Abstract
The influence of chemical modification on the functional–group composition of humic acids derived from Tisul’sk lignite (Kansko-Achinsk Basin) is studied. The structural changes of the humic acids as a result of destructive alkylation of n-butanol and oxidation of hydrogen peroxide are compared, by means of IR, Raman, ESR, and NMR spectroscopy. Alkylation of the humic acids changes the composition and content of the aliphatic fragments in their structure. Oxidation by hydrogen peroxide increases the content of acid-bearing groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Humic acids are natural high-molecular compounds of irregular structure found in soil, peat, lignite, and sapropels. They differ in elemental composition; degree of condensation and substitution of the aromatic rings; content of functional groups; and ratio of hydrophilic and hydrophobic fragments. They are highly reactive, with high biological activity. Thanks to these properties, they are of great practical value in agriculture, pharmacology, and ecology. Humic acids are promising for the production of sorbents, biostimulants, pharmaceuticals, organic fertilizers, and structuring agents for soils. Their utility is limited by deficiencies such as low sorptional properties in comparison with synthetic sorbents, insufficient mechanical strength, and solubility in alkaline structures.
Lately, researchers have shown great interest in chemical modification of humic acids, which yields preparations with valuable properties. Modification increases the reactivity and the thermal and chemical stability of humic acids, while decreasing their solubility [1, 2]. Modification of humic acids to change their functional-group composition may improve their sorptional properties [3, 4] and biological activity [5–7] and regulate their redox properties.
The modified humic acids are also of applied interest in environmental protection; detoxification and reconditioning of degraded and contaminated soil; and stimulation of plant growth. In addition, chemical modification of humic acids may be used for detailed investigation of their structure and the influence of different functional groups on their sorption of different cations. It is important to establish a relation between the composition, structure, and properties of humic acids, so as to understand the behavior of humic acids in natural and industrial processes and hence to improve their use.
In the present work, we compare the structure and group composition of humic acids obtained by modification: specifically, oxidation with hydrogen peroxide and alkylation with n-butanol.
EXPERIMENTAL
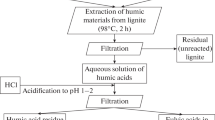
To obtain humic acids, we use lignite samples from the Tisul’sk field in the Kansko-Achinsk Basin and samples of lignite that has been oxidized in the bed. The humic acids are released from the coal by treatment with sodium-hydroxide solution and subsequent deposition with hydrochloric acid [8]. Table 1 presents the characteristics of the lignite samples and the humic acids derived from them. The yield of humic acids is 2.0–2.5 times greater from the oxidized lignite than from the unoxidized lignite. Likewise, the humic acids from the oxidized lignite have higher oxygen content.
The humic acids are modified with hydrogen peroxide as follows [3]: with constant mixing in a mechanical agitator, droplets of hydrogen peroxide (C = 32%, V = 5, 10 mL) are added from a burette to a specific volume of solutions containing 10 g of sodium humate, with precipitation of the product by means of hydrochloric acid. The deposits are filtered, washed with water to the pH of distilled water, dried at 70°C to constant weight, and sent for spectral analysis.
For alkylation, a weighed sample (5 g) of humic acids from the lignite or oxidized lignite is dissolved in 100 mL of n-butyl alcohol, with subsequent acidification to pH \( \approx \)2–3 by means of hydrochloric acid. The solution is boiled for 3 h at the boiling point of butanol and then cooled to room temperature. The mixture is diluted with distilled water (2 L). The deposit obtained is filtered in a Bunsen funnel, washed with water until the butanol odor disappears, dried to constant weight, and then sent for spectral analysis.
High-resolution 13C NMR spectra (13C NMR CP MAS) in the solid state are recorded on a Bruker Avance III 300W instrument by cross polarization with rotation by the magic angle at a frequency of 75 MHz; the frequency of sample rotation is 5 kHz.
The ESR spectra of the samples are recorded at room temperature on a Bruker EMX-m40X instrument at 9.86 GHz. For all the samples, the ESR spectra are recorded in the following conditions: power 1.8–1.9 mW; modulation frequency 100 kHz. To determine the quantity of organic paramagnetic centers, Mn2+ ions in magnesium oxide MgO are used as the standard. The characteristics of the ESR spectra are calculated by means of Bruker Win EPR software.
The Raman spectra are recorded on a Renishaw Invia Basis spectrometer at an exciting wavelength of 514.5 nm, with a light spot of diameter no greater than 2 μm; the diffraction grating has 1800 lines per mm. Measurements are made in the spectral range of the Raman shift: 100–4000 cm–1.
The IR spectra are recorded on an Infralyum-FT 801 spectrometer with Fourier transformation, in tablets with KBr.
RESULTS AND DISCUSSION
The humic acids are characterized by typical absorption bands of different intensity in the IR spectra [10]. The broad band with an absorption peak at ~3400 cm–1 indicates the presence of hydroxyl groups with a hydrogen bond. The bands at 2920–2940 and 2840–2860 cm–1 correspond to valence vibrations of the CH3 and CH2 groups; the band at 1610–1630 cm–1 corresponds to valence vibrations of the C=C conjugate double bonds in aromatic fragments; that at 1370–1450 cm–1 to deformation vibrations of the C–H bond in the aliphatic groups CH3 and CH2; that at 1240–1280 cm–1 to deformation vibrations of the C–O bond in carbonyl acids and esters and the O–H bond of phenols; that at 1030–1100 cm–1 to the C–O bond of cyclic and aliphatic esters and alcohols; and that at 800 cm–1 to the C=C and C–H bonds of aromatic rings. For all the samples of the initial humic acids, bands of different intensity appear at 1710–1720 cm–1, corresponding to valence vibrations of the C=O bond in carbonyl acids.
When the humic acids from unoxidized lignite are treated with hydrogen peroxide, the intensity of the 3400, 1710, 1260, and 1030–1100 cm–1 bands increases. The spectra for the humic acids from oxidized lignite are characterized by lower intensity of the band corresponding to the C=O bond in carbonyl acids.
The IR spectra of alkylated humic acids are characterized by increased intensity of the bands at 2930 and 2870 cm–1 (CH3 and CH2 groups), 1450 and 1380 cm–1 (C–H bond in the CH3 and CH2 groups), and 1240 cm–1 (esters).
As for lignite, which consists mainly of carbon, the main characteristics of the Raman spectra of the humic acids are the D peak (~1350 cm–1) and G peak (~1600 cm–1) [11]. The G band corresponds to sp2-hybridized carbon, and the D band to sp3-hybridized carbon, whose position depends greatly on the number of hexagonal aromatic rings. The intensity ratio ID/IG of the bands characterizes the disorder of the humic acids. If ID/IG > 1, sp3-hybridized carbon predominates. If ID/IG < 1, sp2-hybridized carbon predominates; in other words, the structure has a high content of aromatic carbon.
Table 2 presents the basic characteristics of the Raman spectra of modified and unmodified humic acids. We see that modification of the humic acids, whether by alkylation or oxidation with hydrogen peroxide, changes the ratio of alkyl and aromatic fragments and produces a more ordered structure. Sharper decrease in ID/IG is observed for the humic acids from oxidized lignite after modification with butanol, since alkylation decreases the content of irregular aliphatic fragments [12]. The structure of the humic acids becomes more compact. Sharp decrease in the half-width of the D peak indicates more uniform composition of the alkyl components on account of the replacement of the high-molecular alcohol fragments by n-butyl as a result of reesterification [9].
The ESR spectra of the humic acids are typical of coal and hard polyaromatic compounds of quinoid type [13, 14]. The ESR signals of practically all the samples of modified and unmodified humic acids are symmetric or slightly asymmetric singlets, consisting of a superposition of two signals of different width and intensity. Table 3 present the data for the ESR spectra of modified and unmodified humic acids.
For all of the samples, the g-factor is 2.0034–2.0039, in good agreement with literature data [13, 14]. That may indicate identical structural types of the humic-acid samples.
The number of paramagnetic centers is an order of magnitude smaller for the humic acids from oxidized lignite. Modification with hydrogen peroxide decreases the content of paramagnetic centers in all the samples, since the hydrogen peroxide, which is a source of OH radicals and oxygen, may break down the organic radicals and facilitate the appearance of new functional groups [15]. The ionic nature of alkylation also facilitates decrease in the content of paramagnetic centers, as we see for the humic acids from unoxidized lignite after alkylation with butanol [9].
According to the 13C NMR spectra, the humic acids from oxidized lignite have a higher content of carboxyl groups, as established in [4]. On modification with hydrogen peroxide, various oxidative reactions increase the content of carbonyl groups (187–220 ppm) and also of fragments containing oxygen with carbon (48–90 ppm). The content of aromatic fragments Car–O (145–165 ppm) and Car (108–145 ppm) declines. For humic acids from unoxidized lignite, the content of carboxyl groups (165–187 ppm) increases; the opposite is seen for humic acids from oxidized lignite. This is probably associated with simultaneous decarboxylation of the COOH groups, leading to increase in the content of carbonyl and hydroxyl groups after modification with hydrogen peroxide.
Alkylation of fossil fuels by alcohols largely involves esterification and reesterification with the participation of carboxyl groups, as shown in [9]. That breaks the bonds between molecules in the organic mass and increases the solubility and the yield of extracted materials, such as long-chain aliphatic alcohols. Consequently, the relative content of aromatic fragments in the organic mass increases. The greatest structural changes in the humic acids on modification by butanol would be expected in the NMR spectra in the range 0–100 ppm.
Thanks to the structural heterogeneity of the initial humic acids and the difference in position and coordination, the chemical shift of Calk corresponds to a broad signal in the range 5–48 ppm. In the range 48–90 ppm, we see a broad low-intensity signal corresponding to Calk–O. After alkylation, the spectrum of the humic acids includes an intense signal at 66 ppm (Calk–O) and intense narrow signals at 14.4 ppm (CH3 group) and at 19.7 ppm (CH2 group bound to terminal CH3 group) and 32.0 ppm (CH2 group at the middle of the chain) [16]. The small changes in the spectrum of the modified humic acids in the range 100–200 ppm may be associated with change in coordination of carbon in the aromatic ring and carbonyl carbon in the region 160–180 ppm on account of the participation of carboxyl groups in alkylation.
Thus, chemical modification changes the functional-group composition and hence the properties of humic acids. Modification with hydrogen peroxide increases the content of oxygen-bearing groups, including carboxyl groups. That is promising in terms of the use of such humic acids as sorbents for selective separation and extraction of metal cations from various media and in the treatment of industrial water and wastewater. Destructive alkylation of humic acids changes the proportions of aromatic and aliphatic structure and also of hydrophilic and hydrophobic fragments and thereby regulates the biological activity of the humic acids.
REFERENCES
Lyubchenko, V.I., Dumbai, I.N., Gubanova, E.N., et al., Granulated sorption materials based on lignite humates, Khim. Tverd. Topl. (Moscow), 1999, no. 2, pp. 38–46.
Zherebtsov, S.I., Non-fuel use of Itatsk lignite, Trudy nauchno-tekhnicheskoi konferentsii “Opyt i perspektivy naukoemkikh tekhnologii v ugol’noi promyshlennosti Kuzbassa” (Proc. Sci.-Tech. Conf. “Prospective Use of High Technologies in Coal Industry of Kuzbass”), Kemerovo, 1998, pp. 258–262.
Malyshenko, N.V., Zherebtsov, S.I., Smotrina, O.V., et al., Sorption of zinc cations by modified humic acids, Chem. Sustainable Dev., 2015, vol. 23, no. 4, pp. 451–457.
Zherebtsov, S.I., Malyshenko, N.V., Smotrina, O.V., et al., Sorption of copper cations by native and modified humic acids, Chem. Sustainable Dev., 2016, vol. 24, no. 3, pp. 399–403.
Naumova, G.V., Strigutsky, V.P., Zhmakova, N.A., and Ovchinnikova, T.F., The relation between the molecular structure of humic acids and their biological activity, Solid Fuel Chem., 2001, vol. 35, no. 2, pp. 1–10.
Zherebtsov, S.I., Malyshenko, N.V., Smotrina, O.V., et al., Structural group composition of humic acids in brown coal and their physiological activity, Chem. Sustainable Dev., 2015, vol. 23, no. 4, pp. 439–444.
Zherebtsov, S.I. and Ismagilov, Z.R., Effect of the alkylation of brown coal and peat on the composition and properties of humic acids isolated from them, Solid Fuel Chem., 2012, vol. 46, no. 6, pp. 339–351.
Taits, E.M. and Andreeva, I.A., Metody analiza i ispytaniya uglei (Methods of Analysis and Tests of Coals), Moscow: Nedra, 1983.
Zherebtsov, S.I., Lozbin, V.I., Polubentsova, M.F., Interaction of brown coal of the Aleksandriya coalfield with methanol, Solid Fuel Chem., 2003, vol. 37, no. 2, pp. 1–6.
Shaks, I.A. and Faizullina, E.M., Infrakrasnye spektry iskopaemogo organicheskogo veshchestva (Infrared Spectra of Fossil Organic Matter), Moscow: Nedra, 1974.
Filippov, M.M., Analysis of deeply carbonated organic matter by Raman spectroscopy, Part 1. The general use, Tr. Karel. Nauchn. Tsentra, Ross. Akad. Nauk, 2014, no. 6, p. 115.
Zherebtsov, S.I., Malyshenko, N.V., and Ismagilov, Z.R., Mechanism of the alcohol-mediated alkylation of solid fossil fuels at the low stage of coalification, Chem. Sustainable Dev., 2015, vol. 23, no. 1, pp. 139–145.
Jezierski, A., Czechowski, F., Jerzykiewicz, M., et al., Electron paramagnetic resonance (EPR) studies on stable and transient radicals in humic acids from compost, soil, peat and brown coal, Spectrochim. Acta, Part A, 2000, vol. 56, pp. 379–385.
Ishiwatari, R., Electron spin resonance of sedimentary humic acids in relation to their aromatic character, Geochem. J., 1974, vol. 8, pp. 97–102.
Nonhebel, D.C. and Walton, J.C., Free-Radical Chemistry: Structure and Mechanism, Cambridge: Cambridge Univ. Press, 1974.
Breitmaier, E. and Voelter, W., Carbon-13 NMR Spectroscopy: High-Resolution Methods and Applications in Organic Chemistry and Biochemistry, New York: VCH, 1990, 3rd ed.
ACKNOWLEDGMENTS
Financial support for this research was provided under a grant of the Russian government to the Federal Research Center of Coal and Coal Chemistry, Siberian Branch, Russian Academy of Sciences (project AAAA-A17-117041910148-9, under the direction of S. I. Zherebtsiov).
The research was conducted on equipment provided by the Cooperative-Use Center at the Federal Research Center of Coal and Coal Chemistry, Siberian Branch, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by Bernard Gilbert
About this article
Cite this article
Zherebtsov, S.I., Malyshenko, N.V., Bryukhovetskaya, L.V. et al. Influence of Chemical Modification on the Structure, Composition, and Properties of Lignite Humic Acids. Coke Chem. 61, 396–400 (2018). https://doi.org/10.3103/S1068364X18100083
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068364X18100083