Abstract
The conversion of coal and coal tar over iron–magnesium oxide catalyst produced from natural high-magnesia siderite is investigated. The use of this catalyst in the thermal processing of coal tar increases the yield of lighter fractions (by factors as large as 1.7) and decreases the yield of heavier fractions (by factors as large as 1.8). The catalytic pyrolysis of coal increases the total yield of the lightest materials (by a factor of 1.1–2.3). The nonmagnetic fraction of the solid residue may be added to coking batch, while the magnetic fraction may be used in sintering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The ore reserves in the Bakal fields (Chelyabinsk region, Russia) exceed 1 billion t. This ore has high magnesium content (≥10%). In addition, the ore and its roasting products form a common crystal lattice. Traditional enrichment methods (such as magnetization on roasting and subsequent magnetic separation) are unable to separate components with common crystal lattice. Hence, the magnesium-oxide content in the concentrate is very high. High magnesium-oxide content is undesirable for blast-furnace use (in particular, because of the highly viscous slag formed in the blast furnace [1]), and so concentrate based on Bakal siderites is used in limited amounts in hot-metal production.
However, this deficiency may be regarded as a benefit if the high-magnesia siderite is used to produce a catalyst containing iron and magnesium oxides. The product obtained in the processing of high-magnesia siderite catalyzes the conversion of ethanol by water vapor and the gasification of carbon (as its dioxide), as established in [2–8].
Further research on the catalytic properties of the products of complex processing of high-magnesia siderites: in the Chelyabinsk region, there are large reserves of high-magnesia siderite ore and lignite, as well as coke-plant wastes, whose use is difficult at present for lack of effective and economical processing methods [9–12].
To develop such technology, we may investigate the catalytic conversion of coal concentrates from the Kuznetsk Basin and coal tar from the coke plant at PAO MMK.
In the present work, we investigate the conversion of coal and coal tar over iron–magnesium oxide catalyst produced from high-magnesia siderite.
CHARACTERISTICS OF MATERIALS
Catalyst
Table 1 summarizes the composition of the initial high-magnesia siderite ore and the iron–magnesium oxide catalyst. Electron-microscope and X-ray diffraction data indicate that the roasting products of high-magnesia siderite ore include not only magnesioferrite (MgFe2O4) but also a magnesiowüstite solid solution [FeO]x ⋅ [MgO]1 – x. Small quantities of magnesium oxide in periclase form are also formed. Iron is not only present in magnesioferrite and magnesiowüstite but also in magnetite (Fe3O4). The thermal-decomposition product has magnetic properties. The iron–magnesium oxide catalyst is the magnetic component of the product of ore roasting. After crushing and classification, we obtain the >0.5–2.0 mm class [13–16].
Coal
Concentrate from GZh gas–bituminous Raspadsk coal from the Kuznetsk Basin is subjected to pyrolysis [17].
This concentrate is characterized by relatively high yield of volatiles (37.7%). On heating, the concentrate may pass to the plastic state and undergo sintering. In the experiments, we use a relatively large coal fraction (>1–2 mm). The results of technical analysis are given in Table 2.
Coal Tar
We use coal tar from the coke plant at PAO MMK. Its composition and properties are summarized in Table 3.
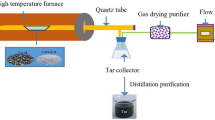
The mass of the material subjected to conversion (coal or coal tar) is 100 g, and the maximum catalyst mass is 60 g. In Fig. 1, we show the experimental apparatus.
Experimental apparatus: (1) tubular electrical furnace; (2) electric plate; (3) device for microprobing of liquid; (4) evaporation bulb; (5) autotransformers; (6) catalyst layer; (7) thermocouples; (8) secondary instrument; (9) descending cooling tube; (10) intake flask; (11) coil; (12) cooling mixture; (13) cooled intake flask; (14) gas meter.
Gaseous Products
The gaseous products are analyzed by gas-adsorption chromatography on a Chromatec Crystal 5000 system using a 3 m × 2 mm HayeSepQ packed column (for CO2 determination) and a 3 m × 2 mm NaX packed column with a Carboxen (0.5 m × 2 mm) precolumn (for CO determination). The carrier gas for the HayeSepQ packed column is helium (15 mL/min); for the NaX and Carboxen columns, we use argon (20 mL/min). The volume of the gas sample introduced is 1 mL. To determine the yield of the components, we analyze the gas mixture by means of thermal-conduction sensors. The chromatograms are analyzed by means of Chromatec Analytic software. In quantitative analysis, we use absolute graduation by means of calibration mixtures. Each chromatographic measurement is made three times. The results are statistically analyzed and averaged.
Liquid Products
The products of coal pyrolysis are identified by means of a Chromatec Crystal 5000 chromatographic system with a ZB5-ms capillary column (5% phenyl arylene + 95% dimethyl polysiloxane), with linear heating at 6°C/min. The carrier gas is nitrogen (15 mL/min, P = 100 kPa). Chromatographic analysis lasts 60 min.
In determining the content of the components, we use absolute calibration with respect to a standard mixture of polycyclic aromatic hydrocarbons. Materials absent from the standard mixture are identified qualitatively by means of systems of retention indices [18]. Their content is quantitatively estimated by the normalization of areas.
RESULTS AND DISCUSSION
Research on coal gasification has shown that mild roasting of high-magnesia siderite produces a material capable of catalyzing the gasification of carbon as its dioxide [7, 8].
On gasification of coal in the presence of iron–magnesium oxide catalyst, we observe a deeper catalytic effect than in experiments with gasification of a mechanical mixture of precarbonized coal and iron–magnesium oxide catalyst. This indicates that the plastic layer increases the coal–catalyst contact in gasification and increases the gasification by a factor of 2–4.
That finding helps determine the next step in investigating the catalytic conversion of carbon-bearing materials. Since the contact with the catalyst is greatest when the carbon-bearing material passes to the plastic state, whereas the contact area declines on heating to the temperatures of semicoke formation and on gasification, the next step is to study catalytic processes in the temperature range where the carbon-bearing material has liquid mobility. In the first stage, the selected process is the catalytic pyrolysis of the carbon-bearing material (coal or coal tar).
We investigate the pyrolysis of coal in the presence of iron–magnesium oxide catalyst and also the pyrolysis of coal tar in the presence of iron–magnesium oxide catalyst. Coal tar is selected with a view to its use as a plasticizer in the pyrolysis of coal.
Coal Pyrolysis
In pyrolysis, the coal is heated to 600°C at 10 K/min, with the continuous sampling of liquid and gaseous reaction products. In this series of experiments, the catalyst mass varies from 10 to 30 g. Table 4 presents the yield of gaseous and liquid products and the solid residue.
The catalyst has practically no effect on the quantitative ratio of the conversion products (gaseous, liquid, and solid phases), whereas chromatographic analysis of the liquid products indicates increase in their content of light fractions and decrease in the content of heavy fractions (Table 5). The light (low-boiling) fractions correspond to groups retained for up to 10 min (group 1) and for 10–20 min (group 2). Analogously, in terms of the retention time, we identify the medium fraction (groups 3 and 4) and the heavy fraction (Table 5, groups 5 and 6). This classification is based on the linear relation established between the retention time and boiling point of the materials.
The greater yield of light fractions than in the noncatalytic process may be explained by the destruction of heavier fractions and the redistribution of the destruction products among the other groups.
The content of the light fraction increases by a factor of 1.1–2.3, with increase in the content of 1-nonene, tert-butylcyclohexane, phenol, 2,6-xylenol, cis-decalin, and quinoline, in particular. At the same time, the proportion of the medium fraction decreases by a factor of 1.1–1.5, with decrease in the content of 1-methylnaphthalene, 2-methylbiphenyl, acenaphthylene, 4-methylbiphenyl, fluorene, and 4,4-dimethylbiphenyl, in particular. For the other identified components, the effect of catalysis is less significant. The lack of selectivity may be the result of inadequate and unstable contact between the coal and the iron–magnesium oxide catalyst. To improve contact, the quantity of liquid phase may be increased, either by adjusting the coal/catalyst ratio in favor of coal (with consequent increase in the content of plastic mass) or by adding a plasticizer, such as coal tar.
Conversion of Coal Tar
In Fig. 2, we illustrate the thermal transformation of coal tar in the presence of iron–magnesium oxide catalyst. Samples of the condensing fractions 1–7 are taken continuously in the course of coal-tar conversion (without stopping the process). The fractions are identified on the basis of the boiling point. The maximum temperature is 500°C; the tar mass is 100 g; and the catalyst mass is 15–60 g [19].
Ignition of the catalyst is observed above 230°C. The greatest increase in the yield is seen for fraction 3, while the greatest decrease is seen for fraction 6. In Fig. 3, we show chromatograms of these fractions; the series of identified materials is noted. The content of these components is presented in Table 6. The process is relatively noncatalytic. In the presence of iron–magnesium oxide catalyst, we note change in the light fraction (retention time less than 20 min) on account of increase in naphthalene, while the change in the medium fraction (retention time 20–40 min) is mainly due to the destruction of phenanthrene (Table 6).
A promising next step is to investigate the combined pyrolysis of coal and a plasticizer (coal tar) over iron–magnesium oxide catalyst produced from high-magnesia siderite ore. The solid residue (an analog of semicoke) formed in the experiments after pyrolysis with the catalyst may be added to sintering batch and used as a lean additive in coking batch. This conclusion is reached after dry magnetic separation of the solid residue into magnetic and nonmagnetic fractions and determination of their ash and carbon content, with the following results.
1. The mass ratio of the magnetic and nonmagnetic components is 2 : 1.
2. The ash content of the solid residue Aa = 22%; it contains 78% C.
3. For the magnetic fraction, Aa = 28%; 72% C (component for sintering batch).
4. For the nonmagnetic fraction, Aa = 13%; 87% C (component for coking batch).
CONCLUSIONS
1. In the presence of iron–magnesium oxide catalyst, the total yield of the light fraction (retention time less than 20 min) in coal pyrolysis is increased by a factor of 1.1–2.3.
2. In the presence of iron–magnesium oxide catalyst, the greatest increase in the yield in the thermal conversion of coal tar is seen for the fraction that boils in the range 230–270°C (by a factor of 1.7), while the greatest decrease is seen for the fraction that boils in the range 410–450°C (by a factor of 1.8). The increased content of the light fraction (230–270°C) is mainly due to the 1.4-fold increase in naphthalene content.
3. By dry magnetic separation, the solid residue formed on pyrolysis in the presence of iron–magnesium oxide catalyst may be divided into a magnetic fraction (suitable for use in sintering batch) and a nonmagnetic fraction (suitable for use in coking batch).
REFERENCES
Yur’ev, B.P., Melamud, S.G., Spirin, N.A., and Shatsillo, V.V., Tekhnologicheskie i teplotekhnicheskie osnovy podgotovki siderlitovykh rud k metallurgicheskim peredelam (Technological and Thermal Engineering Principles of Preparation of Siderite Ores for Metallurgical Treatment), Yekaterinburg: Den’ RA, 2016.
Klochkovskii, S.P., Smirnov, A.N., and Kolokol’tsev, V.M., RF Patent 2536618, 2014.
Smirnov, A.N., Klochkovskii, S.P., Bigeev, V.A., Kolokol’tsev, V.M., and Bessmertnykh A.S., RF Patent 2471564, 2013.
Smirnov, A.N., Klochkovskii, S.P., Krylova, S.A., et al., Possible use of catalysts based on processing products of highly magnesian siderite in ethanol conversion reactions, Aktual’nye Probl. sovrem. Nauki, Tekhn.,Obraz., 2016, vol. 1, pp. 258–261.
Smirnov, A.N., Sysoev, V.I., Krylova, S.A., and Klochkovskii, S.P., Catalytic activity of products of annealing of highly magnesian siderite, Vestn. Bashkir. Univ., 2017, vol. 22, no. 3, pp. 657–665.
Smirnov, A.N., Klochkovskii, S.P., Krylova, S.A., et al., Possible use of oxide ferromanganese contacts in the reaction of water gas, Aktual’nye Probl. sovrem. Nauki, Tekhn.,Obraz., 2017, vol. 2, pp. 66–70.
Smirnov, A.N., Sysoev, V.I., and Krylova, S.A., Investigation of the catalytic activity of the middlings of high-magnesian siderites processing, Recent Pat. Mater. Sci., 2017, vol. 10, no. 2, pp. 136–141.
Smirnov, A.N., Klochkovsky, S.P., Krylova, S.A., and Sysoev, V.I., Gasification of the Kuznetsk Basin coal concentrate using oxide iron-magnesium catalysts, J. Chem. Technol. Metall., 2019, vol. 54, no. 2, pp. 286–291.
Kairbekov, Z.K., Dzheldybaeva, I.M., Kairbekov, A.Z., et al., Catalytic hydrogenation of Oi-Karagai coal, Coke Chem., 2015, vol. 58, no. 1, pp. 1–8.
Proshunin, Yu.E. and Shkoller, M.B., New trends in deep processing of bitumen and lignites in Kemerovo oblast, Koks Khim., 2016, no. 2, pp. 10–15.
Fedorova, N.I., Gavrilyuk, O.M., Zaostrovsky, A.N., and Ismagilov, Z.R., Yield and composition of semicoking tars from low-quality coal, Coke Chem., 2018, vol. 61, no. 9, pp. 338–343.
Romanova, N.A., Leont’ev, V.S., and Khrekin, A.S., Production of commercial naphthalene by coal-tar processing, Coke Chem., 2018, vol. 61, no. 11, pp. 453–456.
Klochkovskii, S.P., Smirnov, A.N., and Savchenko, I.A., Development of physical and chemical principles of the integrated use of highly magnesian siderites, Vestn. Magnitogorsk. Gos. Tekh. Univ. G.I. Nosova, 2015, vol. 49, no. 1, pp. 26–31.
Klochkovskii, S.P., Smirnov, A.N., Savchenko, I.A., et al., Physical and chemical principles of complex processing of highly magnesian siderite ores from Bakal’skoe deposit, Izv. Samar. Nauchn. Tsentra, Ross. Akad. Nauk, 2014, vol. 16, no. 4 (3), pp. 572–575.
Klochkovskii, S.P., Smirnov, A.N., Savchenko, I.A., and Sysoev, V.I., Physicochemical principles of the complex processing of highly magnesian siderite ores, Materialy XIX Mezhdunarodnoi nauchno-tekhnicheskoi konferentsii “Nauchnye osnovy i praktika pererabotki rud i tekhnogennogo syr’ya” (Proc. XIX Int. Sci.-Tech. Conf. “Scientific Principles and Practice of the Processing of Ore and Technogenic Raw Material”), Yekaterinburg, 2014, pp. 154–158.
Krylova, S.A., Sysoev, V.I., Alekseev, D.I., et al., Physicochemical characteristics of highly magnesian siderites, Vestn. Yuzn.-Ural. Gos. Univ.,Ser.: Metall., 2017, vol. 17, no. 2, pp. 13–21.
Sysoev, V.I., Smirnov, A.N., and Krylova, S.A., Processing of carbon-containing raw materials on oxide iron-manganese catalysts, Materialy VII Mezhdunarodnogo Rossiisko-Kazakhskogo simpoziuma “Uglekhimiya i ekologiya Kuzbassa,” Tezisy dokladov (Proc. VII Int. Russ.-Kazakh Symp. “Coal Chemistry and Ecology of Kuzbass,” Abstracts of Papers), Kemerovo: Fed. Issled. Tsentr Uglya Uglekhim., Sib. Otd., Ross. Akad. Nauk, 2018, pp. 95.
Smirnov, A.N., Sysoev, V.I., Krylova, S.A., et al., Calibration of gas chromatographic analysis method for the identification of the products of carbon containing materials processing obtained using iron-magnesian oxide catalyst, Kach. Obrab. Mater., 2018, no. 2 (10), pp. 34–40.
Smirnov, A.N., Krylova, S.A., Sysoev, V.I., et al., Investigation of the process of catalytic conversion of coal tar using an oxide iron-magnesia catalyst, Kach. Obrab. Mater., 2018, no. 1 (9), pp. 44–47.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by B. Gilbert
About this article
Cite this article
Smirnov, A.N., Krylova, S.A., Petukhov, V.N. et al. Conversion of Coal over an Oxide Catalyst Produced from Natural High-Magnesia Siderite. Coke Chem. 62, 468–473 (2019). https://doi.org/10.3103/S1068364X19100119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068364X19100119