Abstract
The efficiency of using rare earth metals largely depends on their impurity composition, which affects the structure and properties of materials. Before the analytical control of materials based on rare earth elements (REEs) and the starting materials for their production, the task is to determine both macrocomponents with high accuracy and impurities with high sensitivity, correctness, and precision. To determine the impurities in REE-based materials in the range from 10–5 to 5.0 wt %, a complex of methods of atomic emission and mass spectral analysis is frequently used. However, the analysis of REE-based materials, even using these modern highly sensitive methods, is a difficult task due to spectral and matrix interferences. Therefore, different separation/preconcentration procedures are needed to determine both rare earth and non-rare-earth impurities. This article reviews publications of preconcentration methods for spectral and mass spectral methods of analysis of materials based on REEs and some other analytical methods. It is shown that the most common approaches are liquid extraction and chromatography. Sorption, cloud-point extraction, and precipitation are also used. There is no universal approach. Each method discussed in this article has its advantages and limitations. The analytical completion of the method confirms the effectiveness of the selected separation/preconcentration method in each specific case.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Rare earth metals are widely used in industries connected with electronics, nuclear engineering, and phosphor materials [1–3]. Impurity elements noticeably affect the properties of materials based on rare earth elements (REEs). Therefore, sensitive, multielement, and reliable methods are required to determine rare earth impurities (REIs) and non-rare-earth impurities (NREIs) to meet the needs for quality control of promising functional materials based on REEs.
The application of solid-state highly sensitive methods (for example, mass spectrometry with different ionization sources) for quantitative analysis is frequently complicated because of the absence of reliable calibration and control samples despite ultimate analytical performance in terms of sensitivity. Therefore, technologies combining high-temperature atomization sources and sample dissolution have been actively implemented in recent decades.
However, these methods (inductively coupled plasma mass spectrometry (ICP-MS) and atomic emission spectrometry (ICP-AES)) have limitations connected with matrix and interelement influence, which is the most typical of REEs having multiline spectra [4, 5]. The required sensitivity and selectivity cannot be reached without preliminary extraction and preconcentration of impurities.
Spectral overlaps at ICP-AES of REE-based materials constitute a significant problem discussed in a large number of works [6–13]. It was shown in [11–13] that the presence of line interferences in REEs results in a noticeable worsening of detection limits of required elements (Na, Ca, Ni, Fe, Co, Cr, Cd, etc.). Dilution of sample solutions, use of comparison elements, and introduction of additives did not give correct results when the content of impurities was lower than 10–3–10–2 wt %. In this case, in the analysis of sample solutions, it is proposed to use calibration solutions with a composition close to the analyzed one and preliminary separation of the matrix to take into account and minimize spectral interferences of the matrix element [11–13].
The main limitations of ICP-MS are the matrix effect and interference from polyatomic ions [14–23]. The matrix effect can be compensated by using an internal standard because the intensity of the analytical signal of its element and analyte of interest decreases simultaneously with an increase in matrix element concentration [24, 25]. However, the interference problem can limit the analytical possibilities of ICP-MS for many REE-based materials, especially high-purity ones. For example, the authors of [23] developed the method of REI, Ba, and Pb determination in Gd-based contrast agents used in magnetic resonance imaging with contrast enhancement as well as in gadolinium oxide used as a precursor. Since in ICP-MS of Gd-based substances nGd16O+ and nGd16O1H+ polyatomic interferences are observed, which interfere with the determination of Tm, Yb, and Lu, a part of the study was devoted to the investigation of the possibility of their calculation. However, it was impossible to solve the problem of detecting low concentrations of these elements even with a higher resolution.
The given examples are far from being an exhaustive set of publications devoted to the problems in determining target impurities in ICP-AES and ICP‑MS. Therefore, it appears to be relevant to review methods and approaches to separation of the matrix and/or preconcentration of rare earth and non-rare-earth impurities concerning the analysis of rare earth metals and materials based on them. This work summarizes the trends in this area with an emphasis on the methods of spectral and mass spectral analysis.
PRECONCENTRATION OF RARE EARTH IMPURITIES
There are many ways to preconcentrate impurities in rare earth materials [26–58]. Procedures based on liquid and liquid-solid-phase extraction, sorption, and coprecipitation are used. Each approach has its own features. Extraction and preconcentration of REEs from rare earth matrices are especially interesting. This is connected with the fact that REEs have similar chemical and physicochemical properties. Even trace amounts of REEs can significantly change the functional characteristics of materials. Table 1 presents summarized information on the application of REE extraction methods combined with spectrometric methods of analysis; below, we will consider them in more detail.
Chromatographic methods are the most widely used for group preconcentration or separation of REIs from the base element owing to their high efficiency and separation rate. They allow one to separate ions with close chemical properties, including REEs. The separation of these ions by common chemical methods is extremely complicated. As seen in Table 1, works are mainly devoted to the determination of impurity elements in different REE oxides, including high-purity ones; ICP-AES and ICP-MS are used. In this case, it is the separation of impurity elements from the analyzed base that makes it possible to solve frequently occurring problems associated with spectral interferences.
Liquid-solid chromatography [26–44] is based on the separation of the liquid (sample solution) and solid (sorbent) phases, after which impurities are extracted when eluted with a suitable solvent (as a rule, diluted inorganic acids in case of REIs). Organic solvents can also be used as eluents for the subsequent ICP-MS or ICP-AES analysis combined with a special input system. α-Hydroxyisobutyric acid (α-HIBA) is one of the effective eluents for the separation of individual REEs [26]. In recent years, chromatography with chelate resins as a stationary phase has attracted growing attention for separation and preconcentration of REIs. The following substances are used: 2-ethylhexyl hydrogenethylhexy phosphonate (EHEHP) [28, 29], 2-ethylhexyl hydrogen 2-ethylhexyl phosphonate (commercial name P507) [32–41], 2-ethylhexyl hydrogen 2-ethylhexylphosphonate (PC-88A) [42], Amberite XAD-7HP resin [44]. Ion exchange resins Dowex AG 1W [48] and Dowex 50W-X8 [52] are also applied.
Thus, the authors of [28, 29] analyzed high-purity Gd2O3 and metal Yb by ICP-MS spectroscopy after chromatographic separation with EHEHP. The use of EHEHP in Gd2O3 analysis eliminated interferences of GdH, GdO, and GdOH in Tb, Tm, Yb, and Lu [28]. The limits of REE determination were 0.002–0.05 µg/g; the accuracy was 1.0–7.5%. The developed approach made the analysis of high-purity (99.99–99.9999%) Gd2O3 possible. Thus, the isobar interferences of atomic and molecular ions due to the matrix element were effectively eliminated.
The authors of [19–21, 30] carried out a number of studies on the determination of trace amounts of REE impurities in a high-purity rare earth matrix, combining high-performance liquid chromatography (HPLC) followed by ICP-MS analysis.
Sorbents used in preconcentration systems for REI determination can be modified chemically by means of complexing reagents [30, 31]. For example, the authors of [11] developed a type of resin impregnated with solvent (ionic liquid) IL-SIR to improve liquid-solid-phase extraction of metal ions based on imidazolium type. In this study, [C8mim] [PF6] containing Cyanex923 was immobilized on XAD-7 resin, which promoted an increase in mass transfer efficiency, i.e., reducing the time of establishing equilibrium from 40 to 20 min, increasing the efficiency of extraction from 29 to 80%. In addition, the new IL-SIR allowed one to conduct efficient separation of Y(III) from Sc(III), Ho(III), Er(III), and Yb(III) by adding a water-soluble complexing agent. Thus, liquid-solid-phase extraction with IL-SIR combined with the method of complexation can be considered as an effective strategy to increase the efficiency of mass transfer and selectivity of extraction based on ionic liquid.
The studies are relevant in creating new sorbents to extract REEs by liquid-solid-phase chromatography. Thus, the authors of [53] developed a new sorbent based on mesoporous silicon oxide and obtained by ion imprinting technology for the selective extraction of dysprosium with liquid-solid-phase chromatography in acidic media. New studies can be expected on the development of ecological solutions for selective preconcentration of REIs in the analysis of REE-based materials.
Liquid extraction is widely used for preconcentration and separation of REIs in REE-based materials. In this method, an analyte is distributed between two immiscible liquid phases (usually aqueous and organic). Before the analysis with spectral methods, as a rule, re-extraction of the released substances from the organic phase into the aqueous one is conducted (most often using dilute solutions of hydrochloric or nitric acids). However, measurement directly from the organic phase is also possible. Liquid extraction of La(III) from geological samples, including monazite sand, was carried out in [49] using calix[4]resorcinarene-hydroxamic acid (C4RAHA) in ethyl acetate, which exhibits a high affinity for La(III). The extract is injected directly into an inductively coupled plasma spectrometer, which greatly increases the sensitivity of the analysis.
When liquid extraction is used, separation and preconcentration are characterized by a high extraction factor. As seen in Table 1, in recent years, liquid extraction has found application in the analysis of REE ores [45–51, 54, 55] and is rarely used in the analysis of oxides of individual REEs. For example, the authors of [54] separated La from Pr and Nd (with subsequent determination by the ICP-AES method), and La and Nd were extracted from monazite in [55].
As seen in Table 1, the following extractants for separating REIs from a rare earth base in the analysis of rare earth ores have been widely used recently: bis-(2-ethyl hexyl) hydrogen phosphate (HDEHP) [43], 2-ethylhexylphosphonic acid mono-2-ethylhexyl ester [45], N-phenyl-(1,2-methanofullerene C60)61-formohydroxamic acid (PMFFA) [46], calix[4]resorcinarene hydroxamic acid (C4RAHA) [49], Cyanex 921 [51], Cyanex 272, D2EHPA, PC88A, and Cyanex 301 [54], trioctylphosphine oxide (TOPO) and trialkylphosphine oxide (TRPO) in kerosene [55]. Liquid extraction has some drawbacks such as the necessity of using a large number of organic solvents, a multistage process, and the formation of wastes harmful to the environment.
Cloud-point extraction by nonionic surfactants (NSA) at a cloud point temperature is an effective preconcentration method that can be used to extract REIs from REE-based materials [50]. Compared with conventional extraction by organic solvents, cloud-point extraction is characterized by increased preconcentration coefficients and operational safety owing to the low flammability of nonionic surfactants. Taking into account the toxicity of most organic solvents, cloud-point extraction is considered as a “green” alternative to traditional liquid extraction. In [50], a spectrophotometric technique was developed using cloud-point extraction of trace amounts of scandium from monazite into the modified micellar phase Triton X-100. The use of spectrophotometric determination in this method is dictated by the very small volume of the phase obtained after extraction and the use of organic reagents to dissolve the analyzed phase, which complicates the analysis by ICP-MS or ICP-AES.
In addition to the methods for REI separation listed above, deposition is also applied when using ICP-MS and ICP-AES. As seen in Table 1, this method is mainly used to analyze ores and concentrates of REEs. The main requirement for this method is that the precipitant must be easily separated from the matrix solution. This can be performed by filtration, centrifugation, and sediment washing. In addition, the precipitant should be pure and readily available. This method is simple, and different analyte ions can be preconcentrated and separated from the matrix simultaneously.
Various inorganic or organic precipitating substances are used precipitation of REEs. For example, in [57], the separation of europium from a mixture of REEs was conducted in two stages. The first stage was the reduction of europium with metal zinc to its bivalent oxidation state. The second stage included the precipitation of the resulting Eu(II) chloride using a sulfate salt in an inert medium, while other rare earth sulfates remained in the solution. The precipitated Eu(II) sulfate was allowed to settle, and the residual Eu content in precipitant was determined by the spectrophotometric method. The disadvantages of this method include the complexity and duration of the process.
A few works describe the application of sorption methods for preconcentration/separation of REIs before the analysis of REE-based materials. For example, the authors of [56] proposed a sorption-luminescence method of terbium determination in natural Transcarpathian clinoptilolite without the use of synthetic organic compounds and toxic solvents. Optimal conditions of preparation of luminophore are sorption of Tb(III) on zeolite in a borate buffer solution with pH 8.25 and subsequent calcination of clinoptilolite–Tb(III) samples at t = 500°C. To excite luminescence, beams with the wavelength λ = 220 nm were used. The luminescence intensity at λ = 545 nm was chosen as an analytical parameter for quantitative estimation of terbium. The determined Tb(III) concentration range is from 3 to 1140 ng/mL. The proposed method can be used to determine the terbium content in the presence of many REEs, including the determination of trace amounts of terbium ions in synthetic aqueous solutions and intermetallic compounds. In this case, the use of the single-element method is justified because the selective extraction of only one component is conducted. On the basis of the region of determined values, ICP-AES and ICP-MS can also be applied in this combined technique.
Work [58] is devoted to the modification of kaolin (natural clay) using radiation polymerization to obtain a polyacrylamide–acrylic acid–kaolin composition for sorption of lanthanum (light REE), europium (heavy REE), and uranium from monazite ore. The studies were conducted with different sorbent weights (0.1 and 0.2 g). The degree of extraction for Eu3+, La3+, and U\({\text{O}}_{2}^{{2 + }}\) was 94.6, 91.6, and 73.4%, respectively. The mechanism of sorption of lanthanum and europium ions on the PAM-AA-K sorbent is mainly in the form of Ln(OH)2+, and it is in the form of U\({\text{O}}_{2}^{{2 + }}\) for uranium. The concentration of each element was measured by ICP-AES.
On the basis of the given studies, it can be concluded that group preconcentration of REIs followed by multicomponent analysis is widely used for REE oxides as precursors for obtaining materials based on them. This approach is suitable to estimate “academic purity” [59] and control correctness in determining the target purity by individual impurities. Methods of individual preconcentration and single-element methods are used for the selective evaluation of a small amount of elements.
PRECONCENTRATION OF NON-RARE-EARTH IMPURITIES
Besides rare earth impurities, an important role in the purity of REE-based materials is taken by non-rare-earth impurities (NREIs). For example, in the manufacture of phosphors and optical ceramics, high-purity rare earth oxides are used, and critical impurities are colored ions Fe, Ni, Cr, Co, Cu, V, Mn, etc., as well as some lanthanides [5, 11–13]. The properties of such materials deteriorate significantly even at a content of these impurities above 10–5 wt %. However, determining the low concentrations of these elements by ICP-MC and ICP-AES is a complicated task because of matrix and spectral phenomena. Therefore, combined methods of analysis are used to find NREIs.
Table 2 summarizes information on the application of methods of NREI extraction combined with spectrometric methods of analysis. As is seen, preconcentration is more frequently used for the subsequent ICP-AES analysis which has good characteristics for the determination of impurities in pure REE oxides: low determination limits, high accuracy, and a broad dynamic range of determined concentrations. Nevertheless, emission spectra of many REEs are complex, which limits the selectivity of this method owing to spectrum overlapping. However, ICP-AES and ICP‑MS are widely used for this purpose in combination with preliminary separation/preconcentration of analytes. It was shown in [73] that direct determination of trace impurities in high-purity rare earth elements by ICP-AES is complicated, and chemical separation and preconcentration of analytes are required. The authors determined trace amounts of Co, Cr, Cu, Fe, Mo, Ni, and Zn in high-purity europium oxide by means of liquid extraction with APDC-IBMK solvent followed by ICP-AES analysis. However, since APDC is useful only for transition metal ions and is not effective for complex formation with aluminum, alkali, and alkaline earth metal ions, this extraction method is inapplicable for simultaneous extraction of these elements.
More versatile and frequently found in articles is the method of liquid-solid-phase chromatography [60–69]. The following sorbents are used for the group chromatographic separation of rare earth impurities from a rare earth matrix: di-(2-ethylhexyl)phosphoric acid (HDEHP) [60, 61], TOPO-Levextrel [62], XAD‑16 resin [63], P507 resin [64, 69], and silica gel with activated carbon [65, 67, 68].
Coprecipitation is also used to extract NREIs. A technique described in [13] is developed for the analysis of pure Y2O3 by ICP-AES with preconcentration of impurities from a nitrate solution of the sample by coprecipitation with a trace amount of yttrium hydroxide. The main advantages of the developed technique of analysis are a wide range of simultaneously determined impurities (28 elements) and the use of available reagents (H2O, HNO3, NH3 aqueous solution).
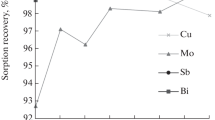
Sorption is a promising method of NREI preconcentration in REE-based materials. This is one of the modern approaches to cleaning the analytical signal of the desired elements in such complex and multicomponent objects. Thus, the efficiency of polymer thioether sulfur- and nitrogen-containing sorbents for separating As, Bi, Sb, Cu, and Te from a rare earth matrix with subsequent arc spectral analysis was demonstrated in [73].
CONCLUSIONS
This review demonstrates that, to eliminate interferences strongly limiting analytical possibilities of spectrometric methods of analysis, methods of separation and preconcentration of impurities are widely used. Liquid extraction and liquid-solid-phase chromatography are the most widespread methods for studying REE-based materials equivalently allowing one to extract the impurities and subsequently determine them by the spectral method of analysis. Precipitation, sorption, and complex formation are also successfully used. The use of preconcentration, undoubtedly, makes it possible to eliminate the problem of the interfering influence of the matrix and, in most cases, to reduce the limits of determination. However, this time-consuming procedure requires highly qualified analysts.
REFERENCES
Li, D., Li, Y., Pan, D., Zhang, Z., and Choi, C.J., Prospect and status of iron-based rare-earth-free permanent magnetic materials, J. Magn. Magn. Mater., 2019, vol. 4691, pp. 535–544.
Baranovskaya, V.B., Karpov, Yu.A., Petrova, K.V., and Korotkova, N.A., Actual trends in the application of rare-earth metals and their compounds in the production of magnetic and luminescent materials: A review, Russ. J. Non-Ferrous Met., 2021, vol. 62, no. 1, pp. 10–31.
Ritter, S.K., A whole new world for rare earths. How the technologically important metals rose from obscurity to ubiquity, Chem. Eng. News, 2017, vol. 95, no. 34, pp. 30–34.
Petrova, K.V., Baranovskaya, V.B., and Korotkova, N.A., Direct inductively coupled plasma optical emission spectrometry for analysis of waste samarium–cobalt magnets, Arabian J. Chem., 2022, vol. 15, no. 1, p. 103501.
Korotkova, N.A., Petrova, K.V., and Baranovskaya, V.B., Analysis of cerium oxide by mass-spectral and atomic-emission methods with inductively-coupled plasma, J. Anal. Chem., 2021, vol. 76, no. 12, pp. 1–12.
Marathe, S.M., Biswas, S.S., Patil, P.B., and Murty, P., An ICP-AES method for the determination of heavy rare earth elements (Eu–Lu) in high purity yttrium oxide, Microchim. Acta, 1992, vol. 109, no. 5, pp. 261–268.
Li, J.Y., Yang, J., and Dong, Z.R., Determination of 14 rare earth elements in high purity europium oxide by ICP-AES, Spectrosc. Spectral Anal. (Beijing, China), 1995, pp. 71–74.
Dong, R. and Xin, R., ICP-AES determination of Co-existing rare-earth impurities in high-purity europium oxide, Phys. Test. Chem. Anal., Part B, 2004, vol. 40, no. 3, pp. 135–137.
Biswas, S.S., Sethumadhavan, A., and Murty, P.S., Determination of Y, Sm, Eu, Gd, Dy, Ho, Er in high purity terbium oxide by ICP-AES, Microchim. Acta, 1991, vol. 103, no. 1, pp. 71–77.
Cai, B., Hu, B., and Jiang, Z., Direct determination of trace rare earth elements in high purity Y2O3 using fluorination assisted electrothermal vaporization inductively coupled plasma atomic emission spectrometry with slurry sampling, Fresenius’ J. Anal. Chem., 2000, vol. 367, no. 3, pp. 259–263.
Evdokimov, I.I. and Pimenov, V.G., Determination of impurities in optical ceramics and its precursors by atomic spectrometry, Vestn. Nizhegorod. Univ. im. N.I. Lobachevskogo, 2013, no. 4 (1), pp. 98–102.
Evdokimov, I.I. and Pimenov, V.G., Determination of impurities in high-purity neodymium-doped yttrium oxide nano-powders by inductively coupled plasma atomic emission spectrometry, Anal. Kontrol, 2013, vol. 17, no. 2, pp. 170–176.
Evdokimov, I.I. and Pimenov, V.G., Analysis of yttrium oxide by inductively coupled plasma atomic emission spectrometry and coprecipitation of impurities, Zavod. Lab., Diagn. Mater., 2016, vol. 82, no. 9, pp. 5–12.
Javis, K.E., Gray, A.L., and Houk, R.S., Handbook of Inductively Coupled Plasma Mass Spectrometry, Glasgow: Blackie, 1992.
Becker, J.S. and Dietze, H.J., State-of-the-art in inorganic mass spectrometry for analysis of high-purity materials, Int. J. Mass Spectrom., 2003, vol. 228, nos. 2–3, pp. 127–150.
Day, J.A., Caruso, J.A., Becker, J.S., and Dietze, H.-J., Application of capillary electrophoresis interfaced to double focusing sector field ICP-MS for nuclide abundance determination of lanthanides produced via spallation reactions in an irradiated tantalum target, J. Anal. At. Spectrom., 2000, vol. 15, pp. 1343–1348.
Kozono, S., Takahashi, S., and Haraguchi, H., Determination of boron in high-purity tantalum materials by on-line matrix separation/inductively coupled plasma mass spectrometry, Analyst, 2002, vol. 127, pp. 930–934.
Zhang, X.Q., Yi, Y., Liu, Y.L., Li, X., Liu, J.L., Jiang, Y.M., and Su, Y.Q., Direct determination of rare earth impurities in high purity erbium oxide dissolved in nitric acid by inductively coupled plasma mass spectrometry, Anal. Chim. Acta, 2006, vol. 555, pp. 57–62.
Pedreira, W.R., Sarkis, J.E.S., Rodrigues, C., Tomiyoshi, I.A., and Queiroz, C.A., Determination of trace amounts of rare earth elements in highly pure praseodymium oxide by double focusing inductively coupled plasma mass spectrometry and high-performance liquid chromatography, J. Alloys Compd., 2001, vol. 49, pp. 323–324.
Pedreira, W.R., Sarkis, J.E.S., Rodrigues, C., Tomiyoshi, I.A., Queiroz, C.A., Queiroz, C.A., and Abrão, A., Determination of trace amounts of rare earth elements in high pure lanthanum oxide by sector field inductively coupled plasma mass spectrometry (HR ICP-MS) and high-performance liquid chromatography (HPLC) techniques, J. Alloys Compd., 2002, vol. 344, pp. 17–20.
Pedreira, W.R., Sarkis, J.E.S., Queiroz, C.A., Rodrigues, C. Tomiyoshi, I.A. and Abrão, A., Determination of trace amounts of rare-earth elements in highly pure neodymium oxide by sector field inductively coupled plasma mass spectrometry (ICP-SFMS) and high-performance liquid chromatography (HPLC) techniques, J. Solid State Chem., 2003, vol. 171, pp. 3–6.
Balaram, V., Strategies to overcome interferences in elemental and isotopic geochemical analysis by quadrupole inductively coupled plasma mass spectrometry: A critical evaluation of the recent developments, Rapid Commun. Mass Spectrom., 2021, vol. 35, pp. 1–29.
Douraied, B.S. and Jean-Alix, B., Determination of rare earth elements in gadolinium-based contrast agents by ICP-MS, Talanta, 2021, vol. 221, p. 121589.
Shabani, M.B. and Masuda, A., Sample introduction by on-line two-stage solvent extraction and back-extraction to eliminate matrix interference and to enhance sensitivity in the determination of rare earth elements with inductively coupled plasma mass spectrometry, Anal. Chem., 1991, vol. 63, pp. 2099–2105.
Adrian, A.A., Amman Inductively coupled plasma mass spectrometry (ICP MS): A versatile tool, J. Mass Spectrom., 2007, vol. 42, pp. 419–427.
Kawabata, K., Kishi, Y., Kawaguchi, O., Watanabe, Y., and Inoue, Y., Determination of rare-earth elements by inductively coupled plasma mass spectrometry with ion chromatography, Anal. Chem., 1991, vol. 63, no. 19, pp. 2137–2140.
Qin, S., Jiang, Z., Hu, B., Qin, Y., and Hu, S., HPLC combined with ICP-MS for the determination of trace amounts of rare earth impurities in high-purity La2O3 by using 2-ethylhexyl hydrogen-2ethylhexylphosphonate resin as a stationary phase, Fresenius’ J. Anal. Chem., 2000, vol. 367, no. 3, pp. 250–253.
Cao, X., Yin, M., and Li, B., Determination of rare earth impurities in high purity gadolinium oxide by inductively coupled plasma mass spectrometry after 2-ethylhexylhydrogen-ethylhexy phosphonate extraction chromatographic separation, Talanta, 1999, vol. 48, no. 3, pp. 517–525.
Zhang, X., Liu, J., Yi, Y., Liu, Y., Li, X., Su, Y., and Lin, P., Determination of rare earth impurities in high purity samarium oxide using inductively coupled plasma mass spectrometry after extraction chromatographic separation, Int. J. Mass Spectrom., 2007, vol. 260, no. 1, pp. 57–66.
Pedreira, W.R., Queiroz, C.A., Abrao, A., Rocha, S.M., Vasconcellos, M.E., Boaventura, G.R., and Pimentel, M.M., Trace amounts of rare earth elements in high purity samarium oxide by sector field inductively coupled plasma mass spectrometry after separation by HPLC, J. Alloys Compd., 2006, vol. 418, nos. 1–2, pp. 247–250.
Sun, X., Peng, B., Ji, Y., Chen, J., and Li, D., The solid-liquid extraction of yttrium from rare earths by solvent (ionic liquid) impregnated resin coupled with complexing method, Sep. Purif. Technol., 2008, vol. 63, no. 1, pp. 61–68.
Yin, M., Li, B., Zhang, Y., and Cao, X.D., Determination of rare earths impurities in high purity Eu 2 O 3 by ICP-MS, Anal. Lab.-Beijing, 1999, vol. 18, pp. 1–6.
Qin, S., Bin, H., Yongchao, Q., Wanjau, R., and Zucheng, J., Determination of trace rare earth impurities in high-purity cerium oxide by using electrothermal vaporization ICP-AES after HPLC separation with 2‑ethylhexylhydrogen 2ethylhexylphosphonate resin as the stationary phase, J. Anal. At. Spectrom., 2000, vol. 15, pp. 1413–1416.
Shuai, Q., Qin, Y., Hu, B., Xiong, H., and Jiang, Z., Determination of rare earth impurities in high-purity lanthanum oxide using electrothermal vaporization/ICP-AES after HPLC separation, Anal. Sci., 2000, vol. 16, pp. 957–961.
Zishu, W., Xiyun, S., and Lijun, L.P., Extraction chromatographic separation and spark source mass spectrometric determination of 14 rare earth impurities in extra-pure Dy2O3, J. Instrum. Anal., 1995, no. 4, pp. 06–09.
Xinjun, Z. and Yongfeng, Z.M.Z., The determination of 14 kinds of rare-earth element as impurity in high pure lutetium oxide by means of P507 chromatographic separation and ICP-AES, Uranium Geol., 1998, no. 1, pp. 6–10.
Lu, Y.Q., Cao, Y.-Q., Wang, L.-H., and Xin, R.-X., Analysis of rare earth elements in high purity europium oxide, Rare Met., 2005, vol. 24, no. 3, pp. 216–220.
Jinying, L., Binghua, G., and Jingsu, G., Determination of 14 rare earth impurities in high purity europium oxide by axis-view ICP-AES with chromatographic separation, Rock Miner. Anal., 1994, vol. 3, pp. 21–28.
Zhiguang, W., Changqing, W., and Xing, W., Chemical preconcentration spectrographic determination of 14 rare earth impurities in 5N Gd2O3, Anal. Lab., 1998, vol. 1, p. 1.
Xigun, S., Zishu, W., and Furong, H., Determination of fourteen rare earths impurities in high purity Nd2O3 by P(507) extraction chromatography separation-spark source mass spectrometry, J. Chin. Mass Spectrom. Soc., 1996, vol. 1, p. 1.
Li, W., Peng, C., Yuan, P., Qi, W., Kuang, Z., and Xu, C., Determination of 14 rare earth impurities in Sm2O3, Eu2O3 and Gd2O3 of ultra-high purity by extraction chromatography atomic emission spectrometry, J. Instrum. Anal., 1998, vol. 1, pp. 18–21.
Kobayashi, S., Wakui, Y., Kanesato, M., Matsunaga, H., and Suzuki, T.M., Chromatographic separation and inductively coupled plasma atomic emission spectrometric determination of the rare earth metals contained in terbium, Anal. Chim. Acta, 1992, vol. 262, no. 1, pp. 161–166.
Premadas, A. and Khorge, C.R., Solvent extraction separation of heavy rare earth elements from light rare earth elements and thorium: ICP-AES determination of REEs and yttrium in monazite mineral, At. Spectrosc., 2006, vol. 27, no. 5, pp. 170–177.
Kim, J.-G., Separation of heavy rare earth elements with extraction chromatography, Curr. Nanosci., 2014, vol. 10, pp. 11–15.
Miranda, P. and Zinner, L.B., Separation of samarium and gadolinium solutions by solvent extraction, J. Alloys Compd., 1997, vol. 249, nos. 1–2, pp. 116–118.
Agrawal, Y.K., Liquid-liquid extraction, separation, preconcentration, and ICP AES determination of lanthanum and cerium with N-Phenyl-(1,2-methanofullerene C60)61-formohydroxamic acid, Fullerenes, Nanotubes, Carbon Nanostruct., 2004, vol. 12, no. 3, pp. 545–570.
Zhao, Z., Lyu, H., Guo, X., Dong, Y., Wang, Y., and Sun, X., The synergistic extraction by combined ammonium and phosphonium type ionic liquids for rare earth elements separation, Hydrometallurgy, 2017, vol. 174, pp. 234–247.
Hastiawan, I., Bings, N.H., and Broekaert, J.A.C., Development and optimization of pre-concentration procedure of rare-earth elements (REEs) in their minerals, using microwave - assisted sample dissolution for ICP-atomic emission spectrometric detection, Procedia Chem., 2015, vol. 17, pp. 93–98.
Jain, V.K., Pillap, S.G., and Mandal, H.C., Liquid-liquid extraction, preconcentration and transport studies of lanthanum(III) with calix [4]resorcinarene-hydroxamic acid (C4RAHA), J. Chil. Chem. Soc., 2007, vol. 52, no. 2, pp. 1177–1181.
Amin, A.S., Kassem, M.A., and Moalla, S.M.N., Determination of scandium in monazite and environmental samples using cloud point extraction coupled with a spectrophotometric technique, RSC Adv., 2016, vol. 6, p. 73797.
Guirguis, L., Orabi, A., and Mohamed, B., Extraction and derivative spectrophotometric assay of Sm(III), Pr(III) and Nd(III) from REEs monazite concentrate, Int. J. Environ. Anal. Chem., 2019, no. 6, pp. 1–20.
Abdou, A.A., Abdelfattah, N.A., and Weheish, H.L., Development of a procedure for spectrophotometric determination of Pr(III) from rare earth elements (REEs) concentrate, SN Appl. Sci., 2019, vol. 1, no. 5, pp. 1–9.
Zheng, X., En-li, L., Zhang, F., Yan, Y., and Pan, J., Efficient adsorption and separation of dysprosium from NdFeB magnets in an acidic system by ion imprinted mesoporous silica sealed in a dialysis bag, Green Chem., 2016, vol. 18, no. 18, pp. 5031–5040.
Banda, R., Jeon, H.S., and Lee, M.S., Solvent extraction separation of La from chloride solution containing Pr and Nd with Cyanex 272, Hydrometallurgy, 2012, vol. 121, pp. 74–80.
El-Nadi, Y.A., Lanthanum and neodymium from Egyptian monazite: synergistic extractive separation using organophosphorus reagents, Hydrometallurgy, 2012, vol. 119, pp. 23–29.
Vasylechko, V.O., Gryshchouk, G.V., Zakordonskiy, V.P., Vasylechko, L.O., Schmidt, M., Leshchack, I.M., Kalychak, Ya.M., and Bagday, S.R., Sorption-luminescence method for determination of terbium using Transcarpathian clinoptilolite, Talanta, 2017, vol. 174, pp. 486–492.
Rabie, K.A., Sayed, S.A., Lasheen, T.A., and Salama, I.E., Europium separation from a middle rare earths concentrate derived from Egyptian black sand monazite, Hydrometallurgy, 2007, vol. 86, nos. 3–4, pp. 121–130.
Metwally, S.S., Hassan, R.S., El-Masry, E.H., and Borai, E.H., Gamma-induced radiation polymerization of kaolin composite for sorption of lanthanum, europium and uranium ions from low-grade monazite leachate, J. Radioanal. Nucl. Chem., 2018, vol. 315, no. 1, pp. 39–49.
Karpov, Yu.A., Churbanov, M.F., Baranovskaya, V.B., Lazukina, O.P., and Petrova, K.V., High pure substances–prototypes of elements of periodic table, Pure Appl. Chem., 2020, vol. 92, no. 8, pp. 1357–1366.
Lee, G.S., Uchikoshi, M., Mimura, K., and Isshiki, M., Separation of major impurities Ce, Pr, Nd, Sm, Al, Ca, Fe, and Zn from La using bis (2-ethylhexyl) phosphoric acid (D2EHPA)-impregnated resin in a hydrochloric acid medium, Sep. Purif. Technol., 2010, vol. 71, no. 2, pp. 186–191.
Yang, X.J., Extractive chromatographic separation and inductively coupled plasma atomic emission spectrometric determination of trace impurities in high purity europium oxide, Talanta, 1994, vol. 41, no. 11, pp. 1807–1813.
Yang, X.J. and Guan, J.S., End-on viewed inductively coupled plasma for the determination of trace impurities in high-purity scandium oxide by extraction chromatography, Anal. Chim. Acta, 1993, vol. 279, no. 2, pp. 261–272.
Choi, K.S., Lee, C.H., Kim, J.G., Kim, W.H., and Kang, J.G., Separating Ag, B, Cd, Dy, Eu, and Sm in a Gd matrix using 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester extraction chromatography for ICP analysis, Talanta, 2007, vol. 71, no. 2, pp. 662–667.
Ruth, W., Zu-cheng, J., Bin, H., Yong-chao, Q., Ying-liang, W., and Xia-shi, Z., Simultaneous determination of trace rare earth elements and other elements in high purity terbium oxide (Tb4O7) by ICP-AES after HPLC separation using P507 resin, Wuhan Univ. J. Nat. Sci., 2002, vol. 7, no. 2, pp. 212–216.
Hongnian, J., Lian, L., Zhenhuan, L., and Zucheng, J., Determination of trace non rare earth metals in high purity lanthanum oxide by ICP AES with preconcentration on active carbon silica gel microcolumn in a flow injection system, J. Anal. Sci., 1996, vol. 12, p. 03.
Hou, L., Wang, S., and Li, J., Determination of 17 trace impurity elements and erbium Zr–U–Er alloy by chromatographic separation with tributyl phosphate and ICP-AES, Spectrosc. Spectral Anal. (Beijing, China), 1996, no. 3, pp. 5–8.
Zucheng, J.H.J., Determination of trace non rare earth elements in high purity rare earth oxides by ICP AES, J. Wuxi Univ. Light Ind., 1999, no. 1, p. 5.
Ji, H., Liao, Z., Sun, J.-G., and Jiang, Z., Study and application of a method for the determination of metallic elements by ICP-AES with preconcentration on an active carbon-silica gel microcolumn in a FI system, Fresenius’ J. Anal. Chem., 1998, vol. 360, no. 6, pp. 721–723.
Wanjau, R., Jiang, Z.-C., Hu, B., and Shuai, Q., Determination of non-rare earth impurities in high purity lanthanum oxide by inductively coupled plasma atomic emission spectrometry after HPLC separation using P507 resin, Chin. J. Rare Earths, 2001, vol. 19, no. 4, pp. 299–303.
Karandashev, V.K., Zhernokleeva, K.V., Turanov, A.N., Baranovskaya, V.B., and Karpov, Yu.A., Determination of admixtures of high-melting metals in rare-earth metals and their compounds, J. Anal. Chem., 2012, vol. 67, no. 4, pp. 340–348.
Agrawal, Y.K. and Vora, S.B., Selective extraction and separation of thorium from monazite using N-phenylbenzo-18-crown-6-hydroxamic acid, Microchim. Acta, 2003, vol. 142, no. 4, pp. 255–261.
Jiafeng, W. and Zhengmin, Z., Determination of impurities in high purity europium oxide by inductively coupled plasma-atomic emission spectrometry after reduction-extraction separation, Metall. Anal., 1998, vol. 18, pp. 1–5.
Koshel’, E.S., Baranovskaya, V.B., and Doronina, M.S., Arc atomic emission analysis of rare earth metals and their oxides with preliminary sorption concentration of impurities, Zavod. Lab., Diagn. Mater., 2018, vol. 84, no. 11, pp. 9–14.
Funding
This study was financially supported by the Russian Science Foundation (project no. 20-13-00180).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by N. Saetova
About this article
Cite this article
Petrova, K.V., Es’kina, V.V., Baranovskaya, V.B. et al. Separation and Preconcentration of Impurities in Rare-Earth-Based Materials for Spectrometric Methods. Russ. J. Non-ferrous Metals 63, 510–525 (2022). https://doi.org/10.3103/S106782122205008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S106782122205008X