Abstract
The thermal transformation of coal and lignite in the presence of Cu(NO3)2 activator (5 wt %) is investigated as a function of the atmospheric composition. The copper nitrate is supported on to the samples by the incipient wetness with Cu(NO3)2 solution in water and alcohol. The effect of the activator is studied as a function of the gas (air/argon) composition, by thermogravimetric analysis with heating at 10°C/min in the range 25–800°C, at atmospheric pressure. With change in gas composition, the activity of Cu(NO3)2 changes. Specifically, the thermal transformation is shifted to lower temperature: Δt = 33°C for lignite and 70°C for coal. With increase in oxidant (air) content in the gas, the activity of the added Cu(NO3)2 increases: that is, Δt increases. By mass spectrometry, the gaseous oxidation products of the coal are analyzed. The upper temperature limits on the peaks of nitrogen-oxide (NOx) liberation are determined as a function of the gas composition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The catalytic combustion of coal is a promising means of converting solid fuel to thermal energy. The degree of coal conversion is high thanks to activation of combustion at relatively low temperature. That also minimizes the pollutant yield in the exhaust gases [1–4]. Most research concentrates on the use of metal oxides to accelerate the oxidation of the organic fuel [5–9].
The precursors of metal oxides (metal salts) have greater action on the thermal conversion of coal, as established in [10–12]. Specifically, the conversion temperature is lowered, and the liberation of volatiles is accelerated. We assume that the intensification of coal oxidation in the presence of precursors is associated with decomposition of the salt in the first stage of sample heating (to 200°C) and the formation of metal oxide (CuOx), which catalyzes the complete oxidation of organic substrates [13].
The catalytic activity of metal oxides may be regulated by adjusting the gas composition (the O2/N2 ratio), according to [14]. The research showed that increase in the O2/N2 ratio in the gas flux (increase in its oxidative character) intensifies the activity of the catalytic oxide additives, which lower the temperature at which intense oxidation begins and hasten the thermal decomposition of coal. For coal samples of little metamorphic development (lignite), the temperature at which volatiles are released under the action of CuSO4 additive changes more significantly in an inert atmosphere, as shown in [11].
In the present work, we study experimentally the influence of added Cu(NO3)2 activator on the thermal conversion of lignite and coal, with variation in gas composition.
EXPERIMENTAL MATERIALS AND METHOD
The initial samples are as follows: 2B lignite (Borodinsk field) and T coal (Alardinsk field). Initial coal samples of size 5–10 mm are crushed in drum mills for 8 h with a 1 : 1 mass ratio of the crushing balls and coal. The samples are then sorted on screens with 80-μm cells. Table 1 presents the size distribution of the coal particles, according to an Analysette 22 laser-diffraction instrument (Fritsch, Germany).
Although the samples are prepared by the same method, the mean particle diameter varies from 18.3 μm (2B lignite) to 26.3 μm (T coal). This may be explained by the different structure and morphology of the coal and lignite [15]. Table 1 also presents the technical analysis and elemental analysis of the samples; standard methods are employed [16]. The content of the basic elements (C, H, N, S, and O) in the samples is determined by means of a Euro EA 3000 analyzer (EuroVector, Italy).
The initial lignite and coal samples are very different in their physicochemical properties (Table 1). For example, the lignite has high content of volatiles (~40%) and a relatively low ash content (no more than 5 wt %). That is associated with the limited metamorphic development. The T coal, by contrast, has low content of volatiles (~13%) and high ash content (16.0 wt %) and carbon content (~80%).
The Cu(NO3)2 ⋅ 3H2O activator is introduced in the samples by incipient wetness procedure [17]. To prevent hydrophobic behavior of the coal powder, a water/alcohol (C2H5OH) mixture (50 : 50, by volume) is employed. The moisture content of the dried coal samples (mL/g) is determined immediately before deposition (Table 1). Then the solution is applied to the coal powder by mechanical dosing. The steeped samples are held in the drying chamber at 105°C for 20 h. The Cu(NO3)2 ⋅ 3H2O content in the modified sample is 5 wt % (recalculated for dry salt). For comparison, we prepare control samples without Cu(NO3)2 activator, which undergo exactly the same treatment. The modified coal and lignite samples are denoted by C/Cu and L/Cu, respectively.
A Jupiter STA 449C synchronous thermal analyzer (Netzsch, Germany) is used to study the thermal transformation of the modified samples in identical conditions at atmospheric pressure. The ~15-mg sample is heated in a corundum crucible with a perforated lid in the range 25–800°C at 10°C/min. The gas (air + argon) is supplied at a rate of 200 mL/min. The air/argon ratio varies as follows: 10/90, 30/70, 50/50, 70/30, and 90/10.
The NOx liberation is recorded by means of a QMS 403D Aeolos (Netzsch, Germany) quadrupole mass-spectrometer.
The following characteristics of thermal transformation are determined: the initial ti and final tf temperatures of the process; the maximum reaction rate wmax at the corresponding temperature tmax; the time Te for which the sample is heated before decomposition begins; the total time Tf of thermal decomposition; and the time Tmax to reach the maximum reaction rate. These parameters are calculated from the measurement results by a graphical method (described in detail in [10]).
For the T coal samples with bimodal differential thermal gravimetric (DTG) curves, this range is divided into two: 1) emission of volatiles; 2) oxidation of the coke residue.
RESULTS AND DISCUSSION
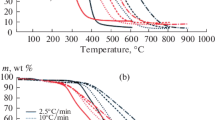
In Figs. 1 and 2, we show TG and DTG curves for the samples in atmospheres of different compositions. We see that, in all cases, the Cu(NO3)2 activator markedly changes the process: the temperature ti at which thermal transformation begins is lowered.
The thermal decomposition of coal in analogous consists of 3–4 main stages (depending on the coal rank), according to [10–12]. These stages are associated with the evaporation of physically adsorbed water, the vaporization and oxidation of volatiles, and the oxidation of the coke residue.
With increase in content of the inert gas (argon), thermal decomposition of the unmodified samples is shifted to higher temperatures (Figs. 1 and 2). In the presence of Cu(NO3)2 activator, the displacement of the TG and DTG curves is less pronounced. That may be associated with activation of the thermal transformation as a result of decomposition of the added Cu(NO3)2 in the low-temperature region (~200°C).
In Figs. 3 and 4, we show the change in the thermal decomposition of the samples with variation in gas composition.
It is evident that, in all cases, the initial ti (Fig. 3a) and final tf (Fig. 3b) temperatures of thermal decomposition are decreased as the oxidant content in the gas increases. Note that the final decomposition temperature cannot be recorded in the gas with the maximum argon content (90%), since the total mass loss in the given range 25–800°C (Fig. 3b) is no more than 70% for 2B lignite and 50% for T coal.
The greatest displacement of ti (Δt = 70°C) is observed for the modified T coal sample with a 90 : 10 air/argon ratio. In that case, it is also found that, with increase in oxygen content in the gas, the activity of the added Cu(NO3)2 declines. For the lignite sample, different behavior is observed: stronger influence of Cu(NO3)2 on the yield of volatiles and their subsequent oxidation. This is most likely associated with the nonuniform lignite structure and a greater quantity of lateral bonds and crosslinks, in the form of oxygen-bearing molecular groups [18]. Such groups actively react with nitrogen oxides to form copper nitrite [19].
Note the symbatic variation of the heating time Te before oxidation of the volatiles and the parameter ti (Fig. 3a), since the temperature at the onset of sample heating is 25°C. The total oxidation time of the active sample mass Tf (oxidation of the volatiles and the coke residue) behaves differently (Fig. 3b). For example, with increase in argon content, tf and Tf increase. That may indicate decrease in the sample’s mean decomposition rate. With maximum air content in the gas mixture, the decrease in tf is greatest (Fig. 3b). Thus, ΔTf is 1 min for the modified T coal sample and 5 min for the modified lignite sample. The increase in transformation rate may be associated with the stronger reaction between the oxidative medium and the liberated nitrogen oxides and the nonstoichiometric carbon oxide formed [13]. That is typical of high activity in the oxidation of a combustible substrate.
With increase in oxidant content in the gas, Tmax is displaced downward (Fig. 4a). The displacement of tmax between the maximum and minimum oxidant content in the gas is 51°C for the unmodified T coal sample and 75°C for the unmodified 2B lignite sample. Note also that, for the samples with bimodal DTG curves, the parameters in Fig. 4 correspond to the first maximum, characterizing the oxidation of volatiles.
For the modified T coal and 2B lignite samples, the change in tmax is practically the same: ~110°C. With increase in argon content in the gas, the difference Δtmax between the modified and unmodified samples decreases (Fig. 4a). Thus, with maximum oxidant content in the gas, Δtmax is 115°C for the T coal and 60°C for 2B lignite. With the minimum oxidant content in the gas, Δtmax is 71°C for the T coal and 30°C for 2B lignite.
With increase in argon content, the time Tmax to reach the maximum oxidation rate for modified samples begins to behave analogously to tmax (Fig. 4a). This is associated with decrease in the influence of the added Cu(NO3)2 on the rate of oxidation of the volatiles. Note also that the change in tmax is greatest for the T coal samples in gas with maximum oxidant content: for modified T coal, ΔTmax = 4.5 min. For modified lignite, ΔTmax = 3 min.
With minimum oxidant content in the gas, ΔTmax becomes negative for T coal. For modified lignite, by contrast, the maximum effect is observed with a 1 : 1 air/argon ratio (Fig. 4a).
With increase in argon content in the gas (Fig. 4b), the maximum oxidation rate wmax declines. This is most evident for the T coal samples, for which the variation in wmax is nonlinear. When the air/argon ratio is 30/70, we note sharp decrease in wmax in comparison with the 50/50 mixture: Δwmax = 1.7%/min. That is comparable with the change in tmax and Tmax (Fig. 4a).
On modifying the samples with Cu(NO3)2, the maximum oxidation rate declines as a result of the intensification of oxidation and its displacement to lower temperatures (Fig. 4b). The difference in wmax on modifying the T coal with Cu(NO3)2 is approximately the same for any gas composition: Δwmax = 0.2%/min.
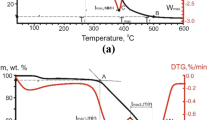
In Fig. 5, we show mass-spectrometric data for the nitrogen oxides formed in the thermal decomposition of the samples. With decrease in oxidant content in the gas, the temperature of maximum NOx liberation (m/z = 30) in Cu(NO3)2 decomposition rises. This is most evident for the modified lignite, for which the temperature shift is 20°C. For the T coal sample, the corresponding shift is 17°C.
Influence of the air/argon ratio in the atmosphere (supplied at 200 mL/min) on the temperature corresponding to the maximum rate of NOx liberation from copper nitrate in the lower range (a) and in the higher range (b), according to mass-spectrometric data: heating rate 10°C/min; temperature 25–800°C.
The mean difference \(\Delta t_{{{\text{N}}{{{\text{O}}}_{x}}}}^{{{{{\max }}_{1}}}}\) between the modified samples is 38.8°C. The difference in the temperatures corresponding to the maximum rate of NOx liberation from copper nitrate may be attributed to the different morphology of the samples. Lignite has a nonuniform structure with numerous open pores and channels and has lower diffusional drag when gas flows past the sample, according to [20]. That permits greater interaction of the oxidant with nitrate integrated into the coal sample by capillary steeping, which intensifies the decomposition.
Comparison of the results from the synchronous thermal analyzer (Fig. 4a) and the mass-spectrometric data (Fig. 5) indicates that Δti is directly correlated with the temperature \(\Delta t_{{{\text{N}}{{{\text{O}}}_{x}}}}^{{{{{\max }}_{1}}}}\) corresponding to maximum NOx liberation from copper nitrate.
For the subsequent NOx liberation in the high-temperature region (400–600°C), the temperature \(\Delta t_{{{\text{N}}{{{\text{O}}}_{x}}}}^{{{{{\max }}_{2}}}}\) increases with decrease in oxidant content in the gas. Note that, for the lignite samples, the activity of the Cu(NO3)2 is increased in the case, as is evident from the increase in the difference \(\Delta t_{{{\text{N}}{{{\text{O}}}_{x}}}}^{{{{{\max }}_{2}}}}\) between the modified and unmodified samples (53–85°C). The temperature shift \(\Delta t_{{{\text{N}}{{{\text{O}}}_{x}}}}^{{{{{\max }}_{2}}}}\) for the T coal samples is practically independent of the gas composition, remaining at 56°C.
CONCLUSIONS
We have investigated the thermal transformation of coal and lignite in the presence of Cu(NO3)2, as a function of the atmospheric composition (the air/argon ratio). We find that increase in the oxidant content in the gas sharply changes the thermal transformation. We may note the following aspects of this change:
(1) shift in the process to lower temperature (Δt = 70°C);
(2) increase in rate of the process (Δw = 1.7%/min);
(3) decrease in heating time to volatile liberation (ΔTe = 7 min);
(4) shift in oxidation to lower temperatures;
(5) greater catalytic effect of Cu(NO3)2: in particular decrease in the initial temperature of active oxidation.
It follows from mass-spectrometric analysis that, with decrease in oxidant content in the gas, the temperature corresponding to maximum rate of NOx liberation from copper nitrate Cu(NO3)2 increases. The peak of the second stage NOx liberation is also shifted to higher temperatures.
REFERENCES
Zyryanova, M.M., Badmaev, S.D., Belyaev, V.D., et al., Catalytic conversion of hydrocarbon raw material into fuel for power plants, Katal. Prom-sti, 2013, no. 3, pp. 22–27.
Sidorov, A.D., Fedorov, I.A., Dubinin, Yu.V., et al., Catalytic thermal systems for industrial heating, Katal. Prom-sti, 2012, no. 3, pp. 50–57.
Parmon, V.N., Simonov, A.D., Sadykov, V.A., and Tikhov, S.F., Catalytic combustion: Achievements and problems, Combust., Explos., Shock Waves, 2015, vol. 51, no. 2, pp. 143–150.
Tikhov, S.F., Simonov, A.D., Yazykov, N.A., et al., Catalytic combustion of brown coal particulates over ceramometal honeycomb catalyst, Catal. Sustainable Energy, 2012, vol. 1, pp. 82–89.
Gong, X., Guo, Z., and Wang, Z., Reactivity of pulverized coals during combustion catalyzed by CeO2 and Fe2O3, Combust. Flame, 2010, vol. 157, pp. 351–356.
Gong, X., Guo, Z., and Wang, Z., Variation on anthracite combustion efficiency with CeO2 and Fe2O3 addition by differential thermal analysis (DTA), Energy, 2010, vol. 35, pp. 506–511.
Gong, X., Guo, X., and Wang, Z., Variation of char structure during anthracite pyrolysis catalyzed by Fe2O3 and its influence on char combustion reactivity, Energy Fuels, 2009, vol. 23, pp. 4547–4552.
Wei, L., Zhang, N., and Yang, T., Effects of alkaline earth metal on combustion of pulverized coal, Adv. Mater. Res., 2012, vols. 516–517, pp. 271–275.
Huang, C.J., Wang, S.J., Wu, F., et al., The effect of waste slag of the steel industry on pulverized coal combustion, Energy Sources,Part A, 2013, vol. 35, pp. 1891–1897.
Larionov, K.B. and Gromov, A.A., Non-isothermal oxidation of coal with Ce(NO3)3 and Cu(NO3)2 additives, Int. J. Coal Sci. Technol., 2019, vol. 6, no. 1, pp. 37–50.
Larionov, K.B., Mishakov, I.V., Vedyagin, A.A., and Gubin, V.E., Effect of an initiating additive of CuSO4 on changes in the characteristics of brown coal oxidation and pyrolysis, Solid Fuel Chem., 2019, vol. 53, no. 2, pp. 120–127.
Larionov, K.B., Mishakov, I.V., Slyusarskii, K.V., et al., Intensification of the oxidation of lignite and coal by an activating additive of Fe(NO3)2, Solid Fuel Chem., 2019, vol. 53, no. 5, pp. 262–269.
Wang, Y., Wang, J., Chen, H., et al., Preparation and NOx-assisted soot oxidation activity of a CuO–CeO2 mixed oxide catalyst, Chem. Eng. Sci., 2015, vol. 135, pp. 294–300.
Liu, G., Liao, Y., Guo, S., et al., Thermal behavior and kinetics of municipal solid waste during pyrolysis and combustion process, Appl. Therm. Eng., 2016, vol. 98, pp. 400–408.
Epshtein, S.A., Crack formation in different type coals, Gorn. Inf.-Anal. Byull., 2009, no. 9, pp. 71–76.
Tabakaev, R., Kanipa, I., Astafev, A., et al., Thermal enrichment of different types of biomass by low-temperature pyrolysis, Fuel, 2019, vol. 245, pp. 29–38.
Tokareva, I.V., Mishakov, I.V., Korneev, D.V., et al., Nanostructuring of the carbon macrofiber surface, Nanotechnol. Russ., 2015, vol. 10, pp. 158–164.
Wang, D.M., Xin, H.H., Qi, X.Y., et al., Reaction pathway of coal oxidation at low temperatures: A model of cyclic chain reactions and kinetic characteristics, Combust. Flame, 2016, vol. 163, pp. 447–460.
Morozov, I.V., Znamenkov, K.O., Korenev, Yu.M., and Shlyakhtin, O.A., Thermal decomposition of Cu(NO3)2 · 3H2O at reduced pressures, Thermochim. Acta, 2003, vol. 403, pp. 173–179.
Strakhov, V.M., Kashlev, I.M., Soloviev, M.A., and Surovtseva, I.V., Changes in lignite on industrial storage, Coke Chem., 2017, vol. 60, no. 2, pp. 47–54.
ACKNOWLEDGMENTS
Financial support was provided by the Russian President (project NSh-2513.2020.8) and the Russian Ministry of Education and Science (project 0303-2016-0014).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by B. Gilbert
About this article
Cite this article
Larionov, K.B., Mishakov, I.V., Slyusarskiy, K.V. et al. Influence of Cu(NO3)2 Activator and Gas Composition on the Thermal Decomposition of Coal and Lignite. Coke Chem. 63, 114–119 (2020). https://doi.org/10.3103/S1068364X20030035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068364X20030035