Abstract
The effect of two bacterial strains, B. subtilis 26D and 11VМ, on three cultivars of wheat Triticum aestivum L., Omskaya 35, Kazakhstanskaya 10 (spring wheat), and Volzhskaya Kachestvennaya (winter wheat) was tested. The character of plants’ response to endophytic inoculation depended on the strain of the microorganism, the concentration of cells in the preparation, and the wheat cultivar used in the experiment in Petri dishes. Both the strains showed a strong growth-stimulating effect when seeds were inoculated with bacterial suspensions at a concentration of 106 cells/mL. There was no growth-stimulating effect when seeds were inoculated with bacteria at a concentration of 109 cells/mL. Plants of the Omskaya 35 cultivar were most responsive to inoculation with endophytes. This cultivar was well responsive to the inoculation with bacterial cells at different concentrations. The Volzhskaya Kachestvennaya cultivar had the lowest growth stimulation. Plants of this cultivar responded well when grown in soil, unlike experiments in Petri dishes. The Kazakhstanskaya 10 cultivar was less responsive when growing plants in Petri dishes. There was no difference between the size of seedlings of inoculated and noninoculated plants of the Kazakhstanskaya 10 cultivar, and only stimulation of root growth was observed. It was concluded that there is pronounced responsiveness of wheat cultivars to the effect of endophytic strains of B. subtilis 26D bacteria, the basis of biofungicide (Fitosporin-M), and this should be considered when using this biofungicide for wheat cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Endophytic B. subtilis strains are isolated from various plant species and can be used as PGPB (plant growth promoting bacteria) to stimulate growth, protect against pathogens, increase resistance of the macroorganism to adverse environmental factors, and for land reclamation [1–3]. Endophytic bacteria B. subtilis 26D, the basis of the Phytosporin-M biofungicide, are actively used by the BashIncom company as a part of various preparations for plant growing. Some properties of this strain, as well as those of B. subtilis 11VМ deposited in the collection of the All-Russian Research Institute of Agricultural Microbiology (the ability to synthesize hormones, organic acids, antibiotics, etc.), were investigated. It was proven that these bacteria have growth-stimulating activity and are able to penetrate the roots and shoots of seedlings of various plants in a relatively short time (in the tissues of corn and pea seedlings, endophytic bacterial populations were found on the third day after inoculation, while that in pumpkin and beans was found on the fifth day) [4].
Bacteria B. subtilis strains 26D and 11VМ belong to the same species, but phytohormone-like activity of the strains is different [4], and the response of crop cultivars to inoculation with their endophytes may vary. There is very little information on this subject in the literature. It is known, for example, that, out of seven studied bacterial strains of the Bacillus genus, S50 isolate stimulated stem growth in Triticum durum Desf. (cultivar Marzak (V1)), while isolate S48 promoted both lengthening of the root and increase in wet and dry mass of the plant. The greatest stimulation of the growth in Karim (V2) wheat plants was revealed upon inoculation with S35 isolate [5].
It is known that the nature of the response of plants under the action of any phytohormone as a signal molecule is determined by its concentration. In this case, the dose-response curve of the plant to the use of exogenous auxin may take the form of a two-vertex curve [6]. If the concentration of the hormone is not optimal, it enhances plant growth at lower concentrations [7, 8] and reduces it at high concentrations [8, 9]. Also, the same bacterial strain can stimulate and inhibit the growth of plants of certain species depending on the level of the hormone it synthesizes [7]. Therefore, the physiological response of plants may vary depending on the concentration of bacterial cells in the inoculum. The right choice of the PGPR strain and the most “appropriate” host plants can improve the quality and productivity of various crops without the use of mineral fertilizers [5].
In this regard, we investigated the responsiveness of various wheat cultivars to seed treatment with cells of B. subtilis 26D and 11VМ strains depending on the inoculum concentration, 105–109 cells in 1 mL of the preparation.
MATERIALS AND METHODS
The study was carried out using spring soft wheat (Triticum aestivum L.) of cultivars Omskaya 35 and Kazakhstanskaya 10 and winter wheat of the Volzhskaya Kachestvennaya cultivar. The Omskaya 35 cultivar is medium late, resistant to lodging (4.7–5.0 points), medium dry, and its growing season is 87–90 days. It is moderately susceptible to wheat leaf rust, susceptible to loose smut and highly susceptible to common bunt, stem rust, powdery mildew, and root rot. The Kazakhstanskaya 10 cultivar is early ripening, highly productive, resistant to lodging, shedding, and germination on root, and its growing season is 66–90 days. It is strongly affected by loose smut and moderately by leafy diseases. The Volzhskaya Kachestvennaya cultivar is midripening, drought tolerant, susceptible to wheat leaf rust, highly susceptible to snow mold and root rot, and its growing season is 304–348 days [10].
In the experiments, we employed two B. subtilis Cohn. Strains: 26D (collection of the All-Russia Research Institute of Agricultural Microbiology, VNIISKhM no. 128) and 11VM (VNIISKhM, no. 519) [11, 12]. The first strain was isolated from superficially sterilized tissues of cotton plants and the second strain was isolated from soft spring wheat [11]. In the experiments, calibrated seeds with a germination rate of at least 90% were used. Before germination, the seeds were washed in running tap water and then in freshly prepared distilled water [13]. The seeds were treated with bacteria in a laminar box. In the experiments, we used a 20-h bacterial culture grown in a shaker in meat-peptone broth at 37°C in 250-mL flasks. The cell suspension was adjusted to the required concentration with a solution of 0.01 M KCl by the optical density of the culture. Seeds were treated by shaking for 30 s in a round bottom flask at a ratio of 20 μL of the bacterial suspension/g of seeds. After treatment, the seeds were dried in an open flask for 1 h. The seeds of the control plants were treated with distilled water according to the same procedure. Before sowing, Petri dishes of various diameters along with filter paper were sterilized in an oven (GP-49-3 ZUPP, Vityaz, Belarus) for 45 min at a temperature of 120°C. Each Petri dish then received 30 seeds and freshly prepared distilled water in such a volume that the paper remained moist for 5 days during germination. Plants were grown in the dark at a 18–20°C.
When growing plants in the soil (leached chernozem), part of the soil was put into plastic vessels followed by seeds, which were covered with a 1-cm layer of the remaining soil. The soil was watered in such a way as to achieve 70% of the total field water capacity (TFWC). Plants were grown under illumination with fluorescent lamps (12 kL) and a 16-hour photoperiod for 30 days.
All experiments were carried out in three biological repetitions. Statistical processing of the results was carried out using standard programs of the Microsoft Excel package. In the tables, the data are presented as the arithmetic mean of the repetitions and the standard deviation. To identify statistically significant differences between plants inoculated and noninoculated with bacteria, Student’s t-test was used. Differences between the control and experimental variants were evaluated as statistically significant at a significance level of p < 0.05.
RESULTS AND DISCUSSION
Both the strains showed the strongest growth-stimulating effect when the seeds were inoculated with the bacterial suspension at a concentration of 106 cells/mL (Table 1). The treatment of seeds with endophytic cells at a concentration of 109 cells/mL was not effective for stimulating the growth of the Kazakhstanskaya 10 and Volzhskaya Kachestvennaya wheat cultivars.
Our results are consistent with literature data on the optimal concentrations of endophyte cells in the range of 105–108 cells/mL for inoculation of plants such as elephant grass (Pennisetum purpureum Schumach) and Pennisetum hybrids using bacteria of the species Sphingomonas, Pantoea, Bacillus, and Enterobacter (Li et al., 2016).
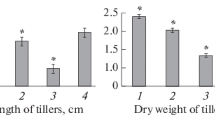
Most responsive to endophyte inoculation were plants of the Omskaya 35 cultivar. Thus, when treating seeds with bacteria at a concentration of 106 cells/mL, the shoot length increased by 28.6% when treated with cells of strain 26D and 40% when treated with cells of strain 11VМ as compared to control plants. Growth stimulation of the above-ground part was higher than that of the roots. However, the ratio of root length to shoot length was more than one (1.0) (Table 2). The cultivar was responsive to inoculation over a wide range of cell concentrations in the bacterial suspension.
Sprouts of the Kazakhstanskaya 10 wheat cultivar responded to inoculation of seeds in a narrower concentration range: 105–108 cells/mL of strain 26D and 105–106 cells/mL of strain 11VM. The root growth stimulation in the plants of this cultivar when treating seeds at a concentration of 106 cells/mL was higher than that of the Omskaya 35 cultivar. When the concentration of cells of strain 11VМ was more than 108 cells/mL, the growth of wheat seedlings of the Kazakhstanskaya 10 cultivar was inhibited. In Petri dishes, the least responsive to inoculation of seeds with bacteria were winter wheat plants of the Volzhskaya Kachestvennaya cultivar; both the strains inhibited growth in seedlings when using preparations at a concentration of 109 cells/mL. When seeds were inoculated with cells of strain 11 VM, plant growth was inhibited several times stronger than when treated with cells of strain 26D.
It is known that damage or underdevelopment of a plant organ is compensated by increased growth of a similar organ or the emergence of new ones [14]. It is logical to assume that the root growth prevailing over the shoot growth in the seedling allows the plant to provide its water needs as the main necessary component for the further building of the organism; therefore, the ratio of the length or mass of the root/shoot towards the root prevalence can serve as one of the indicators of the plant’s adaptation potential under the action of adverse factors.
The analysis of the root and shoot length ratio in wheat seedlings (Table 2) revealed that the root length to the shoot length ratio in all the cultivars increased in comparison with control plants when seeds were treated with cells of bacteria of strain 11VМ at a concentration of 109 cells/mL and was greater than that in the strain 26D. This is consistent with the inhibition of the growth of wheat seedlings of the Kazakhstanskaya 10 and Volzhskaya Kachestvennaya cultivars when using a high concentration of bacterial cells (Table 1). Thus, these data confirm the opinion of the authors of [14] and may indicate a more “tough” effect of cells of the 11VМ strain, and also serve as a sign of activating the coordination reaction of physiological processes upon adverse environmental conditions, in this case, an increase in the density of bacterial cells in the inoculum.
When growing plants in soil under conditions of optimal load of bacterial cells on seeds (106 cells/mL), regular stimulation of seedling growth was observed (Table 3), similar to that obtained in experiments in Petri dishes. When seeds of the Omskaya 35 wheat cultivar were treated with bacterial strains 26D and 11VМ, the length of shoots increased by 10–12% and that of roots by 41 and 18%, respectively, in comparison with control plants. In plants of the Volzhskaya Kachestvennaya cultivar, bacteria of strains 26D and 11VM stimulated shoot growth by 10% and root growth by 24.9 and 18%, respectively. The length of shoots of the Kazakhstanskaya 10 wheat cultivar did not significantly differ when treating seeds with bacteria from that indicator in control plants. Seed inoculation with cells of strain 26D stimulated root growth by 18% and with cells of strain 11VM by only 2%. Stimulation of root growth by inoculation with bacteria in all the cultivars was higher than that of shoots (Table 4).
In contrast to experiments with 5-day-old seedlings in Petri dishes, the Volzhskaya Kachestvennaya winter wheat cultivar, as well as the Omskaya 35 spring cultivar, responded to seed treatment by stimulating root growth, while there was almost no difference between the inoculated and control seedlings of the Kazakhstanskaya 10 cultivar in terms of the size of shoots and only the stimulation of root growth was observed. This effect may be associated with the known precocity of this cultivar. Over 30 days of growth, plants could approach developing maximum genetic organ sizes at a certain stage of ontogenesis, while late-ripening cultivars were still realizing their growth potential.
Thus, the studied wheat cultivars differ in the nature of their response to seed treatment with cells of the studied endophytic bacterial strains. The range of growth-stimulating concentrations of endophyte cells is narrower for plants of the early ripening cultivar Kazahstanskaya 10 in comparison with the midripening cultivar Omskaya 35. For an accurate assessment of the effective concentration of bacterial cells, it is better to use the experimental setup in a model close to the field one, growing plants in soil. In this case, the growth stimulation can be affected not only by the production of a phytohormone (growth regulator) by the bacterium or its induction of the synthesis of phytohormones by the plant [11] but also by the ability of the microorganism to mobilize nutrients in the soil.
REFERENCES
Liu, D.F., Zhao, L., Gao, Y.Z., Ren, H.X., and Liu, X.L., Conversion of furfural residue to biofertilizer using Bacillus subtilis l7 by solid-state fermentation method, J. Chem. Pharm. Res., 2015, vol. 7, no. 3, pp. 2491–2496.
Mingshuang Xu, Jiping Sheng, Lin Chen, Yejun Men, Lin Gan, Shuntang Guo, and Lin Shen, Bacterial community compositions of tomato (Lycopersicum esculentum Mill.) seeds and plant growth promoting activity of ACC deaminase producing Bacillus subtilis (HYT-12-1) on tomato seedlings, World J. Microbiol. Biotechnol., 2014, vol. 30, no. 3, pp. 835–845.
Yu, H., Xing, Y., Lei, F., Liu, Z., Liu, Z., and Jiang, J., Improvement of the enzymatic hydrolysis of furfural residues by pretreatment with combined green liquor and ethanol organosolv, Bioresour. Technol., 2014, vol. 167, pp. 46–52.
Egorshina, A.A., Khairullin, R.M., Luk’yantsev, M.A., Kuramshina, Z.M., and Smirnova, Yu.V., Phosphate-mobilizing activity of endophytic strains of Bacillus subtilis and their influence on the degree of mycorrhization of wheat roots, Zh. Sib. Fed. Univ., Ser. Biol., 2011, no. 2, pp. 172–182.
Chrouqi, L., Ouahmane, L., Jadrane, I., Koussa, T., and Najib Alfeddy, M., Effects of plant growth promoting rhizobacteria (PGPRs) product IAA on the growth of two Moroccan wheat varieties (Triticum durum Desf.), Res. J. Pharm. Biol. Chem. Sci., 2017, vol. 8, no. 3, pp. 2296–2302.
Taiz, L. and Zeiger, E., Plant Physiology, Sinauer Associates Inc. Sunderland, 2010.
Barazani, O. and Friedman, J., Is IAA the major root growth factor secreted from plant-growthmediating bacteria, J. Chem. Ecol., 1999, vol. 25, pp. 2397–2406.
Pattern, C.L. and Glick, B.R., Role of Pseudomonas putida indole acetic acid in development of the host plant root system, Appl. Environ. Microbiol., 2002, vol. 68, pp. 3795–801.
Xie, H., Pasternak, J.J., and Glick, B.R., Isolation and characterization of mutants of the plant growth promoting rhizobacterium Pseudomonas putida GR12-2 that overproduce indoleacetic-acid, Curr. Microbiol., 1996, vol. 32, pp. 67–71.
Kharakteristika sortov sel’skokhozyaystvennykh kul’tur, vklyuchennykh v Gosreestr po Respublike Bashkortostan (Posobiye dlya agronomov) (Characterization of Crop Cultivars Included in the State Register for the Republic of Bashkortostan (Manual for Agronomists)), Ufa: Bashk. Nauchn.-Issled. Inst. Zemledel. Sel. Polevykh Kul’t., Akad. Nauk Resp. Bashkortostan, 1997.
Egorshina, A.A., Khairullin, R.M., Sakhabutdinova, A.R., and Luk’yantsev, M.A., Involvement of phytohormones in the formation of relations of wheat seedlings with the endophytic strain Bacillus subtilis 11BM, Fiziol. Rast., 2012, vol. 59, no. 1, pp. 148–155.
Khairullin, R.M., Minina, T.S., Irgalina, R.Sh., Zagrebin, I.A., and Urazbakhtina, N.A., The effectiveness of new endophytic strains of Bacillus subtilis in increasing the resistance of wheat to disease, Vestn. Orenb. Gos. Univ., 2009, no. 2, pp. 133–137.
Bezrukova, M.V., Fatkhutdinova, R.A., Lubyanova, A.R., Murzabaev, A.R., Fedyaev, V.V., and Shakirova, F.M., The participation of lectin in the formation of wheat resistance to the toxic effect of cadmium, Fiziol. Rast., 2011, vol. 58, no. 6, pp. 907–914.
Shaposhnikov, F.I., Morgunov, A.I., Akin, B., Makarova, N.M., Belimov, A.A., and Tikhonovich, I.A., Comparative characteristics of root systems and root exudation in synthetic, primitive and modern wheat cultivars, S-kh.Biol., 2016, vol. 51, no. 1, pp. 68–78.
Funding
The studies were carried out with partial funding for research within the state assignment no. AAAA-A16-116020350027-7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by K. Lazarev
About this article
Cite this article
Kuramshina, Z.M., Khairullin, R.M. & Smirnova, Y.V. Responsiveness of Triticum aestivum L. Cultivars to Inoculation with Cells of Endophytic Bacillus subtilis Strains. Russ. Agricult. Sci. 46, 1–5 (2020). https://doi.org/10.3103/S1068367420010073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068367420010073