Abstract
The aqueous solution with iron nanoparticles investigated by a microwave stripline sensor based on the optimized double quadratic-shape design. Due to real-time near-field electromagnetic interaction between microwaves and sample S11 reflection coefficient of the sensor was changed depending on iron nanoparticles concentration in the aqueous solution at resonant frequencies. In this work we examined the iron nanoparticles concentration in the 0–20 µg/L concentration range at an operating frequency of about 1.7 GHz. The measured minimum detectable signal was 0.035 dB/(µg/L) and 0.25 MHz/(µg/L) and the measured minimum detectable concentration was 1.4 µg/L and 0.2 µg/L, respectively. The microwave response of sensor systems can be explained by the additional structural changes of water clusters due to the metal nanoparticles ablation. This implemented method has approachable development process and the accuracy of measurement is high, thus it can be applied as a physiochemical sensor for non-invasive monitoring of metal nanoparticles in complex liquids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 INTRODUCTION
The rapid deterioration in water quality has become a global concern. Water pollution becomes a critical issue around the world and environmental protection and remediation of water pollution are of special concern around the world [1, 2]. The main contaminants in water are mainly composed of inorganic pollutants such as heavy metals (metals with atomic numbers greater than 20) and arsenic ions, organic pollutants such as detergents, pesticides, pharmaceuticals, and biomaterials [3, 4]. Heavy metals pollution has become a severe environmental and public health problem because the accumulation of non-biodegradable heavy metals in the human body would lead to serious diseases. Generally, chemical, physical and biological strategies have been applied to remedy the problem of toxic elements, organic contaminations, and biomaterials from water [5, 6].
Transition metal or metal oxides nanoparticles are broadly used to remove heavy metal ions in wastewater. Because of their unique features, iron nanoparticles have been widely applied to the removal of heavy metals in water and determination of the iron nanoparticles concentration in the water solution is an important task [7].
The microwave resonant sensors have been shown high sensitivity for concentration measurement in liquid solutions [8, 9]. In this work we explored the determination of the concentration of the iron nanoparticles in an aqueous solution with the resolution of about 1 µg/L. In this study was used real-time non-destructive near-field electromagnetic interaction technique and the experimental process was implemented out by microwave microstrip sensor based on a double quadratic shape design with 1.7 GHz resonant frequency. The quadratic shape design successfully used for development of sample stripline biosensors in our latest works for glucose [10], NaCI [11] concentration measurement, and Ag nanoparticles investigation in aqueous solutions [12]. The results show that the microwave sensor proposed has high accuracy to detect the concentration of the iron nanoparticles in an aqueous solution and the implemented method is applicable for non-destructive biophysical and chemical sensing applications.
2 MATERIALS AND METHODS
The enrichment of de-ionized (DI) water with iron nanoparticles was implemented by the laser ablation technique [12]. The iron target immersed in a glass cuvette filled with DI water was irradiated by beam of Multimode YAG:Nd pulse laser thereby enriched solutions with iron nanoparticles. More details about this laser ablation technique are given in Ref. [12] in which the same ablation procedure was used for making silver nanoparticles/DI water solution.
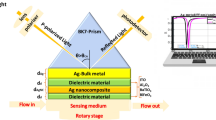
Figure 1 shows the structural configuration of the experimental setup. The microwave sensor preparation procedure and utilized materials are the same as for the sensor used in Ref. [10] for non-invasive sensing of glucose aqueous solution.
In the experiment, we placed a quartz cuvette with iron nanoparticles/DI-water solution i.e. material under test (MUT) on the sensor and the response (microwave reflection coefficient S11) was detected by a vector network analyzer (VNA:HP8753D). We explored the concentrations from 0 µg/L (i.e. DI water) to 20 µg/L with intervals of 10 µg/L. The volume of the aqueous solution was 2 mL. The temperature probe recorded a change in temperature of MUT during measurement. We registered the changes of the reflection coefficient S11 of the sensor which is the consequence of the change of the iron nanoparticles concentration in MUT. Note that the effect of the empty quartz cuvette on the change of the system impedance was negligible and the sensor was calibrated with DI water giving an S11 minimum of –28.5 dB at about 1.75 GHz. The data acquisition time for iron nanoparticle’s for real-time monitoring was 0.2 seconds and experiments were done at an environment temperature of 25°C. The formed iron nanoparticles sizes were evaluated to be within 50–500 nm range.
The operational principle of our suggested system is based on the shift in the microwave reflection coefficient S11 (both magnitude and frequency) due to changes in the dielectric permittivity and electric conductivity of the MUT. The replacement of the MUT brings the changes in the total impedance of the whole system due to the change of the dielectric permittivity and electrical conductivity of the MUT (for example due to change nanoparticle concentration in liquid). The Maxwell Garnett model [13] describe the effective dielectric permittivity for dielectric medium which contain conducting particles (metals) by the following way
where εd is the relative permittivity of dielectric (DI water), εi is the relative permittivity of the ith sort of inclusions (iron nanoparticles), fi is the volume fraction occupied by the inclusions of the ith sort, Nij are the depolarization factors of the ith sort of inclusions, and the index j = 1, 2, 3 corresponds to X, Y, Z coordinates. In our study, the inclusions are iron nanoparticles which are conducting materials and the complex relative permittivity are described as
where \(\varepsilon _{i}^{'}\) and \(\varepsilon _{i}^{{''}}\) are real and imaginary part of complex dielectric permittivity of MUT and σi is the bulk conductivity of MUT, ω is operating frequency, and ε0 is the dielectric permittivity of vacuum.
3 RESULTS AND DISCUSSION
Figure 2 shows the dependence of the measured microwave reflection coefficient profile of the MUT with different concentrations of iron nanoparticles in aqueous solution: 0, 10, and 20 µg/L. The changes in the microwave resonance curve were observed as the iron nanoparticles concentrations were increased. As was expected the changes in the MUT concentration bring the change in the S11 reflection coefficient for both magnitude and resonant frequency. As we can see from Fig. 2 (b) the measured S11 reflection coefficient had shown increasing behavior at resonant frequency (1.75 GHz) when the concentration of the nanoparticles in MUT was increased.
Figure 2b shows that resonant frequency also sensitive to the change of MUT concentration and by increasing the nanoparticles concentration in MUT the resonant frequency shifts with decreasing. The relationship between S11 and nanoparticles concentration is definitely which is crucial for accurate sensor development. The S11 trend variate with a slope of 0.0352 dB/(µg/L) while the resonant frequency variate with a slope of 0.25 MHz/(µg/L). The measured sensitivity of the system is 0.05 dB, thus the minimum detectable concentration of the developed sensor is about 1.4 and 0.2 µg/L by ΔS11 and Δf measurement, respectively. Note that the experiment with the empty cuvette did not have any essential role while when the cuvette was fallen with MUT, the resonant frequency has a shift of around 25 MHz due to the strong change in impedance of the whole system as shown in Fig. 2 (a). In the experiment, the reference intensity level (–28.54 dB at 1.75 GHz) was taken when cuvette filled with 2 mL DI water.
The microwave parameters of the sensor are also changed by changing the volume and temperature of the MUT. Figure 3 shows the dependence of microwave reflection coefficient change ΔS11 and resonant frequency shift Δf on volume of MUT for various concentrations of iron nanoparticles. As follows from Fig. 3, the changes is biggest at 2 mL MUT volume for S11 both magnitude and frequency parameter. Thus, it was decided to make further measurements with the volume of 2 mL, because in this case, the changes in the microwave response of the sensor are greatest.
Figure 4 shows the dependence of the minima of the measured microwave reflection coefficient S11 on the temperature for the different MUT: (a) DI water, Ag aqueous solution with concentration of 20 µg/L, and (c) Fe aqueous solution with concentration of 20 µg/L at the resonant frequency near 1.75 GHz and 2 mL MUT. The effect of temperature on the S11 of the sensor is slight at relatively low temperatures (up to 36°C), while at relatively high temperatures (higher than 36°C) is increased drastically. The slope of this dependence is 0.13, 0.08, 0.17 dB/°C (0.13 dB/°C in average) in the range of 25–36°C and 0.44, 0.4, 0.39 dB/°C (0.41 dB/°C in average) in the range of 37–50°C for the DI water, Ag/DI water solution (20 µg/L), Fe/DI water solution (20 µg/L), respectively. The growth rate 37–50°C range is higher about 3.2 time compare with 25–36°C range.
The shift of the measured microwave reflection S11 for different metals is 0.5 dB for Fe vs. 1.2 dB for Ag at low temperatures (<27°C). This change is possible if the dielectric permittivity of MUT change by Δεeff = 0.02 for Fe and Δεeff = 0.05 for Ag according to transmission line theory [14]. While, the estimated change in dielectric permittivity of MUT due to Fe or Ag nanoparticles is in order 10–8 according to Eq. (1). Therefore, the change in measured signal cannot cause only by changes in effective permittivity of MUT. We assume that the measurable microwave response is due to structural clusters in water enhanced by metal nanoparticles. The additional formed clusters in water close to sizes of microwave exposure wavelength. This fact is confirmed by the temperature behavior of MUT. The microwave signal for different MUTs is significant differs at temperatures <36°C, and almost same at >36°C: with increasing of temperature the formed structures are destroyed and microwave signal going to be same even of DI water. Therefore, the microwave response from MUT caused by structured clusters in size of wavelength additionally enchased by metal nanoparticles.
4 CONCLUSION
In this work, we demonstrated a microwave stripline resonant sensor based on a double quadratic profile for determination the concentration of the iron nanoparticles in an aqueous solution by a non-destructive method.
The microwave response of the sensor to the change of the iron nanoparticles concentration in the aqueous solution is 0.035 dB/(µg/L) for S11 and 0.25 MHz/(µg/L) for Δf. The minimum detectable concentration of the iron nanoparticles is about 1.4 µg/L (by S11) and 0.2 µg/L (by Δf) for a sample volume of 2 mL.
We suppose that the microwave response of MUT/sensor system is caused by structural clusters in water in size of operating wavelength caused by the presence of iron nanoparticles. The results showed the ability of the developed microwave sensor to serve as a biosensor for the determination of the concentration of impurities and detergents in liquid environments.
REFERENCES
Adejumoke, A.I., Babatunde, O.A., Abimbola, P.O., Tabitha, A.A.-A., Adewumi, O.D., and Toyin, A.O., Water Challenges of an Urbanizing World, Ch. 3: Water Pollution: Effects, Prevention, and Climatic Impact, https://doi.org/10.5772/intechopen.72018
Owa, F.D. Mediterranean J. Soc. Sci., 2013, vol. 4, p. 2039.
Sharma S. and Bhattacharya A., Appl. Water Sci., 2017, vol. 7, p. 1043.
Rashmi V. and Pratima, D., Recent Res. Sci. Technol., 2013, vol. 52, p. 98.
Arif, T.J., Azam, M., Siddiqui, K., Ali, A., Choi, I., and Rizwanul Haq, Q.M., Int. J. Mol. Sci., 2015, vol.16, 29 592.
Rasalingam, Sh., Peng, R., and Koodali R.T., J. Nanomater., 2014, Article ID 617405.https://doi.org/10.1155/2014/617405
Yang, J., Hou, B., Wang, J., Tian, B., Bi, J., Wang, N., Li, X., and Huang, X., Nanomaterials, 2019, vol. 9, p. 424.
Gennarelli, G., Romeo, S., Scarfi, M.R., and Soldovieri, F., IEEE Sensors J., 2013, vol. 13, p. 1857.
Odabashyan, L., Babajanyan, A., Baghdasaryan, Zh., Kim, S., Kim, J., Friedman, B., Lee, J.-H., and Lee, K., MDPI Sensors, 2019, vol. 19, p. 5525.
Hovhanisyan, B., Hambaryan, D., Odabashyan, L., and Babajanyan, A., Proc. Yerevan State University: Phys.Math. Sci., 2019, vol. 53, p. 132.
Minasyan, B.J., Odabashyan, L.A., Baghdasaryan, Zh.A., Babajanyan, A.Zh., and Lee, K., Proc. Yerevan State University: Phys.Math. Sci., 2019, vol. 53, p. 60.
Abrahamyan, T., Khachatryan, R., Hambaryan, D., Hovhannisyan, B., Minasyan, B., Odabashyan, L., and Babajanyan, A., J. Contemp. Phys. (Armenian Ac. Sci.), 2019, vol. 54, p. 196.
Markel, A.V., J. Opt. Soc. Am.: A, 2016, vol. 33, p. 1244.
Pozar, D.M., Microwave Engineering, 4th ed., John Wiley & Sons, New York, 2012.
ACKNOWLEDGMENTS
The authors are grateful to Kh.V. Nerkararyan for useful discussions and comments.
Funding
The State Committee for Science and Education of the Ministry of Education and Science of the Republic of Armenia supported this work in the framework of the Research Project # 18T-1C114.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
About this article
Cite this article
Odabashyan, L., Margaryan, N., Ohanyan, G. et al. Detection of Iron Nanoparticles in Aqueous Solutions by Microwave Sensor. J. Contemp. Phys. 55, 171–175 (2020). https://doi.org/10.3103/S1068337220020097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068337220020097