Abstract
This paper provides the first part of a study on the effect of magnesium on the structural phase composition and physical and mechanical properties of nanostructured aluminum–magnesium composite materials with the composition AlxMgy + 0.3 wt % C60 fullerene. Composite powders are obtained by the simultaneous mechanical activation of the initial materials in a planetary ball mill in an argon atmosphere. It is found that the powders have a complex hierarchical structure made up of 50–200 μm aggregates consisting of 5–10 μm strong high-density agglomerates, which in turn are a combination of nanoscale (30–60 nm) crystallites. It is found that the increase in magnesium concentration in the composite up to 18 wt % makes it possible to obtain crystallites with an average size of less than 30 nm during mechanical activation, while the size of aggregates is less than 50 μm. The maximum solubility of magnesium in aluminum with a crystallite size of 30–70 nm during mechanical activation is 15 wt % (17 at %). Using the differential scanning calorimetry method, it is found that nanostructured composites undergo irreversible structural phase transformations during heat treatment in a temperature range of 250–400°C: recrystallization, decomposition of the α-solid solution of magnesium in aluminum, and the formation of intermetallic β-(Al3Mg2), γ-(Al12Mg17) and carbide (Al4C3) phases. In addition, the Raman spectra contain peaks that, according to some sources, correspond to covalent compounds of aluminum with C60 fullerene—aluminum–fullerene complexes. The data that have been obtained will be used in further research to determine the parameters for the thermobaric treatment of nanocomposite powder mixtures in order to obtain and test bulk samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Today, one of the promising ways to improve the strength properties of composite materials is to reduce the size of the matrix grains to a nanometer scale in conjunction with additional modification with various nanosized high-modulus and reactive particles—oxides, nitrides, carbides, carbon nanostructures (CNS), etc. Such metal-matrix nanostructured composite materials (NCMs) can surpass traditional metals and alloys in a series of properties and characteristics [1–4].
In papers [5–7], the possibility of obtaining high-strength aluminum-matrix alloys modified with C60 fullerene by powder metallurgy methods, including high-energy processing in a planetary ball mill and subsequent hot extrusion, is shown. In the process of such processing, not only are the components mixed and the matrix material ground, but the intensive coldworking of grains also occurs, leading to an increase in their physical and mechanical properties. High strength properties (ultimate tensile strength up to 750 MPa, bending strength up to 900 MPa, and hardness up to 2200 MPa) were achieved when using strain-hardened aluminum alloy of AMg6 grade as a matrix material and C60 fullerene as a strengthening phase. In this case, the determined optimum concentration of fullerene was 0.3 ± 0.05 wt %.
The choice of AMg grade as a matrix material is due to the fact that, depending on the magnesium content, alloys of this series are characterized by high strength, ductility, corrosion resistance, temperature stability, good weldability, etc. The main alloying component of this group of alloys is magnesium, which forms an α-solid solution in the aluminum lattice in a wide concentration range and various intermetallic phases.
Commercially available alloys of the AMg type contain up to 11 wt % magnesium. If its concentration reaches 6 wt %, then the alloys are classified as structural strain-hardenable; if there is more magnesium, then they are usually used in a cast state with additional heat treatment. This conditional division is due to the fact that, at room temperature in aluminum with a grain size of 100–200 μm, about 7 at % Mg can be present in the form of a supersaturated α-solid solution [8]. A further increase in the concentration of magnesium in a solid solution is possible only by quenching the supersaturated of α-solid solution up to 16.23–18.60 at % [9, 10]. An increase in the magnesium content in α-solid solution leads to an improvement in the strength characteristics, but the plasticity of the alloys sharply decreases [11].
An α-solid solution much more supersaturated with magnesium can be obtained by various methods. Thus, in [12], a solid solution with a magnesium content of up to 20 wt % was prepared by mechanoactivation treatment; in [13], by crystallization under pressure up to 3 GPa, the concentration of magnesium in the solution reached 25 wt %; and [14] shows the possibility of increasing it up to 40 at %. At the same time, the authors note the dependence of the limiting Mg concentration on the type of starting materials and the conditions for the implementing the processes of mechanoactivation and mechanoalloying [15, 16]. It is worth noting that the resulting extreme solutions were metastable and, at insignificant heat exposure, sharply decomposed with the formation of elemental magnesium, γ-(Al12Mg17), and an intermediate β' phase.
The use of carbon nanostructures is an additional strengthening factor of NCM. As shown in [5, 6, 17], molecules of fullerene C60 under certain conditions form strong and stable covalent bonds with aluminum atoms. In addition, during grinding, the presence of CNS promotes the refinement of Al grains and, during subsequent heat treatment, it prevents recrystallization processes [17, 18].
Thus, the combination of various hardening mechanisms makes it possible to achieve a significant increase in a number of key physical and mechanical properties of NCM; however, the mechanisms and features of interfacial interactions and transformations with a characteristic size of structural elements on the order of several tens of nanometers now require additional comprehensive study.
This work is aimed at investigating the effect of the magnesium concentration on the structural phase composition of powders of nanostructured aluminum–magnesium composite materials modified with fullerene C60.
RESEARCH METHODOLOGY
We used lathe chips of AMg3 and AMg6 alloys (GOST 4784–97) with an average size of 3 × 3 × 1 mm as the starting materials for manufacture of NCM. To change the Mg concentration during grinding, 3, 6, 9, 12, 15, and 18 wt % magnesium chips were added to the AMg6 alloy chips (STP TU KOMP 3-303-10, chemical pure, purity is 99.9%, and size is 5 × 15 × 0.5 mm). Hereinafter, these materials are designated as AMg 3, 6, 9, 12, 15, and 18, respectively.
As a modifying phase, 0.3 wt % of C60 fullerene (99.5%, powder fraction is 100 μm) was additionally introduced into the NCM composition.
Cooperative processing of raw materials, mechanoalloying (MA), was carried out in an AGO-2U planetary ball mill. The total MA time reached 60 min (in 5-min cycles with 3-min interruptions) at a rotation speed of carrier of 1800 rpm. The ratio of the masses of the processed material and the grinding bodies was 1 : 20.
To reduce the degree of chafing in the MA process, the containers and grinding bodies were prelined with the original AMg3 aluminum alloy, which made it possible to minimize the contamination of the NCM with foreign impurities.
To prevent oxidation and the occurrence of other undesirable reactions, all manipulations with the starting materials and the resulting powders were carried out in an insulating glove box filled with argon, which maintains the purity of the atmosphere on oxygen and water vapors at least 0.1 ppm.
Structural studies of the obtained samples were carried out using optical (Olimpus BX51), scanning (JSM-7600F), and transmission (JEM-2010) electron microscopy. The X-ray phase analysis (XPA) of the powders was carried out on a PANalytical Empyrean diffractometer in CuKα-radiation. On the basis of the obtained diffraction patterns using the MAUD program, the size of the coherent scattering regions (CSR)—the average crystallite size—was determined by the method of functional parameters. Studies using Raman scattering (RS) spectroscopy were carried out using a setup based on a TRAIX 552 spectrometer and a CCD Spec-10 detector, 2KBUV 2048×512. BeamLok 2065-7S lasers (Spectra-Physics) with a wavelength of λ = 512 nm and WaveTrain lasers with λ = 257 nm were used as the exciting radiation. The thermal stability of the obtained NCMs was studied by differential scanning calorimetry (DSC) on a PerkinElmer DSC8000 setup.
RESULTS AND DISCUSSION
In the process of Ma, the average CSR size of aluminum decreases from the initial value of 200–250 nm to 25–80 nm, depending on the magnesium concentration (Fig. 1). As can be seen from the presented data, at the initial stage of grinding (the first 10–20 min) with an increase in the Mg concentration, the average CSR size of all the materials under study decreases from the initial 200–250 to 85–90 nm for the AMg3 + 0.3% C60 sample, and up to 50–55 nm for AMg18 + 0.3% C60. Further, in a time interval from 20 to 60 min, the grinding rate slows down. After 60 min of MA, the average sizes of NCM crystallites are from 20 to 70 nm, depending on the magnesium concentration. Further processing has no significant effect on the average CSR size, which can be explained by the achievement of a certain critical value, which requires an increase in the energy loading of the MA process to overcome it. It should be noted that the average CSR size for NCMs containing 18 wt % Mg was 18–20 nm, which, according to [19], is close to the theoretically possible value for aluminum crystallites at room temperature.
An increase in the intensity of grinding of NCM crystallites with an increased concentration of magnesium can be associated with a local increase in the content of the intermetallic phase and magnesium concentration in a supersaturated α solid solution [12, 13], which leads to a decrease in the mobility of dislocations; a decrease in the plasticity powder particles; and, as a consequence, to material embrittlement.
According to XRPA data, no changes in the phase composition were found in NCM powders after grinding (Fig. 2). As the magnesium concentration increases, broadening of the aluminum peaks is observed, as well as their shift to the region of smaller angles of 2θ, which is associated with a decrease in the average crystallite size and an increase in the lattice parameter of aluminum due to an increase in the concentration of the solid solution of magnesium in aluminum [20]. According to Refs [20, 21], an increase in the lattice parameter by 0.0046 Å corresponds to dissolution of 1 at % Mg in aluminum. According to XRPA data (Fig. 2) and [20, 21], the shift of X-ray lines completely correlates with the stoichiometry of the NCM, indicating the formation of a supersaturated α-solid solution with the Mg concentration of 15 wt % (17 at %). A further increase in the magnesium content up to 18 wt % (20 at %) does not lead to a noticeable increase in the concentration of Mg in the solid solution, as is evidenced by the absence of a shift of the characteristic lines of Al relative to the NCM sample with 15 wt % Mg.
Thus, based on the XRPA data, the solubility limit of magnesium in nanosized aluminum grains under the given MA conditions was determined, which was 17 at %, which is close to the equilibrium solubility limit at t = 450°C [22].
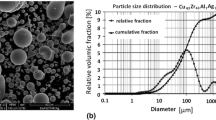
According to XRPA, TEM, and SEM data, the NCM powders have a complex hierarchical structure. Crystallites 20–70 nm in size are combined into strong high-density agglomerates (Fig. 3).
According to the TEM results, the average CSR size of aluminum, determined by XRPA, corresponds to the size of crystallites. The agglomerates, which have a predominantly quasi-globular shape, form aggregates whose sizes decrease with an increase in the magnesium content in the NCM powders (Fig. 4).
Nanostructured materials, as a rule, are in a metastable state and turn to more energetically favorable state upon the application of any external influence to them (heating, pressure, irradiation, etc.). In the case of nanostructured metallic materials, such processes are mainly manifested in the form of recrystallization and other structural phase transformations.
To study the thermal stability of the obtained NCM powders and to establish the critical parameters of further processing (consolidation), studies were carried out using DSC (Fig. 5). As can be seen from the presented data, in the temperature range of 250–400°C, the occurrence of irreversible transformations in NCM is observed, accompanied by endo- and exothermic effects. It was established by Raman scattering and XRPA that the observed thermal effects are associated with recrystallization of the matrix alloy, the decomposition of the supersaturated solid solution, the destruction of aluminum–fullerene complexes, and the formation of aluminum carbide Al4C3. The effects in the region of t = 450°С are associated with the melting of the Al–Al3Mg2 eutectic [22].
It should be noted that the structural phase composition of the studied NCM can also be influenced by the modifying phase—C60 fullerene. Its molecules are very stable by themselves; however, under conditions of mechanosynthesis and heat treatment, they can degrade to elemental carbon and form aluminum carbide Al4C3. The appearance of a carbide phase can negatively affect the strength characteristics of NCM due to the structural features of the carbide (acicular shape of particles and tendency to hydrolysis).
The Raman spectra of NCM powders after milling did not exhibit peaks of Al4C3 (ν = 492 and 857 cm–1) (Fig. 6, spectrum 1). The formation of aluminum carbide was recorded only when the samples were heated to temperatures above 320°C (Fig. 6, spectrum 2).
It should be noted that the Raman spectra also contain broad peaks in the region of ν = 750 and 1500 cm–1, which, according to some sources, can be characteristic of the so-called aluminum–fullerene complexes—covalent compounds of the Al–C60 type [5]. According to the calculations carried out in [5], the strength of such compounds can be higher than the Al–Al bond, which can have a positive effect on the strength properties of bulk NCM.
CONCLUSIONS
Thus, comprehensive studies of the effect of magnesium concentration on the structure and phase composition of nanostructured strain-hardened aluminum–magnesium alloys modified with C60 fullerene are performed, including at elevated temperatures. Based on the results, the following conclusions have been drawn.
(1) The dynamics of changes in the average size of aluminum crystallites in NCM during mechanical activation depends on the magnesium concentration, which may be due to the embrittlement of the material due to the increased magnesium content in the supersaturated α-solid solution and the precipitation of nanosized inclusions of Al–Mg intermetallic compounds at grain boundaries.
(2) The limit of saturation of the aluminum lattice with magnesium atoms in the process of mechanical activation was 17 at %. The resulting supersaturated solid solution is stable at room temperature.
(3) The structural phase composition of the NCM samples under study was stable up to a temperature of 270°C, and its excess led to the occurrence of irreversible structural phase transformations.
(4) NCM powders have a complex hierarchical structure in which large particles (aggregates) prevail, consisting of strong high-density agglomerates, which, in turn, are a set of nanosized crystallites.
Based on the data, the optimal parameters of the consolidation of mechanically activated powder mixtures of NCM will be selected.
REFERENCES
Estrin, Y., Murashkin, M., and Valiev, R., Ultrafine-grained aluminium alloys: processes, structural features and properties, in Fundamentals of Aluminium Metallurgy, Woodhead Publ., 2011, pp. 468–503.
Lloyd, D.J., Particle reinforced aluminium and magnesium matrix composites, Int. Mater. Rev., 1994, vol. 39, no. 1, pp. 1–23.
Markushev, M.V., Bampton, C.C., Murashkin, M.Yu., and Hardwick, D.A., Structure and properties of ultra-fine grained aluminium alloys produced by severe plastic deformation, Mater. Sci. Eng., A, 1997, vols. 234–236, pp. 927–931.
Murashkin, M.Yu., Kil’mametov, A.R., and Valiev, R.Z., Structure and mechanical properties of an aluminum alloy 1570 subjected to severe plastic deformation by high-pressure torsion, Phys. Met. Metallogr., 2008, vol. 106, no. 1, pp. 90–96.
Popov, M., Medvedev, V., Blank, V., Denisov, V., Kirichenko, A., Tat’yanin, E., and Zaitsev, V., Fulleride of aluminum nanoclusters, J. Appl. Phys., 2010, vol. 108, no. 9, pp. 094 317-1–094 317-6.
Evdokimov, I.A., Perfilov, S.A., Pozdnyakov, A.A., Blank, V.D., Bagramov, R.Kh., Perezhogin, I.A., Kulnitsky, B.A., Kirichenko, A.N., and Aksenenkov, V.V., Nanostructured composite materials based on Al–Mg alloy modified with fullerene C60, Inorg. Mater.: Appl. Res., 2018, vol. 9, no. 3. pp. 472–477.
Choi, K., Shin, S., Bae, D., and Choi, H., Mechanical properties of aluminum-based nanocomposite reinforced with fullerenes, Trans. Nonferrous Met. Soc. China, 2014, vol. 24, pp. 47–52.
Mal’tseva, T.V., Ozerets, N.N., Levina, A.V., and Ishina, E.A., Tsvetnye metally i splavy. Uchebnoe posobie (Nonferrous Metals and Alloys. Student’s Book), Yekaterinburg: Ural, 2019.
Starink, M.J. and Zahra, A.M., β' and β precipitation in an Al–Mg alloy studied by DSC and TEM, Acta Mater., 1998, vol. 46, no. 10, pp. 3381–3397.
Siyuan, L., Fusheng, P., Kainer, K.U., Yafang, H., and Wei, K., Progress in Light Metals, Aerospace Materials and Superconductors, Trans Tech Publ., 2007.
Mukai, T., Higashi, K., and Tanimura, S., Influence of the magnesium concentration on the relationship between fracture mechanism and strain rate in high purity Al–Mg alloys, Mater. Sci. Eng., A, 1994, vol. 176, nos. 1–2, pp. 181–189.
Schoenitz, M. and Dreizin, E.L., Structure and properties of Al–Mg mechanical alloys, J. Mater. Res., 2003, vol. 18, no. 08, pp. 1827–1836.
Jie, J.C., Zou, C.M., Wang, H.W., Li, B., and Wei, Z.J., Mechanical properties of Al(Mg) solid solution prepared by solidification under high pressures, J. Alloys Compd., 2012, vol. 510, no. 1, pp. 11–14.
Scudino, S., Sakaliyska, M., Surreddi, K.B., and Eckert, J., Mechanical alloying and milling of Al–Mg alloys, J. Alloys Compd., 2009, vol. 483, nos. 1–2, pp. 2–7.
Calka, A., Kaczmarek, W., and Williams, J.S., Extended solid solubility in ball-milled Al–Mg alloys, J. Mater. Sci., 1993, vol. 28, pp. 15–18.
Al-Aqeeli, N., Mendoza-Suarez, G., and Drew, R.A.L., XRD and TEM characterization of Al–Mg-based nanocomposite alloys, Rev. Adv. Mater. Sci., 2008, vol. 18, pp. 231–235.
Evdokimov, I.A., Khayrullin, R.R., Perfilov, S.A., Pozdnyakov, A.A., Bagramov, R.H., Perezhogin, I.A., and Blank, V.D., Nanostructured aluminum matrix composite materials, modified by carbon nanostructures, Mater. Today: Proc., 2018, vol. 5, no. 12, pp. 26 153–26 159.
Aborkin, A.V., Evdokimov, I.A., Vaganov, V.E., Alymov, M.I., Abramov, D.V., and Khor’kov, K.S., Influence of mechanical activation mode on morphology and phase composition of Al–2Mg–nC nanostructured composite material, Nanotechnol. Russ., 2016, vol. 11, pp. 297–304.
Gryaznov, V.G., Kaprelov, A.M., and Romanov, A.E., Size effects of dislocation stability in small particles and microcrystallities, Scr. Metall., 1989, vol. 23, pp. 1443–1448.
Rabinovich, M.K., Markushev, M.V., and Murashkin, M.Yu., Special features of formation of the submicrocrystalline structure in strain-heat treatment of aluminum alloy 1420 in different initial states, Met. Sci. Heat Treat., 1997, vol. 39, no. 4, pp. 172–176.
Lapovok, R., Timokhina, I., McKenzie, P.W.J., and O’Donnell, R., Processing and properties of ultrafine-grain aluminium alloy 6111 sheet, J. Mater. Process. Technol., 2008, vol. 200, nos. 1–3, pp. 441–450.
Totten, G.E. and MacKenzie, D.S., Handbook of Aluminum, vol. 1: Physical Metallurgy and Processes, Marcel Dekker, 2003.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation as part of Grant Agreement no. 075-15-2019-1307 (no. 14.574.21.0162) dated June 14, 2019, agreement identifier RFMEFI57417X0162.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by Sh. Galyaltdinov
About this article
Cite this article
Evdokimov, I.A., Khayrullin, R.R., Bagramov, R.K. et al. Nanostructured Strain-Hardened Aluminum–Magnesium Alloys Modified by C60 Fullerene Obtained by Powder Metallurgy: Part 1. The Effect of the Magnesium Concentration on the Structure and Phase Composition of Powders. Russ. J. Non-ferrous Metals 62, 132–137 (2021). https://doi.org/10.3103/S1067821221010089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821221010089