Abstract
Results of investigations carried out by the oscillation spectroscopy and quantum chemistry methods (B3LYP/6-31++G(d,p)) to study composition, structure, formation energy, and relative stability of hydrogen-bonded molecular complexes occurring in some aqueous and nonaqueous binary solutions are summarized and analyzed. Common complex formation regularities are established for these solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
To study composition, structure, and properties of stable heteroassociates (HAs) containing molecules of two substances and formed by medium-strong hydrogen bonds is of fundamental and practical interest. This allows gaining a better insight into processes of self-organization and features of interaction of molecules, estimating stability of solvated forms of neutral and charged particles, and obtaining data on microstructure and equilibrium compositions of binary liquids systems (BLSs). The structure of the H-bonded molecular complexes to be found in gases and matrices is determined by comparing vibrational spectra obtained experimentally and calculated by the quantum chemistry method. In matrices, together with the most stable isomers, a small number of less stable ones are sometimes found. The gas phase is characterized by a diversity of HAs with similar compositions.
Stoichiometric ratios of molecular complexes formed in binary solutions are determined by various experimental methods, but it is believed that quantum-chemical calculation cannot help establish their structural characteristics, since it is performed for the gas phase. Therefore, little is known about the structure of HAs in the liquid phase, and the data available in the literature are often incorrect. It is only recently that experimental-and-computational studies of the structure, relative stability, and formation condition for H-bonded complexes observed in aqueous and nonaqueous binary solutions have been carried out on the common logic basis [1–15]. The goal of this work is to generalize the results of these studies and establish regularities of complex formation in BLSs.
2 RESULTS AND THEIR DISCUSSION
Data on the structure and properties of HAs in the solutions of HF in organic solvents (Solv) [1–8], sulfuric acid in 2-pyrrolidone (Pyr) [9], and carboxylic acids [10–14] and dimethylacetamide (DMAA) [15] in water were obtained by determining stoichiometric ratios of the HAs and using the method for studying complexes in gases and matrices developed to apply to the liquid phase. The components of the analyzed solutions are nonelectrolytes or weak electrolytes. Exceptions are the H2SO4–Pyr and CF3COOH–H2O systems. In these systems, dissociation of molecules is observed in particular concentration ranges, but in this work we consider solutions with the compositions at which molecules do not dissociate but combine into HAs.
All investigated H-bonded complexes are found by the oscillation spectroscopy method, i.e., their lifetime is no shorter than 10–12 s. Quantum-chemical calculations, which allowed the structure, formation energy, and IR spectra of different HAs to be determined and their relative stability to be estimated, were performed using the density functional method (DFM) and the GAUSSIAN-09 code [16]. The conclusions about the composition and structure of the H-bonded complexes in solutions are drawn, as a rule, from the comparison of the vibrational spectra of the solutions with the calculated spectra of HAs with different topology and agree with the data of the known experiments performed using other methods. The accuracy of the reproduction of the solution spectra in the calculations is characterized by two parameters, the average relative deviation of the calculated frequencies from the measured ones and the mutual arrangement of the bands in the spectrum.Footnote 1
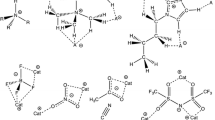
The study of stable molecular complexes in HF–organic solvent systems showed that HAs of three kinds were usually found in them [1–8]. The fact that solutions do not have such a variety of complexes as the gas phase made it possible to determine the structure of those of them for which molar ratios of components are exactly known. This was done for the first time with the HF–acetonitrile BLS [1]. Heteroassociates with the stoichiometric ratios 1 : 1, 4 : 1, and ≥10 : 1 (hereinafter referred to as 1 : 1 HA, 4 : 1 HA, etc.) are formed in it. For each of these complexes, the range of concentrations at which it is observed in the solution and the positions of the HF valence vibration bands (νHF) were determined. Optimal configurations, formation energies (∆Е), and IR spectra of 19 \(m{\text{HF}} \cdot n{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CN}}\) HAs (m = 1–6, n = 1–2) were calculated by the DFM (B3LYP/6-31++G(d,p)), and their relative stability and structure were investigated. It was inferred from the comparison of the calculated and experimental results that the 1 : 1 HA (∆Е = 30.4 kcal/mol) and 4 : 1 HA (∆Е = 46.4 kcal/mol) are stable cyclic complexes \(2{\text{HF}} \cdot 2{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CN}}\) (with alternating molecules) and \(4{\text{HF}} \cdot {\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CN}}\) (Figs. 1a and 1b, respectively).
Similarly, another four HF–Solv BLSs (Solv = (C2H5)2O, (CH3)2NCOH, (CH3)2CO, and (C2H5)2CO) were investigated [2–5]. The first of them is also a cyclic 2HF · 2Solv HA with alternating molecules, and the second is a three-cycle 8HF·2Solv complex formed on the basis of the first by adding two 3HF fragments (see, for example, Figs. 1c–1f). The structure of this complex can be described in another way: it consists of two 4 : 1 HAs linked by two H bonds. The real composition of the second complex probably depends on the proton affinity (PA) of the Solv molecule. Comparison of the PA of the CH3CN molecule (193 kcal/mol) and of the molecules of the other four solvents (202–218 kcal/mol) suggests that the threshold PA value, beginning with which 4:1 HAs are combined into double-composition complexes, is about 195–200 kcal/mol.
In the IR spectra of the solutions containing the 2HF·2Solv and 8HF·2Solv HAs, there are wide intense vibration bands νHF. Integral intensities and positions of peaks of these bands can be found by decomposing the spectrum into the Lorentz functions and Gauss functions, respectively (Fig. 2). The values of two frequencies νHF are much smaller than in the spectrum of liquid hydrogen fluoride (~3390 cm–1), which indicates high strength of hydrogen bonds in the complexes. The strongest interaction is between the HF and Solv molecules in the 8HF · 2Solv HA: it leads to a decrease in the corresponding frequency νHF by ~1550 cm–1. The conclusion about strong hydrogen bonds in mHF·nSolv complexes also follows from the calculations: the formation energies of 1 : 1 HA and 4 : 1 HA are 32.8–39.2 kcal/mol and 86.4–120.0 kcal/mol, respectively.
Infrared spectrum of the HF–(C2H5)2CO solution with 4 : 1 HAs decomposed into the Lorentz functions (a) and Gauss functions (b): the experimental spectrum (1), absorption bands of the HF molecules (2) incorporated in HAs, total absorption of vibrations νCH (3), and the spectrum that is a sum of functions into which decomposition was performed (4).
The analysis of the IR spectra of the HF–Solv BLS showed that each of the observed HAs was formed in solutions with a wide range of molar ratios of components and that complexes of three kinds coexisted in some concentration ranges. This fact served as a basis for the study of equilibrium compositions of HF–Solv solutions. It was carried out using experimental data for three systems containing molecules of acetone, diethyl ketone, and N,N-dimethylformamide (DMFA) [6–8]. In the IR spectra of these molecules, there is a carbonyl group vibration band (νCO), whose frequency and intensity can be measured as the molecule stops being “free”Footnote 2 and becomes part of any HA. Concentration dependences of the fractions of free solvent molecules and those incorporated in the 1 : 1, 4 : 1, 12 : 1 HAs were obtained for each system in two independent ways, from the analysis of the IR spectra (Fig. 3a, symbols) and from the calculations of the material balance (Fig. 3a, lines). The results of all three experiments are in good agreement. In addition, they agree well with the data obtained from the calculation of the material balance up to the solution component ratio 6 : 1. Consequently, it is possible to experimentally determine the equilibrium composition of a solution in this wide range of concentrations.
From the dependence of the fraction of the HF–Solv solution molecules, free and incorporated in various complexes, on the HF mole fraction (Fig. 3b) it is evident that, beginning with the equimolar solution, at least 90% of the molecules are incorporated in HAs. Interestingly, in solutions with the maximum concentration of complexes of one kind ~80% of molecules add to these complexes, ~10% of molecules add to complexes with the “neighbor” ratio, and ~10% molecules add to complexes with the second “neighbor” ratio or remain free. It follows from the above data that equilibrium compositions of solutions in which complexes with identical stoichiometry form are described by a unified set of concentration dependences. Consequently, they will apply to all HF–Solv systems in which the structure of the Solv molecules does not prevent formation of 1 : 1, 4 : 1, and 12 : 1 HAs. These dependences are universal and valid for any binary solutions containing complexes with the stoichiometric ratios mentioned, since the results of the material balance calculations do not depend on the nature of the molecules.
On the basis of the joint analysis of the results [1–8], a number of regularities common for BLS HF–Solv BLSs were established. In these systems, stable molecular complexes \(m{\text{HF}} \cdot n{\text{Solv}}\) with three stoichiometric ratios are usually observed. Each of them is most stable in the series of isomers, has a cyclic or polycyclic structure, contains unstrained H-bonds, and occurs in a solution in a wide range of concentrations. The first to appear (when the acid concentration increases), if the structure of the Solv molecule does not interfere, is the \(2{\text{HF}} \cdot 2{\text{Solv}}\)HA with the alternating HF and Solv molecules. The second, depending on the proton affinity value of the Solv molecule, is either the \(4{\text{HF}} \cdot {\text{Solv}}\) complex or the \(8{\text{HF}} \cdot 2{\text{Solv}}\) complex formed by addition of two chains of three HF molecules to the \(2{\text{HF}} \cdot 2{\text{Solv}}\) HA. The structure of the second complex naturally arises from the structure of the first one. The third HA is most often \(12{\text{HF}} \cdot {\text{Solv}}\). Equilibrium compositions of solutions in which HAs with identical stoichiometric ratios form are described by a unified set of concentration dependences. In solutions with the highest concentrations of complexes of one kind only ~80% of molecules are combined into these complexes. Half the rest of the molecules are in the HAs with the “neighbor” ratio, and the other half are free or in the HAs with the second “neighbor” ratio.
A rare situation is observed in the sulfuric acid–2‑pyrrolidone (Pyr) binary system. Analysis of IR spectra showed that two different complexes with the stoichiometric ratio 1 : 1 coexisted in the equimolar H2SO4–Pyr solution [9]. The concentration of one of them decreased with increasing acid concentration, while the concentration of the other increased. Calculations revealed that it was a quasi-ion pair with incomplete transfer of a proton to the base molecule \({\text{S}}{{{\text{O}}}_{2}}(\text{OH}){\text{O}} \cdots {\text{H}} \cdots {\text{O = C(C}}{{{\text{H}}}_{2}}{{)}_{3}}{\text{NH}}\) and its dimer (Figs. 4a and 4b, respectively). The first of these complexes forms predominantly in solutions with the excess acid; the second, in solutions with the excess base. According to the calculations, all HAs observed in the experiment have a cyclic or polycyclic structure, contain unstrained H-bonds (Fig. 4), and are found and coexist in wide concentration ranges. Two of them, \({{{\text{H}}}_{2}}{\text{S}}{{{\text{O}}}_{4}} \cdot {\text{Pyr}}\) (∆Е = 20.4 kcal/mol) and \({{{\text{H}}}_{2}}{\text{S}}{{{\text{O}}}_{4}} \cdot 2{\text{Pyr}}\) (∆Е = 39.0 kcal/mol), are the most stable in the series of isomers, and the \({\text{2}}{{{\text{H}}}_{2}}{\text{S}}{{{\text{O}}}_{4}} \cdot 2{\text{Pyr}}\) complex (∆Е = 57.2 kcal/mol) results from the interaction of two \({{{\text{H}}}_{2}}{\text{S}}{{{\text{O}}}_{4}} \cdot {\text{Pyr}}\) HAs (it stems from the feature of the given BLS, namely, two OH groups in the Н2SO4 molecule). When the molar ratio of the solution components changes, the structure of the next appearing complex naturally arises from the structure of the previous one. The presented facts allow a conclusion that regularities of complex formation in the H2SO4–Pyr system are the same as in the HF–Solv systems.
(Color online) Structure of the complexes \({{{\text{H}}}_{{\text{2}}}}{\text{S}}{{{\text{O}}}_{4}} \cdot {\text{Pyr}}\) (a), \({\text{2}}{{{\text{H}}}_{{\text{2}}}}{\text{S}}{{{\text{O}}}_{4}} \cdot 2{\text{Pyr}}\) (b), and \({{{\text{H}}}_{{\text{2}}}}{\text{S}}{{{\text{O}}}_{4}} \cdot 2{\text{Pyr}}\) (c).
The study of the hydration features of the molecules and anions of three carboxylic acids, trifluoroacetic, tribromoacetic, and acetic, has shown that the same regularities are valid for aqueous solutions containing not only hydrates of molecules but also hydrates of anions of these acids (they are present in aqueous solutions of the corresponding sodium salts) [10–13]. Let us consider two systems as examples.
In the CF3COOH–H2O BLS, hydrates of thrifluoroacetic molecules with the stoichiometric ratios 1 : 1 and 1 : 2 are found by the oscillation spectroscopy method [10]. According to the quantum-chemical calculations, the most stable among 1 : 1 HAs are two cyclic complexes \(2{\text{C}}{{{\text{F}}}_{{\text{3}}}}{\text{COOH}} \cdot 2{{{\text{H}}}_{2}}{\text{O}}\). In one of them, the acid and water molecules are arranged two in a row (∆Е = 36.8 kcal/mol), and in the other they are arranged alternately (∆Е = 36.7 kcal/mol) [11]. As follows from the analysis of the optical densities of the angle vibrations of the COH molecules of CF3COOH, HAs with pairwise arranged CF3COOH and H2O molecules form in the solutions (Fig. 5a). These hydrates occur in the CF3COOH–H2O system in a wide range of concentrations from 100% CF3COOH to the CF3COOH : H2O ratio of 1 : 2. The most stable 1 : 2 HA (∆Е = 25.2 kcal/mol) also has a cyclic structure (Fig. 5b).
In the investigations of aqueous solutions of acetic acid [13], the results of the quantum-chemical calculations were compared to a limited set of experimental data: Raman light scattering spectra of diluted CH3COOH–H2O and NaCH3CO2–H2O solutions and dependences of the rates of the spin–lattice relaxation of the 2H nuclei in the NaCH3CO2–H2O solutions. Therefore, the data on the structure of the hydrates of the \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{COOH}} \cdot n{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) molecules and the hydrates of the \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CO}}_{2}^{ - } \cdot n{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) anions are not as complete and strictly established as in the above cases. However, they are rather interesting: with the excess of water, there are two stable complexes, \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{COOH}} \cdot 2{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) and \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{COOH}} \cdot 8{{{\text{H}}}_{{\text{2}}}}{\text{O}}\), in the СН3СООН–Н2O solutions (Figs. 6a, 6b), and three HAs, \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CO}}_{2}^{ - } \cdot 2{{{\text{H}}}_{{\text{2}}}}{\text{O}}\), \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CO}}_{2}^{ - } \cdot 6{{{\text{H}}}_{{\text{2}}}}{\text{O}}\), and \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CO}}_{2}^{ - } \cdot 16{{{\text{H}}}_{{\text{2}}}}{\text{O}}\), in the NaCH3CO2–H2O solutions (Figs. 6c–6e). The study of the structure of these cyclic and bulk hydrates revealed another regularity: HAs with the maximal molecular packing density among the complexes with similar n form in the solutions.
(Color online) Structure of the hydrates of \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{COOH}} \cdot 2{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) molecules (а), \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{COOH}} \cdot 8{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) molecules (b), \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CO}}_{2}^{ - } \cdot 2{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) anions (c), \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CO}}_{2}^{ - } \cdot 6{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) anions (d), and \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{CO}}_{2}^{ - } \cdot 16{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) anions (e).
On the basis of the assumption that the complex formation regularities in question are also valid for the HCOOH–H2O BLS, an objective was set in [14] to determine the equilibrium composition of aqueous solutions of formic acid in the entire range of concentrations using only the quantum-chemical calculations. It was found from the study of the structure and properties of 43 cyclic and polycyclic HAs that in the HCOOH–H2O system there are three concentration-structural zones. The first is with the main structural fragment \({\text{2HCOOH}} \cdot 2{{{\text{H}}}_{{\text{2}}}}{\text{O}}\), in which HCOOH and H2O molecules alternate (Fig. 7a), the third is with the main structural fragment \({\text{HCOOH}} \cdot 2{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) (Fig. 7b), and the second zone is with both these complexes. With the excess of acid or water, all hydrates most stable in the series of isomers (except 2 : 1 and 1 : 3 HAs, which do not arise in the solution) are polycyclic solvates of one of the main fragments (see, for example Figs. 7c, 7d). The conclusions drawn from the calculations were supported by the results of a specially conducted experiment.
From the IR spectra of aqueous dimethylacetamide (DMAA) solutions, it follows that hydrates with four stoichiometric ratios 2 : 1, 1 : 1, 1 : 2, and 1 : 4 successively appear in them [15]. The calculations showed that they are polycyclic 2 DMAA · \({{{\text{H}}}_{{\text{2}}}}{\text{O}}\), 4 DMAA · \(4{{{\text{H}}}_{{\text{2}}}}{\text{O}}\), 2 DMAA · \(4{{{\text{H}}}_{{\text{2}}}}{\text{O}}\), and 2D DMAA · \(8{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) HAs, whose structures naturally pass into one another when there arise “excess” DMAA or water molecules (Fig. 8). The average strength of H-bonds in this series of complexes monotonically increases from 3.3 to 6.1 kcal/mol. The structure of the mDMAA · \(n{{{\text{H}}}_{{\text{2}}}}{\text{O}}\) hydrates and the high cooperative interaction ability of water molecules suggest that there are hydrophilic and hydrophobic microregions in the DMAA–H2O solutions with the ratios 1 : 1–1 : 4.
The results obtained in [1–15] give also an idea about relative stability of complexes observed in binary solutions. Table 1 presents average energies of the H-bonds, the parameter that allows comparing strengths of HAs with different structures and different molecules.
It is seen that rather stable molecular complexes with an average H-bond energy of about 7–10 kcal/mol form in all systems considered, except aqueous DMAA solutions. It is worth noting that in all investigated equimolar solutions there are 1 : 1 HAs. (There are no corresponding data for the СBr3СООН–Н2O system because at the component ratio of 1 : 1 it is in the solid phase and the equimolar СН3СООН–Н2O solution was not considered).
3 CONCLUSIONS
All the investigated aqueous and nonaqueous binary solutions in which the structure and properties of HAs were determined, on the one hand, have their own features stemming from the specificity of molecules and, on the other hand, obey the following common regularities:
—In BLSs there are stable (lifetime ≥10–12 s) H‑bonded molecular complexes with several stoichiometric ratios, one of which is 1 : 1 HA.
—These complexes are most stable in the series of isomers and have a cyclic or polycyclic structure, unstrained H-bonds, and the highest packing density of molecules among the HAs with a similar number of molecules in the solvate shell.
—When the concentration of the solution changes, the structure of the next complex appearing in it naturally arises from the structure of the previous one.
—Each HA occurs in the solution in a wide range of molar ratios of component, and in some concentration intervals there coexist 2–3 HAs.
—Equilibrium compositions of solutions in which complexes with identical stoichiometry form are described by a unified set of concentration dependences.
Notes
This parameter was estimated by taking the frequency interval Δ, into which all analyzed vibration bands fall, as 100%, expressing in percent the distances (νj – νi) between all neighbor pairs of bands, and calculating the average deviation of the calculated (νj – νi)/Δ from the experimental ones.
By “free” are meant all Solv and HF molecules that occur in the solution but are not part of an HA, irrespective of what intermolecular interactions they participate in.
REFERENCES
E. G. Tarakanova and G. V. Yukhnevich, “Composition and structure of heteroassociates formed in binary liquid system HF–CH3CN,” J. Struct. Chem. 49 (4), 679–687 (2008). https://doi.org/10.1007/s10947-008-0094-4
E. G. Tarakanova and G. V. Yukhnevich, “Structure of heteroassociates formed in the HF–(C2H5)2O liquid system,” J. Struct. Chem. 51 (1), 69 (2010). https://doi.org/10.1007/s10947-010-0010-6
E. G. Tarakanova and G. V. Yukhnevich, “Composition and structure of heteroassociates formed in the HF–N,N-dimethylformamide binary liquid system,” Russ. Chem. Bull. 60 (1), 81–88 (2011). https://doi.org/10.1007/s11172-011-0011-4
E. G. Tarakanova and G. V. Yukhnevich, “Structure of heteroassociates formed in the HF–(CH3)2CO binary liquid system,” J. Struct. Chem. 53 (3), 495–502 (2012). https://doi.org/10.1134/S0022476612030110
E. G. Tarakanova and G. V. Yukhnevich, “Structure of hydrogen fluoride complexes with diethylketone in a HF–Et2CO solution,” J. Struct. Chem. 57 (4), 684–690 (2016). https://doi.org/10.1134/S0022476616040089
G. V. Yukhnevich, E. G. Tarakanova, and S. I. Kargov, “Relationship between free molecules and molecules involved in various heteroassociates in HF–Me2CO solution,” Russ. Chem. Bull. 61 (6), 1079–1083 (2012). https://doi.org/10.1007/s11172-012-0146-y
G. V. Yukhnevich and E. G. Tarakanova, “Relationship between variously associated molecules in the HF–Et2CO solution,” J. Struct. Chem. 55 (2), 270–276 (2014). https://doi.org/10.1134/S0022476614020115
E. G. Tarakanova and G. V. Yukhnevich, “Equilibrium compositions of HF solutions in N,N-dimethylformamide,” Russ. J. Inorg. Chem. 63 (4), 549–554 (2018). https://doi.org/10.1134/S0036023618040216
V. D. Maiorov, I. S. Kislina, and E. G. Tarakanova, “Structure of complexes in the H2SO4–2-pyrrolidone system as determined by IR-spectroscopy and quantum-chemical calculations,” Russ. J. Phys. Chem. B.11 (1), 37–48 (2017). https://doi.org/10.1134/S1990793117010080
V. D. Maiorov, G. I. Voloshenko, and I. S. Kislina, “Composition and structure of complexes formed in aqueous solutions of trifluoroacetic acid according to IR spectroscopy data,” Russ. J. Phys. Chem. B.12 (2), 185–191 (2018). https://doi.org/10.1134/S1990793118020197
E. G. Tarakanova and G. V. Yukhnevich, “Structure of molecular complexes formed in aqueous solutions of trifluoroacetic acid,” J. Struct. Chem. 55 (8), 1409–1418 (2014). https://doi.org/10.1134/S0022476614080058
E. G. Tarakanova and G. V. Yukhnevich, “Hydration of CBr3COOH molecules and \({\text{CB}}{{{\text{r}}}_{3}}{\text{CO}}_{2}^{ - }\) anions in aqueous solutions,” J. Struct. Chem. 56 (6), 1104–1111 (2015). https://doi.org/10.1134/S0022476615060128
E. G. Tarakanova and G. V. Yukhnevich, “Composition and structure of hydrates of CH3COOH molecules and \({\text{CB}}{{{\text{r}}}_{3}}{\text{CO}}_{2}^{ - }\) anions in aqueous solutions,” J. Struct. Chem. 58 (7), 1357–1367 (2017). https://doi.org/10.1134/S0022476617070125
E. G. Tarakanova, G. I. Voloshenko, I. S. Kislina, V. D. Mayorov, G. V. Yukhnevich, and A. K. Lyashchenko, “Composition and structure of hydrates formed in aqueous solutions of formic acid,” J. Struct. Chem. 60 (2), 255–267 (2019). https://doi.org/10.1134/S0022476619020100
V. D. Maiorov, G. I. Voloshenko, I. S. Kislina, and E. G. Tarakanova, “Composition and structure of hydrates formed in aqueous solutions of dimethylacetamide,” Russ. J. Phys. Chem. B.14 (1) (2020) (in press).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. C. Normand, K. Raghavachari, A. Rendell, Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision A.02 (Gaussian Inc., Wallingford CT, 2009).
Funding
The work concerning the establishment of the regularities of complex formation in binary solutions was done within the State Assignment for the Institute of General and Inorganic Chemistry. The work concerning the recording and analysis of IR spectra was performed within the framework of the Program of Fundamental Research of the Russian Academy of Sciences for 2013–2020, topic no. 46.13 0082-2014-0007. The state registration number of the Center of Information Technologies and Systems for Executive Power Authorities (CITIS) is AAAA-A18-118020890105-3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Potapov
About this article
Cite this article
Tarakanova, E.G., Yukhnevich, G.V., Kislina, I.S. et al. Structure and Regularities of Formation of H-Bonded Complexes in Aqueous and Nonaqueous Binary Solutions. Phys. Wave Phen. 28, 168–175 (2020). https://doi.org/10.3103/S1541308X2002017X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1541308X2002017X