Abstract
This paper proposes a method for quickly estimating the average flotation capacity of minerals according to the kinetic experiment without finding the flotation spectrum at which the first moments of distribution are calculated by the coefficients of the polynomial approximation of the logarithmic form of the kinetics. Using the example of copper-nickel ore, it is shown that the application of this method is effective in the multiparameter problem of comparative assessment of reagents. The ten parameters assessed included the average floatability of the target minerals (chalcopyrite and pentlandite), pyrrhotite and rock; the flotation selectivity coefficients of target minerals relative to pyrrhotite and rock; and the levels of copper and nickel losses from bulk flotation tailings. A visual representation of the interdependencies of parameters is achieved using diagrams showing the effect of flotation reagents on groups of parameters: average flotation, selectivity coefficients, metal losses, and selectivity relative to rock. The influence of butyl xanthate, Aeroflot, diesel fuel, and waste rock depressants—carboxymethyl cellulose (CMC) and acidified water glass (with a total consumption of 130 g/t collectors, 5–10 g/t diesel fuel, 200 g/t CMC, acidified water glass—500 g/t)—on the estimated parameters under conditions of collective flotation is determined. It is found that the addition of Aeroflot and diesel fuel to the main reagent collector xanthate increases the selectivity of pentlandite and chalcopyrite flotation relative to pyrrhotite and rock-forming component. The introduction of acidified liquid glass into the reagent mode increases the selectivity of the flotation of nickel and copper sulfides relative to the rock. CMC additives impair the selectivity of copper flotation. The quantitative effects of each individual parameter are taken into account in the integral rating assessment of the prospects of using reagent combinations for copper-nickel ore in terms of a set of ten parameters. The proposed method can be further used for a mass comparative evaluation of flotation reagents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
When optimizing flotation technology, the problem of a compromise arises between the desire to minimize the loss of metal with tailings and to obtain a rich concentrate in a simple flotation scheme, since the selectivity of flotation and the collecting capacity of the reagent mode, which determines the quality of the concentrate, are to a certain extent opposed. For ores containing several useful components, which are processed according to developed selective and collective-selective schemes, the problem is especially difficult if the selectivity of the reagents is evaluated directly by the quality of the foam products.
In this work, it is proposed to carry out a comprehensive assessment of the action of the reagents simultaneously by the level of losses of the valuable component with tailings and by the kinetic characteristics of the flotation of various ore minerals. The kinetics of flotation is a multifactorial process influenced by many components: the size of the floated grains [1‒5], the shape of the particles [6, 7], the size of the bubble [8], the hydrophobicity of the mineral surface [9], the reagent mode [10–12].
For the first time, the idea of modeling based on the use of the distribution of mineral particles by the first-order flotation rate constant (flotation spectrum) was proposed in [13]. In the future, the approach was widely developed. The technique for studying the flotation spectrum used in this work is based on the dependence of the flotation kinetics on the fractional composition [14, 15]:
or
where k is the constant of the flotation rate (floatability), 1/min; γu(k) is the spectrum of material floatability; γ(k) is the regular component of the flotation spectrum; γ0 is the singular component of the floatability spectrum (nonfloatable residue); L[γ(k)] is the Laplace transform of the function γ(k); \(\bar {\gamma }(t)\) is the kinetics of flotation (extraction or release of a component into foam); and δ(k) is the Dirac delta function.
EXPERIMENTAL PROCEDURE
This study was carried out on copper-nickel ore; the main ore minerals are represented by chalcopyrite, pentlandite, and pyrrhotite. The rock-forming component is dominated by quartz, amphiboles, layered silicates, olivine, and iron hydroxides. The main bulk flotation operation was simulated. Grinding size before flotation is 58–60% of class –0.044 mm. Flotation experiments were carried out on a 237 FL laboratory flotation machine with a chamber volume of 0.75 L. To record the kinetic characteristics, the fractional removal of the foam was carried out at times from 15 s to 40 min. Foam and chamber products were analyzed by X-ray fluorescence with a determination of the mass fraction of copper, nickel, and sulfur. The content of chalcopyrite, pentlandite, and pyrrhotite minerals was determined by recalculating elemental analysis data using the concentrations of elements in minerals established by microprobe analysis. The rock grade was determined to be residual from these three minerals.
The effect on the flotation of additives to xanthate of reagents—collectors of Aeroflot and diesel fuel—as well as depressants carboxymethyl cellulose (CMC) and acidified water glass (WG), was studied. As the basic reagent mode, the flotation mode of collective flotation was adopted, including the following reagent consumption: potassium butyl xanthate 85 g/t and Aeroflot NBA 45 g/t. An additional apolar collector (diesel fuel) in the amount of 5 and 10 g/t and depressants CMC [16] and WG [17] in the amount of 200 and 500 g/t, respectively, were dosed to the basic regime. A comparison with flotation with potassium butyl xanthate alone in the presence of T-92 blowing agent with a flow rate of 60 g/t is carried out.
PROCESSING AND ANALYSIS OF EXPERIMENTAL DATA
Mathematical processing of the experimental kinetics of flotation was carried out by two methods differing in complexity and volume of data. The problem of finding the floatability distribution k (flotation spectrum) on the kinetics of flotation is the problem of inverting the Laplace transform of this distribution [18]. Methods for inverting the Laplace transform were developed in detail in [19]. Like many inverse problems, the inverse of the Laplace transform is classified as ill-posed [20]. In practice, this means that, with the actually achievable accuracy and reproducibility of the flotation experiment, conventional methods of treatment will give an unstable solution. It is necessary to use regularization methods in one form or another. In its general form, such an algorithm is applied, for example, in [21]. A review of methods for inverting the Laplace transform in the study of flotation kinetics and the solution of the problem by the method of expansion in Laguerre polynomials are given in [22].
There are simpler methods for obtaining a stable solution to Eq. (1) based on the approximation of the expected distribution by some convenient function, on the basis of a priori assumptions about the form of the floatability spectrum. Approximation by the gamma function is often used, which satisfactorily approximates the distributions of many properties of mineral particles [13, 23]. Other approximating functions were also used. The solution of the equation in this case is reduced to finding the parameters of the corresponding distribution.
Since previous studies have shown the presence of two components significantly different in terms of the flotation rate in copper-nickel ore, the bimodal gamma distribution was taken as a model [24]. The flotation spectrum and flotation kinetics in this model are represented by the following expressions:
where G is Euler’s gamma function.
Fitting of the model was carried out by selecting parameters a1, a2, p1, p2, γ1. The indices for the parameters correspond to the numbers of fractions: fraction 1 is a material with a higher flotation rate, fraction 2 is a material with a lower one. The nonflotation residue was determined experimentally at a maximum flotation time of 40 min.
Figures 1 and 2 show the calculation results: flotation spectra and fractional composition of minerals for one of the experiments, illustrating the capabilities of the method. The average deviation of the model kinetics from the experimental data does not exceed 2 rel %. The most significant deviations (up to 10%) are observed at a flotation time of 15 s, i.e., at the very beginning of the process, when the level and rate of foam release are unstable.
Thus, the approximation of the flotation spectrum by an adequate model, which in this case was a bimodal gamma distribution, makes it possible to obtain detailed and comprehensive information on the fractional composition of the material. However, in the case of mass research, such a composition of information appears to be redundant. This complicates the analysis of the results and increases the complexity of the calculations.
When setting up studies on the effect of the reagent regime on the kinetics of flotation, information on the law of distribution of flotation is redundant in most cases. For an integrated assessment, as will be shown below, it is sufficient to determine the average floatability of the mineral (the first moment of the floatability spectrum). This problem, in contrast to the general one, is correctly formulated, which makes it possible to obtain a stable estimate without using a model spectrum, even under conditions of significant errors in the flotation kinetic experiment.
The method used for calculating the moments of the flotation spectrum is based on the relationship of the Laplace transform \(L\left[ {\gamma (k)} \right]\) and the generating function of moments \(M(t)\) flotation spectrum [25].
The logarithm of the generating function of moments is expanded into a power series:
where \({{\chi }_{i}}\) is the ith semi-invariant distribution \(\gamma (k)\).
Thus, taking into account (2), we obtain
or
The resulting expression corresponds to the tradition of presenting the kinetics of flotation in a logarithmic form, accepted in the enrichment, and makes the physical meaning of the decomposition coefficients transparent. Using the definitions of semi-invariants, we can write
where \(\bar {k}\) is expected floatability k (average floatability), σ2 is variance of flotation k, and g1 is the coefficient of asymmetry of the distribution γ(k).
Thus, the proposed formula allows one to find the mathematical expectation, variance, asymmetry coefficient, and other characteristics of the flotation spectrum by the coefficients of the polynomial approximating the curve of the flotation kinetics in a logarithmic form.
The ratio of the average floatability of minerals in vapors is further used as an indicator of selectivity:
(i) chalcopyrite (pentlandite)/pyrrhotite—pyrrhotite selectivity;
(ii) chalcopyrite (pentlandite)/rock—rock selectivity.
DISCUSSION
Based on the experimentally obtained flotation kinetics in each reagent mode, the average floatability of minerals and the selectivity coefficients of the flotation of chalcopyrite and pentlandite relative to pyrrhotite and rock were calculated and the contents of copper and nickel in the chamber product were determined.
Figures 3–5 show the average floatability of minerals, selectivity coefficients, and losses of metals with tailings, respectively. Each point in the diagram represents a kinetic flotation experiment. A flotation mode based on xanthate and a foaming agent (ButX + T-92) was chosen as the starting point. One modification of this mode is the replacement of the foaming agent and part of the xanthate with Aeroflot (ButX + Aeroflot). The rest of the experiments were the addition of acidified liquid glass, CMC, or diesel fuel (DF) to this collective mixture. The development of the reagent mode from xanthate and blowing agent to more complex formulations is represented by connecting arrows. The course of each line shows a simultaneous change in two parameters: the average floatability of two minerals (Fig. 3), selectivity for rock and pyrrhotite (Fig. 4), or selectivity for rock and loss of metals in tailings (Fig. 5). For convenience, the arrows also indicate the numerical axes on which the corresponding parameters are displayed.
The data in Fig. 3 show that the additions of all the studied reagents increase the floatability of chalcopyrite. The floatability of pentlandite changes ambiguously with a change in the flow rate of the apolar collector, and the addition of depressants somewhat increases the rate of its flotation. The floatability of the rock decreases with the addition of both collectors and depressants. Apolar collector additives have an ambiguous effect on the floatability of pyrrhotite. Additives of depressants increase the floatability of pyrrhotite and pentlandite.
Data on changes in the flotation ability of the mineral do not yet allow drawing conclusions about the selectivity of the process or the level of metal losses. Additional information is provided by selectivity coefficients, defined as the ratio of floatability of target minerals and rock or pyrrhotite (Fig. 4).
Figure 4 shows that additives of all investigated collectors (Aeroflot and diesel fuel) increase the selectivity of flotation of both pentlandite and chalcopyrite relative to both pyrrhotite and rock. In general, the best selectivity results are demonstrated by the combined use of xanthate, Aeroflot, and diesel fuel. Aeroflot’s selectivity can be interpreted by the known mechanism of joint action of collectors with different functional groups. The selectivity of diesel fuel is explained by the traditional mechanism of action of apolar collectors, fixing on hydrophobic grains and accelerating their flotation.
The effect of depressants on flotation selectivity is ambiguous. Liquid glass increases rock selectivity for both metals. CMC worsens the flotation selectivity in the case of chalcopyrite and somewhat improves in the case of pentlandite. Both depressants significantly impair the flotation selectivity with respect to pyrrhotite. Compared to Fig. 3, this is associated with a significant increase in the floatability of pyrrhotite.
A comparison of rock selectivity and tailing loss provides additional information (Fig. 5). The data in this chart demonstrates the reduction in losses when using collector combinations, with the use of Aeroflot making the largest contribution. The effect of liquid glass on metal losses is different for copper and nickel: copper losses are significantly reduced, while nickel losses remain at the same level, which indicates a different nature of the losses of these two minerals. The effect of CMC on metal losses is insignificant.
Conclusions about the multidimensional action of flotation reagents during the collective flotation of copper-nickel ore are conveniently summarized in the form of a rating assessment (see Table 1). This assessment was carried out according to the following characteristics of the action of the reagents: an increase in the rate of flotation of the mineral, an increase in the selectivity of flotation of metals to pyrrhotite and rock, and a decrease in the loss of metals with tailings. Rating scores range from – – – (a strong negative effect) to +++ (a strong positive effect). Evaluation (+ –) means a small uneven-sign effect at different costs; evaluations (0+) and (0–) mean no action or a weak positive or negative action, respectively. For a convenient comparison of indicators, it was taken into account that, within the framework of the model of material composition, where copper is represented entirely by chalcopyrite and nickel, by pentlandite, the copper flotation rate is equal to the chalcopyrite flotation rate and the nickel flotation rate is equal to the pentlandite flotation rate. Therefore, the flotation rate and selectivity are assigned to the respective metal.
This rating assessment makes it possible to assess the prospects of various reagent modes in relation to the subsequent research and development of an industrial scheme. An unambiguous conclusion can be made regarding the prospects for the use of carboxymethyl cellulose. The effect of CMC on rock selectivity with an insignificant quantity of layered silicates in the ore is insignificant, but the pyrrhotite flotation rate increases significantly and the corresponding selectivity indices fall. Further work with this reagent is impractical.
Aeroflot seems to be the most versatile reagent in this regard. It increases the selectivity of both chalcopyrite and pentlandite flotation with respect to both pyrrhotite and rock. At the same time, it significantly reduces the loss of metals with tailings. The use of diesel-fuel additives to a mixture of xanthate and Aeroflot at a rate of 5–10 g/t provides a significant increase in selectivity while maintaining the level of losses with tailings. However, the use of this reagent can have a negative effect on the subsequent selection of the collective concentrate, if carried out. It is not excluded that the simultaneous use of acidified liquid glass will further improve the selectivity and copper losses.
To fully exploit the possibilities of increasing the selectivity of flotation, it is necessary to change the configuration of the collective cycle scheme.
CONCLUSIONS AND RECOMMENDATIONS
Using the example of studies in the collective flotation of copper-nickel ore, a method is proposed for quickly assessing the average flotation of minerals according to the kinetic experiment, in which the first moments of distribution are calculated by the coefficients of polynomial approximation of the logarithmic form of kinetics.
Ten parameters were estimated: average floatability of target minerals (chalcopyrite and pentlandite), pyrrhotite and rock; flotation selectivity coefficients of target minerals relative to pyrrhotite and rock; and levels of copper and nickel losses from bulk flotation tailings. Based on the results of the studies, the effects of the tested reagents—Aeroflot, diesel fuel, liquid glass, and CMC—were ranked. It has been established that the selectivity of pentlandite and chalcopyrite flotation relative to pyrrhotite and the rock-forming component is achieved by introducing Aeroflot and diesel fuel into the reagent mode to the main reagent-collector (xanthate). Additives to the reagent regime of gangue depressants, in particular acidified water glass, increases the selectivity of the flotation of nickel and copper sulfides relative to the rock minerals, while the introduction of CMC reduces the selectivity of copper flotation.
A comprehensive assessment of flotation reagents with the simultaneous use of the characteristics of metal losses in the chamber product and the selectivity indices of flotation of valuable minerals relative to pyrrhotite and rock made it possible to systematize information on the potential of using flotation reagents. The low labor intensity and reliability of the proposed method, relative to the well-known method for calculating the full range of flotation with a subsequent calculation of the average flotation, make it possible to recommend it for mass comparative tests of reagents.
This study revealed the possibilities of increasing the selectivity of collective flotation of copper–nickel ore. Under the conditions of the simplest collective cycle, which includes one or two operations, it is impossible to realize potential selectivity. In this regard, it is possible to recommend the formulation of studies of a more developed flotation scheme, which assumes the possibility of separating a flow of material that concentrates unopened aggregates of minerals for subsequent regrinding and re-flotation. Such a scheme will make it possible to achieve the capabilities of the reagents and improve the quality of the collective concentrate.
REFERENCES
Ai, G., Yang, X., and Li, X., Flotation characteristics and flotation kinetics of fine wolframite, Powder Technol., 2017, vol. 305, pp. 377–381. https://doi.org/10.1016/j.powtec.2016.09.068
Yalcin, E. and Kelebek, S., Flotation kinetics of a pyritic gold ore, Int. J. Miner. Process., 2011, vol. 98, pp. 48–54. https://doi.org/10.1016/j.minpro.2010.10.005
Zhang, J. and Subasinghe, N., Development of a flotation model incorporating liberation characteristics, Miner. Eng., 2016, vol. 98, pp. 1–8. https://doi.org/10.1016/j.mineng.2016.05.021
Rahman, R.M., Ata, S., and Jameson, G.J., The effect of flotation variables on the recovery of different particle size fractions in the froth and the pulp, Int. J. Miner. Process., 2012, vols. 106–109, pp. 70–77. https://doi.org/10.1016/j.minpro.2012.03.001
Vinnett, L., Marion, C., Grammatikopoulos, T., and Waters, K.E., Analysis of flotation rate distributions to assess erratic performances from size-by-size kinetic tests, Miner. Eng., 2020, vol. 149, article no. 106229. https://doi.org/10.1016/j.mineng.2020.106229
Szczerkowska, S., Wiertel-Pochopien, A., Zawala, J., Larsen, E., and Kowalczuk, P.B., Kinetics of froth flotation of naturally hydrophobic solids with different shapes, Miner. Eng., 2018, vol. 121, pp. 90–99. https://doi.org/10.1016/j.mineng.2018.03.006
Ma, G., Xia, W., and Xie, G., Effect of particle shape on the flotation kinetics of fine coking coal, J. Cleaner Prod., 2018, vol. 195, pp. 470–475. https://doi.org/10.1016/j.jclepro.2018.05.230
Eskanlou, A., Huang, Q., Chegeni, M.H., Khalesi, M.R., and Abdollahy, M., Determination of the mass transfer rate constant in a laboratory column flotation using the bubble active surface coefficient, Miner. Eng., 2020, vol. 156, article no. 106521. https://doi.org/10.1016/j.mineng.2020.106521
Kowalczuk, P.B. and Zawala, J., A relationship between time of three-phase contact formation and flotation kinetics of naturally hydrophobic solids, Colloids Surf., A, 2016, vol. 506, pp. 371–377. https://doi.org/10.1016/j.colsurfa.2016.07.005
Nikolaev, A.A., So, Tu., and Goryachev, B.E., Investigation of the regularities of the kinetics of flotation of non-activated sphalerite with compositions of sulfhydryl collectors by the flotation method, Gorn. Inf.-Anal. Byull., 2015, no. 9, pp. 86–95.
Zhu, H., Li, Y., Lartey, C., Li, W., and Qian, G., Flotation kinetics of molybdenite in common sulfate salt solution, Miner. Eng., 2020, vol. 148, article no. 106182. https://doi.org/10.1016/j.mineng.2020.106182
Wang, Z., Si, J., Song, Z., Zhang, P., Wang, J., Hao, Y., Li, W., Zhang, P., and Miao, S., Precise and instrumental measurement of thermodynamics and kinetics of froth flotation by langmuir-blodgett technique, Colloids Surf., A, 2020, vol. 605, article no. 125337. https://doi.org/10.1016/j.colsurfa.2020.125337
Imaizumi, T. and Inoue, T., Kinetic considerations of froth flotation, Proc. 6th Int. Mineral Processing Congress, Cannes, 1963, pp. 581–593.
Tikhonov, O.N., Zakonomernosti effektivnogo razdeleniya mineralov v protsessakh obogashcheniya poleznykh iskopaemykh (Regularities of the Effective Separation of Minerals in the Processes of Mineral Processing), Moscow: Nedra, 1984.
Rubinshtein, Yu.B. and Filippov, Yu.A., Kinetika flotatsii (Flotation Kinetics), Moscow: Nedra, 1980.
Sibanda, V., Khan, R., and Danha, G., The effect of chemical reagents on flotation performance of a pentlandite ore: An attainable region approach, Powder Technol., 2019, vol. 352, pp. 462–469. https://doi.org/10.1016/j.powtec.2019.04.062
Feng, B., Zhang, W., Guo, Y., Peng, J., Ning, X., and Wang, H., Synergistic effect of acidified water glass and locust bean gum in the flotation of a refractory copper sulfide ore, J. Cleaner Prod., 2018, vol. 202, pp. 1077–1084. https://doi.org/10.1016/j.jclepro.2018.08.214
Kapur, P.C. and Mehrotra, S.P., Estimation of the flotation rate distributions by numerical inversion of the Laplace transform, Chem. Eng. Sci., 1974, vol. 29, pp. 411–415.
Ryabov, V.M., Chislennoe obrashchenie preobrazovaniya Laplasa (Numerical Inversion of the Laplace Transform), St. Petersburg: St. Petersburg State Univ., 2013.
Tikhonov, A.N. and Arsenin, V.Ya., Metody resheniya nekorrektnykh zadach (Methods for Solving Ill-Posed Problems), Moscow: Nauka, 1986.
Konovalov, S.A. and Tikhonov, O.N., Flotometric analysis by using the variational principle in the regularization method, Tsvetn. Metall., 1982, no. 1, pp. 100–105.
Pascual, R.L. and Whiten, W.J., The determination of floatability distribution from laboratory batch cell tests, Miner. Eng., 2015, vol. 83, pp. 1–12. https://doi.org/10.1016/j.mineng.2015.08.007
Harris, C.C. and Chakravarti, A., Semi-batch froth flotation kinetics: species distribution analysis, Trans. Soc. Min. Eng. AIME, 1970, vol. 247, pp. 162–172.
Bu Xiangning, Xie Guangyuan, Peng Yaoli, Ge Linhan, and Ni Chao, Kinetics of flotation. Order of process, rate constant distribution and ultimate recovery, Physicochem. Probl. Miner. Process., 2016, vol. 53, pp. 342–365.
Korolyuk, V.S., Portenko, N.I., Ckorokhod, A.V., and Turbin, A.F., Spravochnik po teorii veroyatnostei i matematicheskoi statistike (Handbook on Theory of Probability and Mathematical Statistics), Moscow: Nauka, 1985.
Funding
This work was carried out as part of project no. 0287-2021-0014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
About this article
Cite this article
Bragin, V.I., Burdakova, E.A., Usmanova, N.F. et al. Comprehensive Assessment of Flotation Reagents by Their Influence on Metal Losses and Flotation Selectivity. Russ. J. Non-ferrous Metals 62, 629–636 (2021). https://doi.org/10.3103/S1067821221060055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821221060055


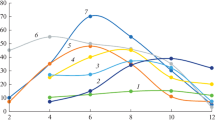



 Fraction 1,
Fraction 1,  fraction 2, and
fraction 2, and  nonflotation residue.
nonflotation residue.

