control level is linked to age-related brain changes (Burzynska et al., 2011; Kochunov et al., 2009) and also to brain activity modulation with difficulty (Angel et al., 2016), we expected that executive control would mediate the age-related changes in neural capacity, in line with the psychogenic effect. This hypothesis is in line with a previous study conducted by Angel et al. (2011), which demonstrated a model in which age-related changes in executive control processes triggered neural changes (degree of lateralization), which in turn mediated episodic memory deficits in aging (Angel et al., 2011). Additionally, we explored the role played by neural capacity in working memory performance. The ability to modulate neural activity efficiently to cope with task difficulty would lead to good working memory performance. However, the expected age-related decline in neural capacity would lead to less efficient working memory functioning.
Methods
Participants
Fifty-one adults, all volunteers and French-speaking, were recruited from the general community to participate in this study. Informed consent was obtained from all individual participants included in the study. Participants were divided into two age groups: 25 young adults (13 women and 12 men) aged 20 to 40 years, and 26 older adults (18 women and 8 men) aged 60 to 80 years. Participants’ characteristics for each age group are summarized in Table 1. Educational level did not differ between groups (t(49) = 0.47, not significant [ns]). However, a marginal age-related difference was observed for the Mill Hill vocabulary test (Deltour, 1993) (t(49) = −1.93, p = 0.06), older adults having higher scores, which is consistent with the aging literature showing that older adults have a higher cultural level than younger ones. The Hospital Anxiety and Depression Scale (HADS, Zigmond & Snaith, 1983) was used to assess levels of anxiety and depression, which could have a significant impact on cognitive functioning; no age-related difference was identified for the global score on this scale (t(49) = −0.73, ns). Additionally, older adults completed the Mini Mental State Examination (MMSE, Folstein et al., 1975); participants who scored below 27 were excluded from this study to reduce the risk of including older adults with cognitive dysfunction or preclinical dementia. All participants stated that they were right-handed and had normal or corrected-to-normal vision. They also reported that they were in good physical and mental health, with no history of neurological or psychiatric illness, and that they were not taking medication affecting the central nervous system. The study was conducted in accordance with the Declaration of Helsinki and it was approved by the local ethics committee of the University of Tours (CER-TP 2018-09-01).
Material and procedure
Participants were tested individually in two sessions in a quiet room. In the first session, they completed the MMSE, the Mill Hill, and the HADS, as well as the Stroop color-word and the N-back tests, which are classically used to assess executive control processes. In the second session, ERPs were recorded while participants completed the probe phase of a Sternberg verbal working memory task (Sternberg, 1966).
Working memory test
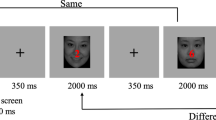
Participants performed a Sternberg verbal working memory task (Sternberg, 1966), comprising two memory set size conditions (2 and 6 items). Stimuli consisted of 11 letters (B, D, F, G, H, J, M, N, Q, R, and T), which were selected because their shapes differ between upper and lower case. As suggested in previous studies (Schneider-Garces et al., 2010; Speer & Soldan, 2015), the letter format (upper vs. lower case) varied between the encoding and the probe phases, so that participants had to rely more on additional cognitive processes as phonological or prelexical processing, rather than on a simple direct visual match. The letters were chosen randomly to create all possible combinations for each set size, and no single letter could be presented twice within the same set. Each trial began by presentation of a fixation cross for 2.5 sec, followed by an encoding phase, in which a memory set was displayed. This consisted of two or six uppercase letters presented simultaneously for 2.5 sec (Fig. 1). Stimuli were arranged in two rows: each one comprising three stimuli. For the trials with two items, empty spaces were replaced by an asterisk to keep the same visual arrangement as for the trials with six items. The encoding phase was followed by a retention interval, in which a blank screen with a fixation point was presented for 5 sec. After that, in the probe phase, a lowercase letter (probe) was displayed for 2 sec. During this interval, participants had to indicate as quickly and accurately as possible whether the probe was included or not in the last memory set, by pressing one of two buttons (“Yes” or “No”). The buttons used for each response were counterbalanced across participants. No feedback was given. All subjects completed ten blocks, including one training block (8 trials, 4 per set size) and nine experimental blocks (136 trials). The first experimental block was shorter than the others and comprised eight trials. This enabled us to check that participants had correctly understood the instructions. As this was the case for all the participants, it was included in the analyses. The other experimental blocks were composed of 8 trials per set size, giving a total of 16 trials per block. For both the training and the experimental blocks, the sets were presented randomly and were counterbalanced across subjects. Within each block, the probe was included in the memory set (match) for 50% of the trials for each set size, whereas for the other 50% it was not (nonmatch). A total of 136 trials were included in the analyses, corresponding to 68 trials for each set size. Figure 1 depicts a schematic sequence of a single trial for the two set sizes.
Performance on the Sternberg task (Sternberg, 1966) was assessed through behavioral accuracy and reaction times (RTs) for each set size. In line with the threshold theories of recognition memory (Pollack & Norman, 1964), behavioral accuracy was estimated by the nonparametric discrimination index A’, which reduces the influence of response bias (Snodgrass & Corwin, 1988). It is computed using the rates of hits (H, proportion of probes correctly recognized as presented in the memory set) and false alarms (FA, proportion of probes incorrectly recognized as presented in the memory set), as follows: A’ = 0.5 + [(H - FA)(1 + H-FA)]/[(4H (1-FA)]. Higher scores reflect greater accuracy. RTs correspond to the time between presentation of the probe and button press for the correct trials. Given that the main goal of the present study was to explore the role played by both executive control processes and neural capacity in age-related working memory deficits, we estimated the individual level of working memory performance across the two memory loads. Thus, we estimated overall performance on the Sternberg task by computing a Mean Accuracy Index (MAI) and a Mean RT Index (MRTI). These indexes were calculated by averaging the A’ indexes and the RTs for the 2- and 6-item sets. MAI and MRTI were used in the correlational and regression analyses as global indicators of working memory performance.
Executive control tests
The Stroop Color–Word Test (SCWT; Stroop, 1935) is extensively used to assess inhibition capacities. We used a blocked paper version, which seems to be suitable to explore the age-related differences in sensitivity to the Stroop interference (Ludwig et al., 2010). In this study, we used two conditions of the standard SCWT: the color condition (C), and the color-word interference condition (CW). Each condition consisted of one card containing 5 columns of 20 stimuli each. In the color condition (C), stimuli were sets of crosses (XXXX) printed in different ink colors (blue, red, or green), and participants were asked to name the color of each set. In the color-word interference condition (CW), stimuli were color names printed in an incongruent ink, and participants had to name the ink color of each word while ignoring the printed color name. For each condition, participants were asked to respond as accurately and quickly as possible within 45 sec. The score for each condition was the total number of correct answers. Answers that did not match the target and the autocorrections were considered as wrong. Previous studies suggest that this Stroop version has adequate internal consistency for both age groups (Ludwig et al., 2010, Cronbach’s Alpha coefficients across conditions: Y = 0.69; O = 0.88) and acceptable test-retest reliability (color condition = 0.82; color-word condition = 0.73) (Golden, 1978). According to Verhaeghen (2011), age-related executive decline could be overestimated in studies that do not consider age-related differences in other general processes, such as attention or processing speed; therefore, they suggested that these baseline differences should be considered. Accordingly, we used a more conservative index to assess Stroop interference (Li & Bosman, 1996), computed as follows: [(Score Color condition – Score Color-word condition/ Score Color condition] (Li & Bosman, 1996). This enabled us to assess interference related change by considering age-related differences in speed processing at baseline; higher scores indicate higher interference and consequently lower inhibition capacity.
The N-back task (Kirchner, 1958) is commonly used to assess updating processes. In the present study, we used an experimental 3-back version developed by our research team.Footnote 1 For this task, the experimenter orally presented a string of 30 letters one-by-one. Starting from the fourth letter, participants had to say (Yes/No) whether or not it matched the one presented three items before. This task consisted of 37% matched and 63% unmatched items. As in previous studies exploring the age-related deficits in up-dating processes (Basak & Veraheghen, 2011; Clarys et al., 2009; Gonçalves & Mansur, 2009; Guerreiro & Van Gerven, 2011; Guerreiro et al., 2013), the score was the number of correct answers.
Data for executive control processes were reduced by calculating a composite index, reflecting the control dimension common to these two tasks. First, we performed a principal component analysis for the executive control scores to verify whether the two tasks loaded on a single factor, even though they measured different dimensions. This was confirmed (eigenvalue > 1), indicating that these two measures had a common executive control factor (−0.82 and 0.82 for the SCWT and the 3-back task respectively). These results suggest that the contribution and the predictive power of these two dimensions in the index are similar. We then computed a composite executive control score for each participant, corresponding to the average of the z-scores of the two measures. A higher Executive Control Index (ECI) indicated a higher level of executive control functioning.
EEG recording
Continuous EEG activity was recorded during the test phase, from 62 electrodes embedded in an elasticated cap, in accordance with the international 10–20 system (Jasper, 1958). Recorded activity was referenced to the left mastoid and re-referenced offline to linked mastoids, and a ground electrode was placed on an anterior site (AFZ). Vertical electrooculogram (EOG) was recorded from two electrodes placed below and above the left eye (VEOG) and horizontal EOG from two electrodes: one at the outer canthus of each eye (HEOG). The EEG and EOG signals were continuously sampled at 250 Hz.
Data analysis
Behavioral data
To determine whether working memory performance varied as a function of age group and set size, we performed a repeated-measures analysis of variance (ANOVA), with age group (young vs. older) as a between-subjects factor, and set size (2 vs. 6 items) as a within-subject factor for both the A’ index and RTs. We performed post-hoc tests using the Newman-Keuls method, with a significance level of p < 0.05.
ERP data
Offline data were segmented with epochs including a 200-ms prestimulus baseline and a 1,000-ms poststimulus interval and filtered with a bandpass of 0.05-40 Hz. Blink artifacts were corrected using Gratton and Colesʼ algorithm (Gratton et al., 1983), and single trials containing muscular or other recording artifacts were rejected manually. ERPs for hits were averaged separately for each age group and for each set size. The mean number of artifact-free epochs in the young group for the 2-item set was 24.40 (SD = 5.00, range 14-30) and for the 6-item set it was 24.64 (SD = 7.06, range 14-38). In the older group, means were 27.54 (SD = 8.50, range 14-46) and 25.38 (SD = 7.45, range 10-37) for the 2- and 6-item sets respectively. The number of trials was the same for both age group [F(1, 49) = 1.30, ns] and for both the 2-item and 6-item sets [F(1, 49) = 0.81, ns]. Moreover, the interaction between age group and set size was not significant [F(1, 49) = 1.28, ns], indicating that no difference was observed in the number of trials in the two age groups for either set size.
For each participant and for each set size, to identify the P300 peak, a semiautomatic peak detection procedure was carried out using Brain Vision analyzer V. 1.0 in a time window of 300- to 800-ms poststimulus. P300 peak was defined as the maximal positive deflection in this window. P300 amplitude was determined at this time point. In line with the goals of this study, ERP amplitudes were quantified at both frontal and parietal sites, with three electrodes at each site: frontal (F1, Fz, F2) and parietal (P1, Pz, P2). In line with the main goal of this study, a Neural Capacity Index (NCI) was calculated at both frontal and parietal sites. It was computed by subtracting the P300 amplitude exhibited for the 6-item set from the amplitude exhibited for the 2-item set and dividing the result by the amplitude for the 2-item set. A positive index corresponded to a decrease in P300 amplitude with task difficulty, and a negative index indicated an increase in P300 amplitude with task difficulty.
For EEG statistical analyses, to explore whether aging and task difficulty modulated the amplitude of the P300 component, we conducted repeated-measures ANOVAs with age group (young vs. older) as a between-subjects factor, and set size (2 vs. 6) and site (Frontal vs. Parietal) as within-subject factors. When necessary, we performed post-hoc tests using the Newman-Keuls method (significance level of p < 0.05), which is a stepwise method enabling all the pairwise comparisons to be performed while considering the multiple comparisons performed successively. It improves the statistical power, while preserving the type I error rate through multiple comparisons (Lee & Lee, 2018)
Finally, for both behavioral and EEG data, we conducted correlational analyses for each age group in order to examine: first, whether executive control was correlated with global working memory performance (MAI and MRTI), and second, whether neural capacity was linked to global working memory performance and to executive control processes. Based on the results of these correlation analyses, we performed stepwise ascendant regression analyses to determine: 1) whether executive control and neural capacity mediated age-related differences in global working memory performance, and 2) whether executive control also mediated the age-related variance in neural capacity.
Results
Behavioral data
Effects of age group and set size on working memory performance
A’ index and RTs for each age group under both set size (2 vs. 6 items) conditions are displayed in Fig. 2A and B, respectively. For accuracy (A’), analyses showed a main effect of age group [F(1, 49) = 4.20, p = 0.04, ɳp2 = 0.08], the accuracy level being higher in young than older adults. A main effect of set size also was observed [F(1, 49) = 34.60, p < 0.001, ɳp2 = 0.41], with greater accuracy for sets with 2 than 6 items. The interaction between age group and set size was not significant [F(1, 49) = 2.00, ns], suggesting that accuracy decreased across set sizes to the same extent for both age groups.
Analysis of RTs showed a main effect of age group [F(1, 49) = 12.65, p < 0.001, ɳp2 = 0.20], young adults exhibiting faster RTs than older adults. The main effect of set size also was significant [F(1, 49) = 397.64, p < 0.001, ɳp2 = 0.89], with shorter RTs for sets with 2 than 6 items. The significant interaction between age group and set size [F(1, 49) = 8.66, p = 0.005, ɳp2 = 0.15] indicated that younger adults had significantly shorter RTs than older adults with both set sizes, but age-related differences were greater for sets with 6 items (p < 0.001) than 2 (p = 0.009). Post-hoc analyses also showed that RTs increased significantly for both groups (p < 0.001), but to a greater extent for older (mean increase = 225.7 ms) than younger adults (mean increase = 167.65 ms). However, given that processing speed declines with aging, RT increase with difficulty could be overestimated for older people. Thus, age-related differences in the low difficulty condition need to be controlled for to have a more reliable estimation of the RT change with difficulty. Thus, we computed an individual RT Change Index (RTCI) corresponding to the difference between RTs for set size 2 and set size 6, divided by RTs for set size 2. Using this formula, higher scores reflect lower RT increase with task difficulty. An independent t-test comparison was conducted to explore whether the RT change index varied as a function of age group. In contrast to the significant interaction between age group and set size, no significant age-related differences were observed for the RTCI, suggesting that RT increase with task difficulty was comparable for the two age groups (Younger: M = −0.19; SD = 0.10; Older: M = −0.22; SD = 0.08; [t(49) = 1.32; ns]). This discrepancy could be explained by the fact that RTCI reflects RT change across set sizes (2 vs. 6) in relation to set size 2.
Hereafter, only MAI and MRTI, reflecting overall task performance, are used in the correlational and regression analyses.
Effect of age on executive control
Executive control results showed lower levels for older than younger adults, reflected by a significant effect of age group for all the executive control measures (Stroop interference index, 3-back, and ECI) (Table 2). It should be pointed out that scores for the color and the color-word condition of the Stroop task were lower for older than young adults. Age-related differences in the color condition mainly reflect a slowing down of processing. It is important to note that the interference Stroop Index used in this study takes this slowing down into account. Only the ECI was used in the following analyses as an indicator of executive control level.
ERP data
Effect of age, site, and set size on P300 amplitude
Mean amplitude per age group, set size, and site are presented in Fig. 3. Surface grand-averaged waveforms reflecting the P300 elicited by hits in the probe phase of the Sternberg task are displayed in Fig. 4A and B. The ANOVAs revealed that neither the main effect of age group [F(1, 49) = 1.27, ns] nor the main effect of set size were significant [F(1, 49) = 3.24, ns]. By contrast, the main effect of site was significant [F(1, 49) = 56.43, p < 0.001, ɳp2 = 0.53]. Results also indicated a significant interaction between age group and site [F(1, 49) = 5.61, p = 0.02, ɳp2 = 0.10] and between site and set size [F(1, 49) = 18.18, p < 0.001, ɳp2 = 0.27]. By contrast, the interaction between age group and set size was not significant [F(1, 49) = 3.63, ns]. Finally, a significant interaction between age group, site, and set size was observed [F(1, 49) = 5.11, p = 0.03, ɳp2 = 0.09]. To examine this interaction in more depth, separate ANOVAs were performed in each age group, with site and set size as within-subject factors. These analyses revealed that the main effect of site was significant for both groups (young: [F(1, 24) = 60.54, p < 0.001, ɳp2 = 0.72]; older: [F(1, 25) = 11.23, p = 0.002, ɳp2 = 0.31]), suggesting that the P300 was greater at the parietal than the frontal site. However, parietal predominance was greater for younger (p < 0.001) than for older adults (p = 0.002). Additionally, for younger adults, the main effect of set size was not significant [F(1, 24) = 0.00, ns], but there was a significant interaction between site and set size [F(1, 24) = 20.48, p < 0.001, ɳp2 = 0.46]. Post-hoc tests revealed that P300 amplitude increased with task difficulty for younger adults at the frontal site (p = 0.003) but decreased at the parietal site (p = 0.004). By contrast, for older adults, results revealed a significant effect of set size [F(1, 25) = 10.37, p = 0.003, ɳp2 = 0.29], indicating that P300 amplitude decreased with task difficulty. The lack of significant interaction between site and set size for this group [F(1, 25) = 2.08, ns] suggests that this decrease was similar at frontal and parietal sites.
A. Voltage maps showing the P300 scalp distribution between 405 and 505 ms, corresponding to the time window in which the P300 peaked per age group and set size. B. Grand-averaged waveforms at frontal and parietal sites showing the P300 component and representing age-related differences for each set size
Given that P300 amplitude varied as a function of site (frontal vs. parietal), the Neural Capacity Index (NCI) was computed independently at frontal and parietal sites (Fig. 5). These neural capacity indexes reflecting changes in P300 amplitude with increasing memory load are used for the following analyses.
Relationship between age, working memory behavioral indexes (MAI, MRTI), executive control index (ECI), and neural capacity indexes (NCI) per age group
Pearson correlations were calculated for the working memory behavioral indexes (MAI, MRTI), the Parietal and Frontal Neural Capacity Indexes (PNCI and FNCI respectively), and the Executive Control Index (ECI) for each age group; results are presented in Table 3. For younger adults, the ECI was correlated negatively with age, confirming that executive control level declines with age. For older adults, correlational analyses indicated that age was negatively correlated with the MAI, the FNCI and the ECI, while it was positively correlated with the MRTI. Thus, increasing age was associated with poorer working memory performance (reflected by lower accuracy scores and higher RTs), with the executive control level decreasing and frontal amplitude increasing more with task difficulty. Furthermore, ECI and FNCI were correlated with MAI and MRTI, revealing that a higher working memory level (high accuracy level and low RTs) was linked to greater executive control and to frontal amplitude increasing less with task difficulty. Finally, the ECI was correlated positively with the FNCI and negatively with the PNCI, indicating that older adults with a high executive level exhibited less frontal amplitude increase and less parietal decrease as task difficulty increased.
Mediating role of executive control (ECI) and frontal neural capacity (FNCI) on age-related variance in working memory performance (MAI, MRTI)
Given that age, ECI, and FNCI were not correlated with the working memory performance of younger adults, regression analyses were carried out only for the older group. Thus, we conducted a series of stepwise ascendant regression analyses, including age, ECI, and FNCI, in order to identify the model that best accounted for the age-related variance in working memory in the older group. In contrast to the FNCI, the PNCI was not correlated with age or working memory performance. Consequently, this variable was not included in the regression analyses. First, we explored whether executive control (ECI) and frontal neural capacity (FNCI) mediated the link between age and working memory performance (MAI, MRTI) (Table 4). Next, we tested the potential mediating role of executive control on the age-related variance in the FNCI (Table 5).
In Models 1, 2, and 3, we explored the independent involvement of age, ECI, and FNCI in working memory performance respectively. When entered alone, age predicted 18% and 34% of the variance of the MAI and the MRTI respectively (Model 1, path A Fig. 6). ECI was a reliable predictor of working memory performance, accounting significantly for 18% and 41% of the MAI and MRTI variance respectively when entered alone (Model 2, Path E in Fig. 6). When entered alone, FNCI accounted significantly for 18% and 26% of the variance related to MAI and MRTI respectively (Model 3, path C in Fig. 6). Model 4 (path A, D, E in Fig. 6) tested the effect of age on working memory performance through executive control. When ECI and age were entered in the equation at the same time, ECI became the only reliable predictor of working memory performance. The fact that the age effect was no longer significant suggests that executive control mediated the age-related variance in working memory performance. Model 5 explored the effect of age on working memory performance through the frontal neural capacity (Paths A, B, C in Fig. 6). When age and FNCI were entered in the model, age no longer accounted reliably for the MAI variance. Therefore, frontal neural capacity mediated the global age-related variance in accuracy. For MRTI, after introducing both age and FNCI in the equation, the age-related variance was significantly reduced by 73% (t (50) = 1.91, p < 0.05), which is consistent with the hypothesis of a partial mediation of FNCI on the age-related variance of overall RT. Model 6 tested concurrently the amount of working memory variance explained by executive control and frontal neural capacity (path E vs. C in Fig. 6). Analyses revealed that when ECI and FNCI were entered in the model, ECI was the only reliable predictor of working memory performance. Thus, FNCI no longer accounted reliably for this variance, suggesting that executive control mediates the variance related to frontal neural capacity in working memory performance. Finally, model 7 tested the effect of age on working memory performance when including both executive control and frontal neural capacity in the equation (paths A, B, C vs. paths A, D, E in Fig. 6). Results revealed that after partialling out ECI, the amount of variance explained by age and FNCI in working memory performance (MAI and MRTI) was reduced to a nonsignificant level. Thus, results suggest that while both ECI and FNCI are reliable mediators of the age-related variance on working memory performance (MAI and MRTI) in older adults, ECI seems to be the best predictor.
Post-hoc power analyses were conducted to examine the power achieved by the final model (Model 7) for both MAI and the MRTI. These analyses indicated that the model showing that executive control processes (ECI) and frontal neural capacity (FNCI) mediated the link between age and working memory performance (MAI, MRTI) achieved an adequate power level for MRTI (effect size = 0.95, α = 0.05, power (1 − β) = 0.98) but not for MAI (effect size = 0.34, α = 0.05, power (1 − β) = 0.62).
We then explored whether the ECI played a potential mediating role in the age-related variance in FNCI for the older adults. In addition to the theoretical argument (Angel et al., 2011), two main statistical findings allowed us to explore this psychogenic effect. First, the variance related to working memory in older adults explained by FNCI was reduced to a nonsignificant level after partialling out ECI. Second, in model 7, including age, neural capacity and executive control processes as predictors, the latter factor was the best predictor of working memory performance. To test this hypothesis, we examined three further models. In model 1, we tested the effect of age on frontal neural capacity (path B in Fig. 6). When entered alone, age accounted for 19% of the FNCI variance. Model 2 examined the link between executive control and frontal neural capacity (path F in Fig. 6). When entered alone, ECI explained 35% of the FNCI variance. In model 3, we explored the effect of age on frontal neural capacity through executive control (paths B, D, F in Fig. 6). Results indicated that when age and ECI were entered in the equation, only ECI emerged as a reliable predictor. Because the age effect was reduced to a nonsignificant level, the hypothesis of a mediating role of ECI on the age-related FNCI variance was validated.Footnote 2 Post-hoc power analyses showed that this model showing the mediating role of executive control on the age-related variance in the FNCI reached a good power level (effect size = 0.55, α = 0.05, power (1 − β) = 0.90).
Discussion
The main goal of this study was to investigate the role played by executive control processes in age-related working memory decline and neural capacity changes. Three main findings emerged. First, results confirm that aging and memory load negatively impact working memory performance, measured through accuracy and RTs. Second, age-related changes in the P300 electrophysiological correlate of working memory varied as a function of the task load and site (frontal vs. parietal). Results also revealed that frontal neural capacity decreased at frontal sites with aging, whereas it remained stable at the parietal site. Third, in line with the main goal of this study, results indicate that both executive control processes and frontal neural capacity reliably predict working memory performance in older adults and are significant mediators of age-related working memory decline. Moreover, we found that executive control also mediates the impact of age on frontal neural capacity in older adults.
Impact of aging and task difficulty on working memory performance
Even if both older and younger adults exhibited a high-level performance in the Sternberg task, in line with previous studies (Gevins et al., 1996; McEvoy et al., 1998; Nagel et al., 2009; Schneider Garces et al., 2010; Speer & Soldan, 2015; Störmer et al., 2013; Rypma et al., 2007; Zarahn et al., 2007), we observed that older adults were slower and less accurate than younger ones. These results suggest that working memory functioning is less efficient with aging especially in tasks requiring coordination of information storage and control processing operations (Babcok & Salthouse, 1990; Dobbs & Rule, 1989; for review see Braver & West, 2008 and Reuter-Lorenz & Sylvester, 2005). In line with the dual process model of recognition (Oberauer, 2005; Oberauer & Kliegl, 2001), deciding whether the probe was presented in the memory set or not is based on information provided by two processes: familiarity and recollection. According to Oberauer (2005), recollection processes, implicated in operations contributing to bind information and context, decline with aging. Therefore, it cannot be used to correct misleading familiarity signals for intrusions. Consequently, working memory in older adults is likely to be overloaded by irrelevant information (Oberauer, 2005), leading to less efficient working memory functioning.
Moreover, accuracy decreased and RTs increased with task difficulty for both younger and older adults, indicating that working memory functioning is less efficient when more information has to be maintained. Thus, supplementary executive control operations must be deployed to manage the multiple representations kept in memory and to regulate potential interference, resulting in reduced working memory performance (Cowan, 2001; Cowan et al., 2006; Sander et al., 2011). The dual process model of recognition (Oberauer, 2005; Oberauer & Kliegl, 2001) offers an alternative explanation of the effect of memory load on working memory functioning. It could be thought that automatic familiarity processes would be more efficient and could provide a higher signal in a low load memory condition. By contrast, when the memory load increases, multiple items must be maintained at the same level, leading to greater interference (Oberauer & Kliegl, 2001). Under these conditions, familiarity processes would be less efficient, and more controlled recollection processes must be implemented to identify the target. However, the measures used in the present study did not allow us to explore the specific contribution of each of these two processes. Our results also indicate that task difficulty overtaxed older more than younger adults (Nagel et al., 2009; Rypma et al., 2007; Speer & Soldan, 2015; Störmer et al., 2013: Zarahn et al., 2007). More precisely, in agreement with Rypma et al. (2007), the oversensitivity to task difficulty in aging was identified only for RTs and not for the accuracy measure. However, it is important to note that no age-related difference was observed when RTs in the low load condition were used as a reference to calculate RT change across set sizes (RTCI in this study). As older adults had slower RTs than the younger ones for the 2-item set, and even though their RTs increased more than those of younger adults with 6 items, the relative RT change in relation to the baseline condition (set size 2) is comparable in the two groups. This indicates the importance of considering the baseline age-related processing speed difference when estimating RT change with difficulty.
Age-related changes in P300 and working memory performance: What about neural capacity?
This study revealed age-related differences in the P300 that were modulated by task difficulty. In line with Daffner et al. (2010), results indicate that parietal P300 amplitude does not decline with aging. Given that at a behavioral level, working memory decreased with aging (reflected by accuracy decrease and RT increase), the same amount of neural activity at parietal sites leads to lower working memory performance by older than younger adults, reflecting an age-related decline in P300 parietal efficiency. By contrast, previous studies found an age-related decline in P300 parietal amplitude (Lubitz et al., 2017; McEvoy et al., 2001; Saliasi et al., 2013; Speer & Soldan, 2015; Wild-Wall et al., 2011). This discrepancy could be explained by the characteristics of the sample. For instance, in the present study and in Daffner et al.’s (2010) study, the older adults had a relatively high educational level, whereas, in contrast to previous studies, we did not include university students in the younger group. It would be interesting to conduct further studies to explore whether age-related changes in P300 could be modulated by these factors. P300 amplitude at the frontal site, in contrast to the parietal site, was greater in older than younger adults (Daffner et al., 2010; Fjell & Walhovd, 2001; Friedman et al., 1997; Reuter-Lorenz et al., 2016). Classically, P300 amplitude is considered to be larger at parietal than frontal sites (Friedman et al., 1997). In line with Pinal et al. (2015b), this parietal predominance of the P300 distribution was observed for both age groups but to a lesser extent in the older adults. This indicates a pattern of brain reorganization with aging that is similar to the PASA model (Davis et al., 2008), suggesting that older adults rely more on frontal resources than younger ones for working memory processing. It is important to note that frontal over-recruitment in aging was concomitant to a lower working memory performance in this group. Thus, recourse to supplementary frontal neural resources did not allow older adults to overcome the age-related working memory decrease.
We also found that P300 amplitude was modulated by task difficulty differentially at parietal and frontal sites and for the two age groups. At the parietal site, P300 decreased with task difficulty for both age groups. It was consistent with the behavioral results suggesting that working memory efficacy decreased with task difficulty at the same extent for both younger and older adults. The P300 parietal amplitude reflects the amount of resources allocated to the stimuli evaluation (Polich, 1996; Wickens et al., 1983). Thus, the P300 amplitude decrease suggests that for both younger and older adults fewer resources are available to process stimuli in tasks with a high level of difficulty, which would lead to a RT increase and an accuracy decrease. In fact, in the high-level condition the resources previously allocated to the stimuli evaluation must been engaged to cope with the increase in task demands (McEvoy et al., 1998; Polich, 2007). By contrast, in line with Daffner et al. (2010), we observed that only the younger adults exhibited an increase in P300 frontal amplitude with task difficulty, reflecting the recruitment of supplementary frontal resources, resulting in decreased parietal predominance. Thus, parietal predominance was lower in older than in younger adults only in the low difficulty condition, whereas in the high difficulty condition, the magnitude of P300 frontal amplitude was similar in younger and older adults. Interestingly, in the present study two main points would suggest that P300 frontal recruitment is linked to a less efficient working memory functioning. First, in the low-level difficulty condition, frontal over-recruitment is observed mainly in older adults, whose have a lesser working memory performance. Second, in the high-level difficulty condition, increased recourse to frontal resources in younger adults was coupled to a decrease of the working memory performance. Friedman (Friedman, 2003; Friedman et al., 1997) suggested that frontal P300 amplitude would be exhibited for novel stimuli that are not well represented and maintained in memory, and also for irrelevant information. Thus, consistent with the context processing hypothesis (Braver & Barch, 2002; Braver et al., 2001, 2005), the results of the present study suggest that, even in a low-load memory condition, older adults have difficulty generating and maintaining an efficient representation of task-relevant information and suppressing irrelevant stimuli. Interestingly, in younger adults the increase in frontal P300 amplitude was only observed in the high-load condition. In this condition, maintaining an efficient representation of task-relevant information is more challenging, because more information must be kept activated, and interfering items must be dealt with. In line with the main purpose and results of this study, we also explored age-related changes in neural capacity. As indicated by the results presented above, we observed an age-related decline in frontal but not parietal neural capacity. Frontal amplitude increased with task difficulty only for younger adults. According to the CRUNCH model (Reuter-Lorenz & Cappell, 2008), only younger adults would recruit supplementary neural resources to react to the increase in task demands. Given their neurocognitive deficits, older adults would use these resources under conditions with a low level of difficulty. Thus, it seems that the parietal to frontal shift reflects a neural mechanism modulated by task difficulty, rather than a neural reorganization pattern observed exclusively in aging. Younger adults would upregulate frontal neural activity as a function of increasing task demands. By contrast, neural functioning would be less efficient in older than in younger adults, reflected by over-recruitment of the frontal areas for low demanding tasks and a difficulty to engage additional resources to cope with task difficulty. It is important to note that in this study, we explored changes in P300 amplitude with task difficulty using two extreme conditions (sets of 2 vs. 6 items). It would be interesting to explore whether the pattern of results observed here would be modified if intermediate difficulty conditions were included. This could help determine whether age-related differences vary gradually with task difficulty. Given that previous studies exploring similar objectives have not reported effect size data, we did not conduct an a priori sample size analysis. We took the sample size used by recent studies exploring similar objectives as a reference (Speer & Soldan, 2015; Pinal et al., 2015a, 2015b). Because this approach has some limitations, we conducted post-hoc power analysis using G*Power to verify whether the sample size was appropriate to detect the significant experimental interaction explored in this study. The results suggest that with N = 51 and alpha set at 0.05 for both behavioral and electrophysiological measures, the analyses achieved at least 98% power.
Role of executive control and neural capacity in working memory functioning in aging
In line with the main purpose of this study, we explored the relationship between executive control, neural capacity and working memory performance. Regarding the association between executive control processes and working memory functioning, as in previous studies exploring episodic memory (Bouazzaoui et al., 2014; Bouazzaoui et al., 2013; Guerrero Sastoque et al., 2020), our results indicate that working memory performance (accuracy and RT indexes) was related to executive control processes for older but not for younger adults. This greater involvement of executive control in memory has been interpreted as a compensation mechanism allowing older adults to cope with age-related memory deficits (Bouazzaoui et al., 2013, 2014; Craik & Rose, 2012; Glisky & Kong, 2008; Gombart et al., 2017). Because working memory capacity decreases with age, older adults would have to rely on executive control processes to maintain effective task performance, even at low levels of difficulty (Isingrini et al., 2015). Interestingly, Isingrini et al. (2015) found that executive control processes also were associated with the working memory performance of younger adults, but only under high difficulty conditions. In line with this result, it is possible that a correlation between executive control and working memory performance would also appear in young adults under conditions with higher difficulty levels than those used here. It is noteworthy to note that in the present study we used a paper version for the two executive control tasks. Recent studies suggest that the format of the task (paper vs. computerized) can modulate not only the age-related decline on the Stroop task but also the cognitive processes underlying the task performance (Ludwig et al., 2010; Penner et al., 2012). Furthers studies are need to explore this issue.
Regarding neural capacity, we showed that the frontal neural capacity was involved in working memory performance exclusively for the older group. For this group, a lower increase in frontal activity with task difficulty, reflecting neural capacity, was linked to greater accuracy and faster RTs. Thus, in line with previous studies (Fabiani & Friedman, 1995; Fabiani et al., 1998; Friedman, 2003; Saliasi et al., 2013), engaging less optimal frontal neural resources when task difficulty increased would reflect less efficient neural functioning, and is observed mainly in individuals with a low performance level (Saliasi et al., 2013; Schmitt et al., 2014; but see Kopp et al., 2014; Lubitz et al., 2017 for different results). In fact, frontal over-recruitment has been associated with less durable memory traces, a weaker working memory capacity, greater susceptibility to workload, and inhibition deficits (Fabiani et al., 1998; Friedman, 2003; Saliasi et al., 2013). Thus, a more efficient neural capacity reflected by a reduction in frontal increase with task difficulty would be linked to better working memory performance in aging. Thus, neural capacity could play a protective role in cognitive aging.
In line with this hypothesis, this study is the first to show that the frontal and parietal neural capacities of older adults are differentially and inversely linked to executive control processes. While frontal neural capacity was correlated positively with executive control processes, parietal neural capacity was correlated negatively. Thus, older individuals with a high executive control level show less frontal increase with task difficulty and more stable parietal amplitude. This result is consistent with previous studies, showing that a high executive control level is associated with P300 predominant mainly at the parietal site, whereas a low executive control level is associated with a more frontal P300 distribution (Fabiani & Friedman, 1995; Fabiani et al., 1998; West et al., 2010; but see Daffner et al., 2005 for different results). Individuals with a high executive control level would have more cognitive and neural resources available, distributed more efficiently between stimulus evaluation and coping with task difficulty, and reflected in a lower P300 parietal decrease with task difficulty and less recourse to less efficient frontal resources. In the present study, executive control processes were not correlated with working memory performance or with neural capacity in the younger group. As suggested above, the task load in the present study may not have been enough to trigger executive control involvement in working memory performance or in its neural correlates. Given the relatively small sample size of the younger group in the present study, an alternative explanation would be that the correlational analyses had a lower statistical power. However, it should be noted that the sample size of the two age groups was similar, but correlations were only observed for the older group. Thus, it seems that the nature of the link between executive control processes and both working memory performance and neural capacity could vary with advancing age.
Finally, the main purpose of this study was to explore the role played by both executive control and neural capacity in the age-related decline of working memory. According to the executive hypothesis (West, 1996) and line with Zuber et al. (2019), our results reveal that executive control mediates the age-related decline in working memory performance. It is well known that executive control deficits in aging lead to less effective inhibition functioning, to higher vulnerability to interference, and to difficulty implementing control related operations involved in retaining information (e.g., chunking), preventing older adults from coping efficiently with the working memory demands of the task (Hasher & Zacks, 1988; Hasher et al., 1999; Lustig et al., 2001). These results are consistent with the context processing hypothesis (Braver & Barch, 2002; Braver et al., 2001, 2005), suggesting that the age-related working memory decline is the result of deficits in the control mechanism underlying efficient context processing. Thus, older adults would have difficulty maintaining and updating representations over time in order to ensure efficient working memory functioning. More specifically, older adults would be more vulnerable to interference and would have more difficulty maintaining task-relevant information. Results also suggest that the decline of executive control in aging slows down working memory processing, and the longer RTs of older adults could reflect the time required to inhibit irrelevant information competing with the correct answer. It should be highlighted that executive control refers to a multidimensional construct involving different cognitive dimensions. According to Verhaeghen (2011), the age-related decline of different executive control dimensions varies widely and deficits are observed mainly in tasks involving dual task coordination and shifting. In the present study, we used a composite index of executive functioning aiming to capture its multidimensional nature. This index was computed by including an inhibition and an updating measure, two important executive processes that are involved in the age-related decline of working memory (Zuber et al., 2019). It would be interesting to conduct further studies to explore the role of these two dimensions in neural capacity, as well as other dimensions that were not evaluated in this study, such as shifting and dual task coordination. Verhaeghen (2011) also suggested that when age-related differences in more basic general processes (e.g., attention, processing speed) are controlled for, executive control decline and its implication in cognitive aging could be reduced or even eliminated. Further studies are needed to better understand the differential contribution of executive control processes and of more basic processes such as attention or processing speed in working memory and neural capacity changes in aging.
Moreover, this study revealed that frontal neural capacity also mediates the age-related decline of working memory. Frontal over-recruitment with task difficulty observed in older adults would reflect recourse to an inefficient neural mechanism to cope with difficulty, which would have a negative impact on working memory functioning. These results are coherent with those of Fabiani et al. (1998), suggesting that frontal over-recruitment reflects a dysfunction in this brain area. In line with this hypothesis, Friedman et al. (1997) proposed that frontal over-recruitment in aging could be the result of: 1) a lower ability to inhibit the frontal novelty activity (P3a component elicited mainly by new stimuli) for stimuli already stored in memory; or 2) a difficulty to integrate in memory the representation of these new stimuli. Finally, our results revealed that executive control mediated the impact of frontal neural capacity on working memory performance in the older adults, and also the age-related variance in frontal neural capacity. These results suggest a neurocognitive model for older adults, in which the individual executive control deficit would lead to a less efficient frontal neural capacity, which in turn would partly explain the working memory decline linked to aging. This model corresponds to a psychogenic interpretation, whereby executive control deficits in aging would trigger neural capacity inefficiency. This model is in line with previous studies (Angel et al., 2011) showing that the executive control processes could cause neural reorganization in aging (HAROLD pattern), which in turn mediates episodic memory deficits in aging. Statistically, the results of the present study are more in keeping with a psychogenic than a neurogenic effect; this interpretation is supported by supplementary analyses exploring the neurogenic effect, which indicated that frontal neural capacity mediated the effect of age on executive control, but only partially. By contrast, in line with the psychogenetic interpretation, results indicated that age-related frontal neural capacity changes are mediated completely by executive control level.
The results of the present study suggest that aging is associated with executive control decline, which would reduce the resources available to cope with cognitive task demands. Thus, as the limits of their neural resources are reached faster, older adults would have to use a less efficient neural network to react to task difficulty, leading to working memory deficits. Consequently, it is conceivable that neural capacity could constitute a neuroprotective mechanism influenced by the executive control level. Thus, older adults with a high level of executive control would have more cognitive and neural resources available, allowing them to cope better with task difficulties (Stern, 2009); they would have more efficient neural functioning with task difficulty, reflected by less frontal recruitment. This suggests that executive control processes are involved in the neural processes underlying the management of task difficulty at a behavioral and a neural level. Executive control processes would enable individuals to recruit more efficient networks for working memory functioning and avoid having to engage less efficient (frontal) ones. An alternative explanation in line with the context processing hypothesis (Braver & Barch, 2002; Braver et al., 2001, 2005) could also be considered. As proposed above, the increase in frontal P300 could reflect deficits in context processing, which would rely on control processes, such as the capacity to inhibit irrelevant information or interferences as well as the ability to update the information maintained in memory over time. Consequently, the age-related executive control decline would lead to less efficient context processing, reflected at the neural level by greater frontal P300 recruitment, especially under high load conditions. These age-related neural changes could thus help to explain the working memory deficits in aging. Given that this model had adequate power for overall reaction times but not for overall task accuracy, the results for the latter measure must be interpreted cautiously. Further studies with a larger sample are needed to verify whether executive control and frontal neural capacity mediate the age-related changes in accuracy. However, given that the Sternberg task is relatively easy and that both younger and older adults achieved high accuracy levels, RT would constitute a better indicator of working memory efficiency in this task. Thus, it would be more pertinent to test the model explored in the present study by using RT as an indicator of working memory functioning.
In sum, the present study confirms that working memory functioning becomes less efficient with aging and that this change is linked to functional neural modifications, assessed here through the P300 component. Interestingly, results suggest that executive control is involved in working memory functioning exclusively for older adults. At the neural level, a neural reorganization pattern consistent with the PASA model is exhibited (parietal to frontal shift) for the P300 component. This pattern would not be exclusively associated with the aging process but would reflect a neural mechanism linked to the task difficulty, as only the younger adults showed an increase in frontal amplitude with task difficulty. This study shows that executive control level and frontal neural capacity are reliable predictors of working memory performance in older adults. Better working memory performance was associated with a higher executive control level and to a lower frontal amplitude increase with task difficulty. Remarkably, results suggest a neurocognitive model in which the age-related decline in executive control processes is responsible for the less efficient frontal neural capacity observed in aging, thereby contributing to working memory decrease (reflected mainly by RT increase with aging). The results of the present study are consistent with the hypothesis that executive control processes could play a protective role in cognitive aging, as they underlie neural mechanisms that modulate age-related cognitive changes.
Notes
We used an alternative split half group method to estimate the reliability of this task. This involved randomly dividing an independent sample into two sub-groups, so that they were matched for age, vocabulary level (Mill-hill score), and educational level. Using this method, a reliable test would not show any differences between two matched groups. No significant differences appeared between the two subgroups for the 3-back score, indicating good reliability (Group 1 (n = 60): M = 21.67; SD = 2.49; Group 2 (n = 59): M = 21.56; SD = 2.20 [t(117) = 0.25, ns]).
Supplementary analyses exploring the neurogenic effect were conducted. These analyses tested whether neural capacity mediated the age-related variance in the executive control index for the older adults. They indicated that both age and frontal neural capacity accounted significantly for the executive control variance (Age: R2C = 0.39, p < 0.001; FNCI: R2C = 0.35, p = 0.001). After introducing both age and FNCI in the equation, the age-related variance was significantly reduced by 59% (t (24) = -2.04, p < 0.05), but was still significant (Age: R2C = 0.16, p = 0.009; FNCI: R2C = 0.12, p = 0.02). Thus, the FNC mediated the age-related variance in the executive control index, but only partially.
References
Angel, L., Bastin, C., Genon, S., Salmon, E., Fay, S., Balteau, E., Maquet, P., Luxen, A., Isingrini, M., & Collette, F. (2016). Neural correlates of successful memory retrieval in aging: Do executive functioning and task difficulty matter? Brain Research, 1631, 53–71. https://doi.org/10.1016/j.brainres.2015.10.009
Angel, L., Fay, S., Bouazzaoui, B., & Isingrini, M. (2011). Two hemispheres for better memory in old age : Role of executive functioning. Journal of Cognitive Neuroscience, 23(12), 3767–3777. https://doi.org/10.1162/jocn_a_00104
Babcock, R. L., & Salthouse, T. A. (1990). Effects of increased processing demands on age differences in working memory. Psychology and Aging, 5(3), 421–428. https://doi.org/10.1037//0882-7974.5.3.421
Baddeley, A. (2003). Working memory: Looking back and looking forward. Nature Reviews Neuroscience, 4(10), 829–839. https://doi.org/10.1038/nrn1201
Baddeley, A. D., & Hitch, G. J. (1974). Working memory. In G. H. Bower (Ed.), The psychology of learning and motivation (Vol. 8, pp. 47–89). Academic Press.
Barulli, D., & Stern, Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17(10), 502–509. https://doi.org/10.1016/j.tics.2013.08.012
Basak, C., & Verhaeghen, P. (2011). Aging and switching the focus of attention in working memory: Age differences in item availability but not in item accessibility. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 66B(5), 519–526. https://doi.org/10.1093/geronb/gbr028
Bopp, K. L., & Verhaeghen, P. (2005). Aging and verbal memory span: A meta-analysis. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 60(5), P223–P233. https://doi.org/10.1093/geronb/60.5.p223
Bouazzaoui, B., Angel, L., Fay, S., Taconnat, L., Charlotte, F., & Isingrini, M. (2014). Does the greater involvement of executive control in memory with age act as a compensatory mechanism? Canadian Journal of Experimental Psychology = Revue Canadienne De Psychologie Experimentale, 68(1), 59–66. https://doi.org/10.1037/cep0000005
Bouazzaoui, B., Fay, S., Taconnat, L., Angel, L., Vanneste, S., & Isingrini, M. (2013). Differential involvement of knowledge representation and executive control in episodic memory performance in young and older adults. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Expérimentale, 67(2), 100–107. https://doi.org/10.1037/a0028517
Braver, T. S., & Barch, D. M. (2002). A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience & Biobehavioral Reviews, 26(7), 809–817. https://doi.org/10.1016/S0149-7634(02)00067-2
Braver, T. S., Barch, D. M., Keys, B. A., Carter, C. S., Cohen, J. D., Kaye, J. A., Janowsky, J. S., Taylor, S. F., Yesavage, J. A., Mumenthaler, M. S., Jagust, W. J., & Reed, B. R. (2001). Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General, 130(4), 746–763. https://doi.org/10.1037/0096-3445.130.4.746
Braver, T. S., Satpute, A. B., Rush, B. K., Racine, C. A., & Barch, D. M. (2005). Context processing and context maintenance in healthy aging and early stage dementia of the Alzheimer’s type. Psychology and Aging, 20(1), 33–46. https://doi.org/10.1037/0882-7974.20.1.33
Braver, T. S., & West, R. (2008). Working memory, executive control, and aging. In The handbook of aging and cognition (3rd ed., pp. 311–372). Psychology Press.
Brébion, G., Smith, M. J., & Ehrlich, M. F. (1997). Working memory and aging: Deficit or strategy differences? Aging, Neuropsychology, and Cognition, 4(1), 58–73. https://doi.org/10.1080/13825589708256636
Burzynska, A. Z., Nagel, I. E., Preuschhof, C., Li, S.-C., Lindenberger, U., Bäckman, L., & Heekeren, H. R. (2011). Microstructure of Frontoparietal connections predicts cortical responsivity and working memory performance. Cerebral Cortex, 21(10), 2261–2271. https://doi.org/10.1093/cercor/bhq293
Cabeza, R., Albert, M., Belleville, S., Craik, F. I. M., Duarte, A., Grady, C. L., Lindenberger, U., Nyberg, L., Park, D. C., Reuter-Lorenz, P. A., Rugg, M. D., Steffener, J., & Rajah, M. N. (2018). Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nature Reviews. Neuroscience, 19(11), 701–710. https://doi.org/10.1038/s41583-018-0068-2
Cabeza, R., Anderson, N. D., Locantore, J. K., & McIntosh, A. R. (2002). Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage, 17(3), 1394–1402. https://doi.org/10.1006/nimg.2002.1280
Cabeza, R., Daselaar, S. M., Dolcos, F., Prince, S. E., Budde, M., & Nyberg, L. (2004). Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex, 14(4), 364–375. https://doi.org/10.1093/cercor/bhg133
Cabeza, R., Nyberg, L., & Park, D. C. (2005). Cognitive neuroscience of aging: Emergence of a new discipline. In cognitive neuroscience of aging: Linking cognitive and cerebral aging (pp. 3–15). Oxford University Press.
Cappell, K. A., Gmeindl, L., & Reuter-Lorenz, P. A. (2010). Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex, 46(4), 462–473. https://doi.org/10.1016/j.cortex.2009.11.009
Carp, J., Gmeindl, L., & Reuter-Lorenz, P. (2010). Age differences in the neural representation of working memory revealed by multi-voxel pattern analysis. Frontiers in Human Neuroscience, 4, 217. https://doi.org/10.3389/fnhum.2010.00217
Chai, W. J., Abd Hamid, A. I., & Abdullah, J. M. (2018). Working memory from the psychological and neurosciences perspectives: A review. Frontiers in psychology (p. 9) https://www.frontiersin.org/article/10.3389/fpsyg.2018.00401
Chaytor, N., & Schmitter-Edgecombe, M. (2004). Working memory and aging: A cross-sectional and longitudinal analysis using a self-ordered pointing task. Journal of the International Neuropsychological Society, 10(4), 489–503. https://doi.org/10.1017/S1355617704104013
Christensen, H., Mackinnon, A. J., Korten, A. E., Jorm, A. F., Henderson, A. S., & Jacomb, P. (1999). Dispersion in cognitive ability as a function of age: A longitudinal study of an elderly community sample. Aging, Neuropsychology, and Cognition, 6(3), 214–228. https://doi.org/10.1076/anec.6.3.214.779
Clarys, D., Bugaiska, A., Tapia, G., Baudouin, A., & and. (2009). Ageing, remembering, and executive function. Memory, 17(2), 158–168. https://doi.org/10.1080/09658210802188301
Collette, F., & Salmon, E. (2014). Les modifications du fonctionnement exécutif dans le vieillissement normal. [executive dysfunction in normal aging.]. Psychologie Française, 59(1), 41–58. https://doi.org/10.1016/j.psfr.2013.03.006
Cowan, N. (1998). Attention and memory: An integrated framework. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780195119107.001.0001
Cowan, N. (1999). An embedded-processes model of working memory. In models of working memory: Mechanisms of active maintenance and executive control (pp. 62–101). Cambridge University Press. https://doi.org/10.1017/CBO9781139174909.006
Cowan, N. (2001). The magical number 4 in short-term memory: A reconsideration of mental storage capacity. The Behavioral and Brain Sciences, 24(1), 87–114, discussion 114-185. https://doi.org/10.1017/s0140525x01003922
Cowan, N., Naveh-Benjamin, M., Kilb, A., & Saults, J. S. (2006). Life-span development of visual working memory: When is feature binding difficult? Developmental Psychology, 42(6), 1089–1102. https://doi.org/10.1037/0012-1649.42.6.1089
Craik, F. I. M., & Bialystok, E. (2006). Cognition through the lifespan: Mechanisms of change. Trends in Cognitive Sciences, 10(3), 131–138. https://doi.org/10.1016/j.tics.2006.01.007
Craik, F. I. M., & Bialystok, E. (2008). Lifespan cognitive development: The roles of representation and control. In the handbook of aging and cognition (3rd ed.pp. 557–601). Psychology Press.
Craik, F. I. M., & Rose, N. S. (2012). Memory encoding and aging: A neurocognitive perspective. Neuroscience and Biobehavioral Reviews, 36(7), 1729–1739. https://doi.org/10.1016/j.neubiorev.2011.11.007
Daffner, K. R., Chong, H., Sun, X., Tarbi, E. C., Riis, J. L., McGinnis, S. M., & Holcomb, P. J. (2010). Mechanisms underlying age- and performance-related differences in working memory. Journal of Cognitive Neuroscience, 23(6), 1298–1314. https://doi.org/10.1162/jocn.2010.21540
Daffner, K. R., Ryan, K. K., Williams, D. M., Budson, A. E., Rentz, D. M., Scinto, L. F. M., & Holcomb, P. J. (2005). Age-related differences in novelty and target processing among cognitively high performing adults. Neurobiology of Aging, 26(9), 1283–1295. https://doi.org/10.1016/j.neurobiolaging.2004.11.007
Davis, S. W., Dennis, N. A., Daselaar, S. M., Fleck, M. S., & Cabeza, R. (2008). Que PASA? The posterior-anterior shift in aging. Cerebral Cortex (New York, N.Y.: 1991), 18(5), 1201–1209. https://doi.org/10.1093/cercor/bhm155
Deltour, J. J. (1993). Échelle de vocabulaire de Mill Hill de J. C. Raven. Adaptation française et normes européennes du Mill Hill et du Standard progressive matrices de Raven (PM 38). Editions L’application des techniques modernes.
Dennis, N. A., & Cabeza, R. (2008). Neuroimaging of healthy cognitive aging. In The handbook of aging and cognition (3rd ed., pp. 1–54). Psychology Press.
Dobbs, A. R., & Rule, B. G. (1989). Adult age differences in working memory. Psychology and Aging, 4(4), 500–503. https://doi.org/10.1037/0882-7974.4.4.500
Donchin, E., & Coles, M. G. (1988). Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences, 11(3), 357–427. https://doi.org/10.1017/S0140525X00058027
Elliott, R. (2003). Executive functions and their disorders. British Medical Bulletin, 65, 49–59.
Engle, R. W., Kane, M. J., & Tuholski, S. W. (1999). Individual differences in working memory capacity and what they tell us about controlled attention, general fluid intelligence, and functions of the prefrontal cortex. In I. A. Miyake (Ed.), Models of working memory: Mechanisms of active maintenance and executive control (pp. 102–134). Cambridge University Press. https://doi.org/10.1017/CBO9781139174909.007
Fabiani, M., & Friedman, D. (1995). Changes in brain activity patterns in aging: The novelty oddball. Psychophysiology, 32(6), 579–594. https://doi.org/10.1111/j.1469-8986.1995.tb01234.x
Fabiani, M., Friedman, D., & Cheng, J. C. (1998). Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology, 35(6), 698–708. https://doi.org/10.1111/1469-8986.3560698
Fjell, A. M., & Walhovd, K. B. (2001). P300 and neuropsychological tests as measures of aging: Scalp topography and cognitive changes. Brain Topography, 14(1), 25–40. https://doi.org/10.1023/a:1012563605837
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198.
Friedman, D. (2003). Cognition and aging: A highly selective overview of event-related potential (ERP) data. Journal of Clinical and Experimental Neuropsychology, 25(5), 702–720. https://doi.org/10.1076/jcen.25.5.702.14578
Friedman, D., Kazmerski, V., & Fabiani, M. (1997). An overview of age-related changes in the scalp distribution of P3b. Electroencephalography and Clinical Neurophysiology, 104(6), 498–513. https://doi.org/10.1016/s0168-5597(97)00036-1
Friedman, D., Simpson, G., & Hamberger, M. (1993). Age-related changes in scalp topography to novel and target stimuli. Psychophysiology, 30(4), 383–396. https://doi.org/10.1111/j.1469-8986.1993.tb02060.x
Gevins, A., Smith, M. E., Le, J., Leong, H., Bennett, J., Martin, N., McEvoy, L., Du, R., & Whitfield, S. (1996). High resolution evoked potential imaging of the cortical dynamics of human working memory. Electroencephalography and Clinical Neurophysiology, 98(4), 327–348. https://doi.org/10.1016/0013-4694(96)00288-x
Glisky, E. L., & Kong, L. L. (2008). Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. Journal of Experimental Psychology. Learning, Memory, and Cognition, 34(4), 809–822. https://doi.org/10.1037/0278-7393.34.4.809
Golden, C. J. (1978). Stroop color and word test: A manual for clinical and experimental uses. Stoelting Company.
Gombart, S., Fay, S., & Isingrini, M. (2017). Connaissances et contrôle exécutif: Deux facteurs cognitifs de protection contre le vieillissement de la mémoire épisodique ? Psychologie Française. https://doi.org/10.1016/j.psfr.2017.03.001
Gonçalves, V. T., & Mansur, L. L. (2009). N-Back auditory test performance in normal individuals. Dementia & Neuropsychologia, 3(2), 114–117. https://doi.org/10.1590/S1980-57642009DN30200008
Grady, C., Maisog, J., Horwitz, B., Ungerleider, L., Mentis, M., Salerno, J., Pietrini, P., Wagner, E., & Haxby, J. (1994). Age-related changes in cortical blood flow activation during visual processing of faces and location. The Journal of Neuroscience, 14(3), 1450–1462. https://doi.org/10.1523/JNEUROSCI.14-03-01450.1994
Gratton, G., Coles, M. G. H., & Donchin, E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. https://doi.org/10.1016/0013-4694(83)90135-9
Guerreiro, M. J. S., Murphy, D. R., & Van Gerven, P. W. M. (2013). Making sense of age-related distractibility: The critical role of sensory modality. Acta Psychologica, 142(2), 184–194. https://doi.org/10.1016/j.actpsy.2012.11.007
Guerreiro, M. J. S., & Van Gerven, P. W. M. (2011). Now you see it, now you don’t : Evidence for age-dependent and age-independent cross-modal distraction. Psychology and Aging, 26(2), 415–426. https://doi.org/10.1037/a0021507
Guerrero, L., Isingrini, M., Angel, L., Fay, S., Taconnat, L., & Bouazzaoui, B. (2021). Effect of self-reported internal memory strategy use on age-related episodic and working memory decline: Contribution of control processes. Canadian Journal of Experimental Psychology/Revue canadienne de psychologie expérimentale, 75(4), 348–361. https://doi.org/10.1037/cep0000240
Guerrero Sastoque, L. F., Bouazzaoui, B., Angel, L., Fay, S., Gombart, S., & Isingrini, M. (2020). Differential protective role of control and representation against age-related memory decline. Canadian Journal of Experimental Psychology = Revue Canadienne De Psychologie Experimentale, 74(1), 44–55. https://doi.org/10.1037/cep0000191
Habib, R., Nyberg, L., & Nilsson, L.-G. (2007). Cognitive and non-cognitive factors contributing to the longitudinal identification of successful older adults in the betula study. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 14(3), 257–273. https://doi.org/10.1080/13825580600582412
Hasher, L., & Zacks, R. T. (1988). Working memory, comprehension, and aging: A review and a new view. In G. H. Bower (Ed.), Psychology of learning and motivation (Vol. 22, pp. 193–225). Academic Press. https://doi.org/10.1016/S0079-7421(08)60041-9
Hasher, L., Zacks, R. T., & May, C. P. (1999). Inhibitory control, circadian arousal, and age. In attention and performance XVII: Cognitive regulation of performance: Interaction of theory and application (pp. 653–675). The MIT Press.
Heinzel, S., Lorenz, R. C., Brockhaus, W.-R., Wüstenberg, T., Kathmann, N., Heinz, A., & Rapp, M. A. (2014). Working memory load-dependent brain response predicts behavioral training gains in older adults. Journal of Neuroscience, 34(4), 1224–1233. https://doi.org/10.1523/JNEUROSCI.2463-13.2014
Holtzer, R., Rakitin, B. C., Steffener, J., Flynn, J., Kumar, A., & Stern, Y. (2009). Age effects on load-dependent brain activations in working memory for novel material. Brain Research, 1249, 148–161. https://doi.org/10.1016/j.brainres.2008.10.009
Isingrini, M. (2001). Fonctions exécutives, mémoire et métamémoire dans le vieillissement normal. In T. Meulemans, F. Collette, & M. Van der Linden (Eds.), Neuropsychologie des fonctions exécutives (pp. 79–108). Solal.
Isingrini, M., Angel, L., Fay, S., Taconnat, L., Lemaire, P., & Bouazzaoui, B. (2015). Age-related differences in the reliance on executive control in working memory: Role of task demand. PLoS One, 10(12), e0145361. https://doi.org/10.1371/journal.pone.0145361
Jasper, H. (1958). The ten-twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology, 10, 371–375.
Kane, M. J., & Engle, R. W. (2003). Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology. General, 132(1), 47–70. https://doi.org/10.1037/0096-3445.132.1.47
Kim, C., Kroger, J. K., Calhoun, V. D., & Clark, V. P. (2015). The role of the Frontopolar cortex in manipulation of integrated information in working memory. Neuroscience Letters, 595, 25–29. https://doi.org/10.1016/j.neulet.2015.03.044
Kirchner, W. K. (1958). Age differences in short-term retention of rapidly changing information. Journal of Experimental Psychology, 55(4), 352–358. https://doi.org/10.1037/h0043688
Kochunov, P., Robin, D. A., Royall, D. R., Coyle, T., Lancaster, J., Kochunov, V., Schlosser, A. E., & Fox, P. T. (2009). Can structural MRI indices of cerebral integrity track cognitive trends in executive control function during normal maturation and adulthood? Human Brain Mapping, 30(8), 2581–2594. https://doi.org/10.1002/hbm.20689
Kopp, B., Lange, F., Howe, J., & Wessel, K. (2014). Age-related changes in neural recruitment for cognitive control. Brain and Cognition, 85, 209–219. https://doi.org/10.1016/j.bandc.2013.12.008
Lara, A. H., & Wallis, J. D. (2015). The Role of Prefrontal Cortex in Working Memory: A Mini Review. Frontiers in Systems Neuroscience, 9, Lara, A. H., & Wallis, J. D. (2015). The Role of Prefrontal Cortex in Working Memory: A Mini Review. Frontiers in systems neuroscience, 9, 173. https://doi.org/10.3389/fnsys.2015.0017310.3389/fnsys.2015.00173
Lee, S., & Lee, D. K. (2018). What is the proper way to apply the multiple comparison test? Korean Journal of Anesthesiology, 71(5), 353–360. https://doi.org/10.4097/kja.d.18.00242
Li, K. Z. H., & Bosman, E. A. (1996). Age differences in stroop-like interference as a function of semantic relatedness. Aging, Neuropsychology, and Cognition, 3(4), 272–284. https://doi.org/10.1080/13825589608256630
Lubitz, A. F., Niedeggen, M., & Feser, M. (2017). Aging and working memory performance: Electrophysiological correlates of high and low performing elderly. Neuropsychologia, 106, 42–51. https://doi.org/10.1016/j.neuropsychologia.2017.09.002
Ludwig, C., Borella, E., Tettamanti, M., & de Ribaupierre, A. (2010). Adult age differences in the color Stroop test: A comparison between an item-by-item and a blocked version. Archives of Gerontology and Geriatrics, 51(2), 135–142. https://doi.org/10.1016/j.archger.2009.09.040
Lustig, C., May, C. P., & Hasher, L. (2001). Working memory span and the role of proactive interference. Journal of Experimental Psychology: General, 130(2), 199–207. https://doi.org/10.1037/0096-3445.130.2.199
McCabe, D. P., Roediger, H. L., McDaniel, M. A., Balota, D. A., & Hambrick, D. Z. (2010). The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology, 24(2), 222–243. https://doi.org/10.1037/a0017619
McEvoy, L. K., Pellouchoud, E., Smith, M. E., & Gevins, A. (2001). Neurophysiological signals of working memory in normal aging. Brain Research. Cognitive Brain Research, 11(3), 363–376. https://doi.org/10.1016/s0926-6410(01)00009-x
McEvoy, L. K., Smith, M. E., & Gevins, A. (1998). Dynamic cortical networks of verbal and spatial working memory: Effects of memory load and task practice. Cerebral Cortex, 8(7), 563–574. https://doi.org/10.1093/cercor/8.7.563
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. https://doi.org/10.1146/annurev.neuro.24.1.167
Morgan, H. M., Klein, C., Boehm, S. G., Shapiro, K. L., & Linden, D. E. J. (2008). Working memory load for faces modulates P300, N170, and N250r. Journal of Cognitive Neuroscience, 20(6), 989–1002. https://doi.org/10.1162/jocn.2008.20072
Nagel, I. E., Preuschhof, C., Li, S.-C., Nyberg, L., Bäckman, L., Lindenberger, U., & Heekeren, H. R. (2009). Performance level modulates adult age differences in brain activation during spatial working memory. Proceedings of the National Academy of Sciences of the United States of America, 106(52), 22552–22557. https://doi.org/10.1073/pnas.0908238106
Oberauer, K. (2005). Binding and inhibition in working memory: Individual and age differences in short-term recognition. Journal of Experimental Psychology: General, 134(3), 368–387. https://doi.org/10.1037/0096-3445.134.3.368
Oberauer, K., & Kliegl, R. (2001). Beyond resources: Formal models of complexity effects and age differences in working memory. European Journal of Cognitive Psychology, 13(1–2), 187–215. https://doi.org/10.1080/09541440042000278
Osaka, M., Osaka, N., Kondo, H., Morishita, M., Fukuyama, H., Aso, T., & Shibasaki, H. (2003). The neural basis of individual differences in working memory capacity: An fMRI study. NeuroImage, 18(3), 789–797. https://doi.org/10.1016/S1053-8119(02)00032-0
Owen, A. M., McMillan, K. M., Laird, A. R., & Bullmore, E. (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. https://doi.org/10.1002/hbm.20131
Park, D. C., Lautenschlager, G., Hedden, T., Davidson, N. S., Smith, A. D., & Smith, P. K. (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17(2), 299–320. https://doi.org/10.1037/0882-7974.17.2.299
Park, D. C., Welsh, R. C., Marshuetz, C., Gutchess, A. H., Mikels, J., Polk, T. A., Noll, D. C., & Taylor, S. F. (2003). Working memory for complex scenes: Age differences in frontal and hippocampal activations. Journal of Cognitive Neuroscience, 15(8), 1122–1134. https://doi.org/10.1162/089892903322598094
Penner, I.-K., Kobel, M., Stöcklin, M., Weber, P., Opwis, K., & Calabrese, P. (2012). The Stroop task : Comparison between the original paradigm and computerized versions in children and adults. The Clinical Neuropsychologist, 26(7), 1142–1153. https://doi.org/10.1080/13854046.2012.713513
Pinal, D., Zurrón, M., & Díaz, F. (2015a). Age-related changes in brain activity are specific for high order cognitive processes during successful encoding of information in working memory. Frontiers in Aging Neuroscience, 7, 75. https://doi.org/10.3389/fnagi.2015.00075
Pinal, D., Zurrón, M., & Díaz, F. (2015b). An event related potentials study of the effects of age, load and maintenance duration on working memory recognition. PLoS One, 10(11), e0143117. https://doi.org/10.1371/journal.pone.0143117
Polich, J. (1996). Meta-analysis of P300 normative aging studies. Psychophysiology, 33(4), 334–353. https://doi.org/10.1111/j.1469-8986.1996.tb01058.x
Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 118(10), 2128–2148. https://doi.org/10.1016/j.clinph.2007.04.019
Pollack, I., & Norman, D. A. (1964). A non-parametric analysis of recognition experiments. Psychonomic Science, 1(5), 125–126. https://doi.org/10.3758/BF03342823
Raz, N. (2000). Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In The handbook of aging and cognition (2nd ed., pp. 1–90). Lawrence Erlbaum Associates Publishers.
Reuter-Lorenz, P. A., & Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177–182. https://doi.org/10.1111/j.1467-8721.2008.00570.x
Reuter-Lorenz, P. A., Festini, S. B., & Jantz, T. K. (2016). Chapter 13—Executive functions and neurocognitive aging. In K. W. Schaie & S. L. Willis (Eds.), Handbook of the psychology of aging (Eighth ed., pp. 245–262). Academic Press. https://doi.org/10.1016/B978-0-12-411469-2.00013-3
Reuter-Lorenz, P. A., & Lustig, C. (2005). Brain aging: Reorganizing discoveries about the aging mind. Current Opinion in Neurobiology, 15(2), 245–251. https://doi.org/10.1016/j.conb.2005.03.016
Reuter-Lorenz, P. A., Stanczak, L., & Miller, A. C. (1999). Neural recruitment and cognitive aging: Two hemispheres are better than one, especially as you age. Psychological Science, 10(6), 494–500. https://doi.org/10.1111/1467-9280.00195
Reuter-Lorenz, P. A., & Sylvester, C.-Y. C. (2005). The cognitive neuroscience of working memory and aging. In cognitive neuroscience of aging: Linking cognitive and cerebral aging (pp. 186–217). Oxford University Press.
Rypma, B., Berger, J. S., & D’Esposito, M. (2002). The influence of working-memory demand and subject performance on prefrontal cortical activity. Journal of Cognitive Neuroscience, 14(5), 721–731. https://doi.org/10.1162/08989290260138627
Rypma, B., Berger, J. S., Genova, H. M., Rebbechi, D., & D’Esposito, M. (2005). Dissociating age-related changes in cognitive strategy and neural efficiency using event- related fMRI. Cortex, 41(4), 582–594. https://doi.org/10.1016/S0010-9452(08)70198-9
Rypma, B., & D’Esposito, M. (2000). Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience, 3(5), 509–515. https://doi.org/10.1038/74889
Rypma, B., Eldreth, D. A., & Rebbechi, D. (2007). Age-related differences in activation-performance relations in delayed-response tasks: A multiple component analysis. Cortex, 43(1), 65–76. https://doi.org/10.1016/S0010-9452(08)70446-5
Rypma, B., Prabhakaran, V., Desmond, J. E., & Gabrieli, J. D. (2001). Age differences in prefrontal cortical activity in working memory. Psychology and Aging, 16(3), 371–384. https://doi.org/10.1037//0882-7974.16.3.371
Saliasi, E., Geerligs, L., Lorist, M. M., & Maurits, N. M. (2013). The relationship between P3 amplitude and working memory performance differs in young and older adults. PLoS One, 8(5), e63701. https://doi.org/10.1371/journal.pone.0063701
Sander, M. C., Werkle-Bergner, M., & Lindenberger, U. (2011). Contralateral delay activity reveals life-span age differences in top-down modulation of working memory contents. Cerebral Cortex, 21(12), 2809–2819. https://doi.org/10.1093/cercor/bhr076
Schmitt, H., Wolff, M. C., Ferdinand, N. K., & Kray, J. (2014). Age differences in the processing of context information. Journal of Psychophysiology, 28(3), 202–214. https://doi.org/10.1027/0269-8803/a000126
Schneider-Garces, N. J., Gordon, B. A., Brumback-Peltz, C. R., Shin, E., Lee, Y., Sutton, B. P., Maclin, E. L., Gratton, G., & Fabiani, M. (2010). Span, CRUNCH, and beyond: Working memory capacity and the aging brain. Journal of Cognitive Neuroscience, 22(4), 655–669. https://doi.org/10.1162/jocn.2009.21230
Shimamura, A. P., & Jurica, P. J. (1994). Memory interference effects and aging: Findings from a test of frontal lobe function. Neuropsychology, 8(3), 408–412. https://doi.org/10.1037/0894-4105.8.3.408
Smith, E. E., Geva, A., Jonides, J., Miller, A., Reuter-Lorenz, P., & Koeppe, R. A. (2001). The neural basis of task-switching in working memory: Effects of performance and aging. Proceedings of the National Academy of Sciences, 98(4), 2095–2100. https://doi.org/10.1073/pnas.98.4.2095
Snodgrass, J. G., & Corwin, J. (1988). Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General, 117(1), 34–50. https://doi.org/10.1037/0096-3445.117.1.34
Speer, M. E., & Soldan, A. (2015). Cognitive reserve modulates ERPs associated with verbal working memory in healthy younger and older adults. Neurobiology of Aging, 36(3), 1424–1434. https://doi.org/10.1016/j.neurobiolaging.2014.12.025
Steffener, J., & Stern, Y. (2012). Exploring the neural basis of cognitive reserve in aging. Biochimica et Biophysica Acta, 1822(3), 467–473. https://doi.org/10.1016/j.bbadis.2011.09.012
Stern, Y. (2003). The concept of cognitive reserve: A catalyst for research. Journal of Clinical and Experimental Neuropsychology, 25(5), 589–593. https://doi.org/10.1076/jcen.25.5.589.14571
Stern, Y. (2009). Cognitive reserve. Neuropsychologia, 47(10), 2015–2028. https://doi.org/10.1016/j.neuropsychologia.2009.03.004
Sternberg, S. (1966). High-speed scanning in human memory. Science (New York, N.Y.), 153(3736), 652–654. https://doi.org/10.1126/science.153.3736.652
Störmer, V. S., Li, S.-C., Heekeren, H. R., & Lindenberger, U. (2013). Normative shifts of cortical mechanisms of encoding contribute to adult age differences in visual-spatial working memory. NeuroImage, 73, 167–175. https://doi.org/10.1016/j.neuroimage.2013.02.004
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643–662. https://doi.org/10.1037/h0054651
Touron, D. R., Oransky, N., Meier, M. E., & Hines, J. C. (2010). Metacognitive monitoring and strategic behaviour in working memory performance. Quarterly Journal of Experimental Psychology (2006), 63(8), 1533–1551. https://doi.org/10.1080/17470210903418937
van Dinteren, R., Arns, M., Jongsma, M. L. A., & Kessels, R. P. C. (2014). Combined frontal and parietal P300 amplitudes indicate compensated cognitive processing across the lifespan. Frontiers in Aging Neuroscience, 6. https://doi.org/10.3389/fnagi.2014.00294
Verhaeghen, P. (2011). Aging and executive control: Reports of a demise greatly exaggerated. Current Directions in Psychological Science, 20(3), 174–180. https://doi.org/10.1177/0963721411408772
Verhaeghen, P., & Salthouse, T. A. (1997). Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological Bulletin, 122(3), 231–249. https://doi.org/10.1037/0033-2909.122.3.231
West, R., Schwarb, H., & Johnson, B. N. (2010). The influence of age and individual differences in executive function on stimulus processing in the oddball task. Cortex, 46(4), 550–563. https://doi.org/10.1016/j.cortex.2009.08.001
West, R. L. (1996). An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin, 120(2), 272–292. https://doi.org/10.1037/0033-2909.120.2.272
Wickens, C., Kramer, A., Vanasse, L., & Donchin, E. (1983). Performance of concurrent tasks: A psychophysiological analysis of the reciprocity of information-processing resources. Science (New York, N.Y.), 221(4615), 1080–1082.
Wild-Wall, N., Falkenstein, M., & Gajewski, P. D. (2011). Age-related differences in working memory performance in a 2-Back task. Frontiers in Psychology, 2. https://doi.org/10.3389/fpsyg.2011.00186
Zarahn, E., Rakitin, B., Abela, D., Flynn, J., & Stern, Y. (2007). Age-related changes in brain activation during a delayed item recognition task. Neurobiology of Aging, 28(5), 784–798. https://doi.org/10.1016/j.neurobiolaging.2006.03.002
Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
Zuber, S., Ihle, A., Loaiza, V. M., Schnitzspahn, K. M., Stahl, C., Phillips, L. H., Kaller, C. P., & Kliegel, M. (2019). Explaining age differences in working memory: The role of updating, inhibition, and shifting. Psychology & Neuroscience, 12(2), 191–208. https://doi.org/10.1037/pne0000151
Acknowledgments
The authors thank Magdalena Leferme for assisting in the collection of the data and Jean Pylouster for his technical assistance for the conception of the material and for data analysis.
Funding
No funding was received for conducting this study or to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data presented in this study are available upon request to the corresponding author.
Code availability
NA
Declarations
Conflict of Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and it was approved by the ethics committee of the University of Tours (CER-TP 2018-09-01).
Consent to participate
All participants provided written informed consent.
Consent for publication
NA
Additional information
Open Practice Statement
Data or materials for the experiments reported here are available upon request to the corresponding authors, as none of the experiments were preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Guerrero, L., Bouazzaoui, B., Isingrini, M. et al. Involvement of executive control in neural capacity related to working memory in aging: an ERP P300 study. Cogn Affect Behav Neurosci 22, 1311–1333 (2022). https://doi.org/10.3758/s13415-022-01018-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-022-01018-8